Abstract
Intracranial vertebral artery (VA) dissection has three clinical presentations: ischemia, hemorrhage, and mass effect. Imaging findings of intracranial VA dissections vary according to clinical presentation. Irregular stenosis or occlusion of the VA is the most common finding in patients with posterior fossa infarction, whereas a dissecting aneurysm is the main feature in those with acute subarachnoid hemorrhage. A chronic, giant, dissecting aneurysm can cause mass effect on the brain stem or cranial nerves, as well as distal embolism. Magnetic resonance imaging is useful for detection of intramural hematomas and intimal flaps, both of which are diagnostic of VA dissection. Multidetector computed tomography angiography is increasingly used for diagnosis of VA dissection. Catheter angiography is still beneficial for evaluation of precise endoluminal morphology of the dissection before surgical or endovascular intervention. Endovascular treatment is now considered a major therapeutic option for patients with a ruptured dissecting aneurysm or a chronic dissecting aneurysm. Anticoagulation therapy is currently considered the initial treatment of choice in patients with posterior circulation ischemic symptoms. Endovascular treatment, such as stent-assisted angioplasty or coil occlusion at the dissection site, can be performed in selected patients with posterior fossa ischemic symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vertebral artery (VA) dissection is an important cause of posterior circulation stroke and a common cause of ischemic stroke in young and middle-aged patients. The annual incidence of spontaneous VA dissection can be estimated at 1–1.5 per 100,000 [1].
The VA is divided into four anatomical segments. V1 extends from its origin to its entry into the transverse foramen of cervical vertebra 6 (C6). V2 extends from the transverse foramen of C6 to C1. V3 extends from the exit from the transverse foramen of C1 to the foramen magnum. V4, the intracranial portion of the VA, extends from the foramen magnum to the basilar artery [2]. The intracranial portion of the VA gives rise to three major branches: the posterior inferior cerebellar artery (PICA), the anterior spinal artery, and the posterior spinal artery. The largest branch of the VA is the PICA, which usually arises near the lower end of the olive and supplies the cerebellar hemisphere, inferior vermis, dorsolateral aspect of the medulla oblongata, choroid plexus of the fourth ventricle, and dentate nucleus. An anterior spinal artery arises near the end of the VA and descends anterior to the medulla oblongata to unite with the other anterior spinal artery from the opposite side at the mid-medullary level. Branches from the anterior spinal arteries are distributed to the medulla oblongata. A posterior spinal artery usually arises from the PICA but may come directly from the VA, near the medulla oblongata. Minute medullary arteries arise from the VA and its branches and are distributed widely to the medulla oblongata [3]. Histologic differences in structure exist between intracranial and extracranial VAs. Intracranial VAs have a thicker internal elastic lamina but lack an external elastic lamina, have a thinner adventitia, and have fewer elastic fibers in the media [4, 5].
VA dissection is most frequently found between the C1 and C2 cervical vertebrae (uppermost part of the V2 segment) and between C1 and the entry site into the dura (V3 segment). These segments are tightly fixed to bones (lateral masses of the C1 and C2 vertebral bodies) and the dural attachment and, thus, are most vulnerable to mechanical stress, such as rotation, hyperextension, or hyperflexion movements of the head [5–7]. Intracranial (V4 segment) VA dissections occur less frequently than extracranial VA dissections.
Dissections of the V4 segment can be classified as either traumatic or spontaneous. In the majority of cases, intracranial VA dissection occurs spontaneously. Several risk factors have been associated with spontaneous cervical artery dissection. Hereditary connective tissue disorders, particularly vascular Ehlers–Danlos syndrome, are known risk factors for spontaneous dissections. Other inherited disorders associated with spontaneous dissections include Marfan syndrome, autosomal dominant polycystic kidney disease, and osteogenesis imperfecta type I. Hyperhomocysteinemia, α1-antitrypsin deficiency, and abnormalities of neural crest cells have also been associated with an increased risk of spontaneous cervical dissections [1, 8, 9]. A recent respiratory tract infection was found to be an independent risk factor for spontaneous dissections [10]. This possibility is supported by the finding of seasonal variation in the incidence of spontaneous dissections, with a peak incidence in the fall [11]. Spontaneous dissections have also been reported in association with fibromuscular dysplasia and cystic medial necrosis. Both fibromuscular dysplasia and cystic medial necrosis, however, are nonspecific abnormalities that are associated with a variety of systemic disorders [1].
The pathogenesis of VA dissection is still controversial. It has been suggested that the acute VA dissection is caused by a sudden disruption of the internal elastic lamina (IEL), the most important layer with regard to the strength of the intracranial arterial wall [12, 13]. Subsequent penetration of circulating blood into the wall of the artery creates an intramural hematoma, the so-called false lumen. Recently, in their pathology study of the vertebral artery in patients with VA dissecting aneurysm, Yamada et al. [14] found areas of severe degeneration and calcification in the IEL of the VA wall, just proximal to the aneurysm. They hypothesized that degeneration of the IEL of this type might constitute a predisposing factor and may have led to vascular wall dissection. They also postulated that increased hemodynamic stress on the VA distal to the cervical flexures and around the VA union might contribute to the genesis of IEL degeneration, although they failed to elucidate the precise etiology of the IEL degeneration in their study. However, in several pathology studies of intracranial VA dissection, an intramural hematoma was found without any connection between the false and true arterial lumen [1, 9, 15, 16], which suggests that some VA dissections might be caused by a primary intramural hematoma. It has been suggested that possible causes of primary intramural hematoma are rupture of the vasa vasorum and rupture of new vessels formed in response to medial necrosis [15, 16].
The plane of dissection extends through the tunica media and can be subintimal or subadventitial. A subintimal dissection tends to result in luminal stenosis or occlusion by intramural hematoma. Arterial dissection may cause a dissecting aneurysm when the major dissection plane is subadventitial and the entire vessel wall is widely disrupted [12, 17, 18].
The pathogenesis of chronic dissecting aneurysm also remains unclear. Recently, Krings et al. [19] reviewed and supported the hypothesis that repeated subadventitial bleeding from the new vasa vasorum plays an important role in the pathogenesis of chronic giant dissecting aneurysm, which had been proposed by Schubiger et al. in 1980s [20, 21]. Chronic dissecting aneurysm can progressively increase in size due to the apposition of multiple layers of intramural hematoma of different ages within the vessel wall [19].
The clinical course and prognosis of patients with intracranial VA dissection range from a relatively benign course with no neurologic complications to death from severe brain injury. Clinical presentations of intracranial VA dissection can be categorized into three groups: posterior circulation ischemia or infarction, acute subarachnoid hemorrhage (SAH), and posterior fossa mass effect on the brain stem or cranial nerves [17, 18]. Imaging findings of intracranial VA dissections are also variable and are highly associated with specific clinical presentations. Recently, endovascular treatment has emerged as a major therapeutic option for patients with intracranial VA dissections, especially patients who presented with acute SAH. In this article we present the clinicoradiologic features of VA dissection involving the V4 segment and discuss the endovascular treatment options for these patients.
Clinical manifestations of intracranial vertebral artery dissection
Posterior circulation ischemic symptoms
Unilateral, occipital headache with or without ipsilateral neck pain, followed by progressive onset of ischemic symptoms, is the hallmark of VA dissection [5, 22]. A prospective study found that headache or neck pain occurred in 57 (85%) of 67 patients with VA dissections [23].
Brain stem or cerebellar infarction is the most common clinical presentation of intracranial VA dissection [1]. These infarctions are mainly caused by distal embolization from the dissection site or thrombotic occlusion of branch vessels, rather than hypoperfusion due to arterial stenosis or occlusion [18, 24]. More than 50% of patients with intracranial VA dissection demonstrate symptoms related to brain stem infarction, the most common of which is lateral medullary infarction (Wallenberg syndrome) (Fig. 1), [1, 25]. Intracranial VA dissection is also an important cause of medial medullary infarction. One study found that, in 21% of patients, medial medullary infarction was related to intracranial VA dissection (Fig. 2) [26].
Images from a 38-year-old man with left-sided facial pain, vertigo, and ataxia. a Axial T2-weighted magnetic resonance (MR) image shows a focal, high-signal-intensity lesion (arrow) in the dorsolateral aspect of the left medulla, consistent with acute infarction. b Axial T1-weighted MR image shows high signal intensity (arrow) in the wall of the left VA, indicative of intramural hematoma. c Anteroposterior left vertebral arteriogram shows long segmental, irregular, stenoses (arrows) involving the intracranial segment of the left VA. Arrowheads indicate the left posterior inferior cerebellar artery. The patient was treated intravenously with heparin for 5 days and, subsequently, by oral administration of warfarin for 3 months. One month after admission he had made a full recovery and was discharged
Images from a 33-year-old man with left-sided hemiparesis and sensory impairment. a Axial T2-weighted magnetic resonance (MR) image shows focal, high signal intensity (arrow) in the ventromedial aspect of the medulla, consistent with acute infarction. b Axial T1-weighted MR image shows crescentic, iso-intensity signal (arrow) in the wall of the left VA, indicative of intramural hematoma. c Anteroposterior left vertebral arteriogram shows fusiform aneurysmal dilatation involving the V4 segment of the left VA. The patient was treated intravenously with heparin followed by oral therapy with warfarin. He was discharged with minimal weakness of the left extremities 2 weeks after admission
Although prognosis depends on the initial severity of neurologic deficits, intracranial VA dissection presenting with posterior circulation ischemic symptoms carries a relatively good prognosis [25, 27, 28]. In one study, more than 90% of patients who presented with ischemic symptoms subsequently returned to their previous lifestyles [27].
Acute subarachnoid hemorrhage
Acute SAH occurs when a dissecting aneurysm is formed and subsequently ruptures in a patient with intracranial VA dissection (Fig. 3). In contrast to intracranial VA dissections presenting with posterior circulation ischemic symptoms, intracranial VA dissections presenting with acute SAH due to ruptured dissecting aneurysms carry a poor prognosis if left untreated. Yamada et al. [29] reported an overall mortality rate of 67% in 24 patients who underwent conservative treatment after acute SAH caused by ruptured VA dissecting aneurysms. Early rebleeding is frequent and is responsible for a high mortality rate in these patients. Rates of rebleeding of ruptured VA dissecting aneurysms range from 30% to 71% [30]. Rebleeding most often occurs during the hyperacute phase: 57%–93% of rebleeding occurs within 24 h of the first hemorrhage. Reported mortality rates for patients with rebleeding are 47%–71% [29, 30].
Images from a 45-year-old man who presented with comatose mental status preceded by severe headache. a Axial, non-contrast, computed tomography scan shows diffuse subarachnoid hemorrhage in the basal cistern and intraventricular hemorrhage in the fourth ventricle. b Anteroposterior left vertebral arteriogram shows focal, irregular, aneurysmal dilatation (arrows) with proximal and distal arterial stenoses (arrowheads) in the V4 segment of the left vertebral artery, which is consistent with dissecting aneurysm. Endovascular coil occlusion of the dissecting aneurysm was performed on the day of admission. The patient developed multifocal cerebellar infarctions 3 days after embolization, despite intravenous therapy with heparin. On follow-up 6 months later, his score on the Glasgow outcome scale was 3 (severe disability)
Posterior fossa mass effect
Posterior fossa mass effect is a rare manifestation of intracranial VA dissection. A chronic, large, dissecting aneurysm can compress the brain stem or cranial nerves and cause symptoms related to mass effect, including headache, occipital pain, vertigo, vomiting, progressive myelopathy, and lower cranial nerve symptoms, such as dysphagia or hearing loss (Fig. 4) [17, 30]. A chronic dissecting aneurysm may also cause recurrent ischemic symptoms due to emboli from intra-aneurysmal thrombus.
Images from a 77-year-old man with chronic headache caused by a chronic dissecting aneurysm. a Axial, non-enhanced, computed tomography (CT) scan shows a mass with a calcified wall (arrows) in the posterior fossa. b A maximum intensity projection image from CT angiography clearly demonstrates a dissecting aneurysm with hematoma (arrows) within the pseudolumen. c Anteroposterior right vertebral artery arteriogram shows a dissecting aneurysm and blood flow (arrow) into the pseudolumen
Radiologic manifestations of intracranial vertebral artery dissection
Catheter angiographic findings
Imaging findings of V4 dissections are variable and are highly associated with specific clinical presentations. The most common angiographic findings in intracranial VA dissections are irregular, long segmental stenosis (Fig. 1) and tapered or abrupt occlusion of the V4 segment. These findings are predominantly observed in patients with ischemic symptoms.
In patients with acute SAH, a dissecting aneurysm with proximal and distal stenosis (pearl and string sign) is the typical angiographic finding (Fig. 3). Fusiform aneurysmal dilatation with or without proximal and/or distal stenosis is also common in these patients. On rare occasions, fusiform dissecting aneurysms also cause posterior fossa infarction due to distal thromboembolism (Fig. 2).
Angiographic findings of intimal flap and retention of contrast medium within the pseudolumen on delayed-phase images are diagnostic of VA dissection (Fig. 5). However, these findings are rarely observed. Another characteristic feature of arterial dissection is that angiographic findings may change rapidly within a period of days or even hours [1].
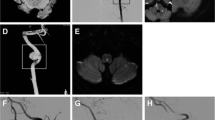
Images from a 66-year-old man who presented with severe headache. a A source image from contrast-enhanced magnetic resonance angiography shows an intimal flap (arrow) in the right VA. b, c Arterial (b) and venous (c) phase images from a lateral right vertebral angiogram show an intimal flap (arrows) in the dissecting aneurysm and retention of contrast material (arrowheads) within the pseudolumen. The patient refused treatment and was lost to follow-up
Magnetic resonance imaging and magnetic resonance angiographic findings
Magnetic resonance (MR) imaging is replacing catheter angiography as the imaging study of choice in the diagnosis of intracranial VA dissection [1]. MR imaging is helpful in differentiating VA dissection from other diseases causing luminal stenosis or occlusion, because MR imaging can directly visualize intramural hematomas and intimal flaps (both diagnostic of VA dissection) within VAs. In addition, MR imaging is the modality of choice in the evaluation of acute ischemic lesions in the posterior cranial fossa.
The typical MR imaging finding in VA dissection is an increased diameter of the affected artery, with eccentric narrowing of the arterial lumen caused by an intramural hematoma [25, 31, 32]. The intramural hematoma appears as an iso-intensity or high intensity signal on T1-weighted spin echo images, depending on the age of the hematoma, and may be crescentic, oval, or circumferential (Figs. 1 and 2). Because the high signal intensity of subacute intramural hematomas (methemoglobin) may be obscured by adjacent fat, a fat-suppressed T1-weighted sequence is recommended in patients suspected of having VA dissection. A T2-weighted sequence is not helpful in the diagnosis of intracranial VA dissection, because the high signal intensity of a subacute intramural hematoma can be obscured by adjacent high-signal cerebrospinal fluid [32]. T2-weighted images may be helpful for detecting decreased signal intensity of an acute-stage intramural hematoma (deoxyhemoglobin).
Various MR angiographic techniques have been used to diagnose VA dissection. Projection images of three-dimensional time-of-flight (3D TOF) MR angiography (MRA) may demonstrate patterns similar to those on catheter angiograms, such as vascular luminal narrowing or occlusion and aneurysmal dilatation. However, these MR angiographic findings are nonspecific. Thus, projection images alone are less useful than MR angiographic source images or T1-weighted images with fat saturation [33]. A double lumen with an intimal flap, a specific sign of arterial dissection, is better visualized on source images than on projection images of 3D TOF MRA (Fig. 5). In addition, projection images of 3D TOF MRA are insufficient to separate residual flow from intramural hematoma with high signal intensity [25, 34]. Contrast-enhanced 3D MRA using gadolinium chelates has the advantage of being able to differentiate residual lumen from intramural hematoma and is thus considered the technique of choice for the evaluation of VA dissection [2, 35].
Essential MR sequences for the diagnosis of intracranial VA dissection include axial, non-contrast, fat-suppressed, T1-weighted, spin echo images; axial, T2-weighted, spin echo images; and contrast-enhanced, 3D, MR angiograms covering the region from aortic arch to circle of Willis.
Computed tomography angiographic findings
With the advent of multidetector helical computed tomography (CT), multidetector CT (MDCT) angiography has been increasingly used in the diagnosis of intracranial VA dissection. Axial source images of MDCT angiography afford high-resolution images of the vessel in cross-section, and post-processed images can provide angiographic images at various angles (Fig. 4). Recently, Chen et al. [36] reported that CT angiography is excellent for detecting VA dissection, having a sensitivity of 100% and a specificity of 98%. The most important CT angiographic criteria the researchers used for diagnosing VA dissection were an increased external diameter and crescent-shaped mural thickening.
CT and MR imaging, non-invasive imaging techniques, are now replacing catheter angiography in the diagnosis of intracranial VA dissection. The choice of initial imaging modality for intracranial VA dissection depends on the clinical presentation of the patient. CT and CT angiography are recommended for patients with acute SAH due to a ruptured VA dissecting aneurysm. MR imaging and MRA are preferred for the diagnosis of VA dissection in patients with posterior fossa ischemic symptoms.
Endovascular treatment of intracranial vertebral artery dissection
Acute subarachnoid hemorrhage
Because mortality rates for patients with rebleeding are high, surgical or endovascular interventions should be promptly performed to prevent rebleeding in patients with ruptured dissecting aneurysms. The goal of treatment is to isolate the dissecting aneurysm from the cerebral circulation to prevent re-rupture. A ruptured VA dissecting aneurysm can be excluded from posterior circulation by either open surgery or endovascular treatment. Surgical treatment options include ligation of the proximal VA and direct clipping or trapping of the aneurysms. It has been reported that open surgical procedures are associated with high mortality rates and high rates of postoperative complications, such as lower cranial nerve palsy and brain stem infarction [29, 30, 37].
Endovascular treatment has largely replaced surgery as the treatment of choice for ruptured VA dissecting aneurysms [37–44]. Initially, proximal occlusion of the VA (parent vessel occlusion) with detachable balloons or coils was attempted [45, 46]. However, these procedures are not always effective in preventing re-rupture, and some investigators have reported rebleeding after proximal occlusion [40, 47]. Complete occlusion of the dissecting aneurysm with detachable coils (internal trapping), and concomitant occlusion of the parent artery, is considered the most effective method for treating ruptured VA dissection in the acute stage (Fig. 6). A high technical success rate and a low complication rate have been reported with this technique [38, 40, 41, 44]. Mortality rates for patients treated with endovascular trapping have been reported to be 0%–38% [38–40, 43, 48–50], whereas those for untreated patients range from 36% to 67% [29, 30, 40]. Poor neurologic grade on admission and early rebleeding are predictive of poor clinical outcome after endovascular treatment in patients with VA dissecting aneurysms [42].
Images from a 40-year-old man who presented with a sudden-onset severe headache. CT scan showed diffuse SAH in the basal cistern (not shown). a Three-dimensional digital subtraction angiogram shows a dissecting aneurysm (arrows) in the left VA, just distal to the origin of the left PICA (curved arrow). b Left anterior oblique view of the left vertebral arteriogram after embolization shows complete occlusion of the left VA, including the dissecting aneurysm (arrows). The PICA (arrowhead) is well preserved. On follow-up 1 year later, the patient remained well, without any neurologic deficits
VA dissection involving the origin of the PICA, bilateral VA dissections, and VA dissection with a hypoplastic contralateral VA remain therapeutic challenges. Treatment of VA dissection involving the origin of the PICA is problematic because inadvertent occlusion of the PICA might cause brain stem or cerebellar infarctions. In these patients, bypass surgery (occipital artery–PICA anastomosis), followed by endovascular internal trapping of the dissecting aneurysm, is generally recommended [44]. Proximal parent artery occlusion, leaving the aneurysm itself, can be performed emergently to prevent rebleeding before elective bypass surgery or when surgery is contraindicated [40]. Use of an intravascular stent is another therapeutic option for patients with dissecting aneurysms involving the origin of the PICA. Placement of intravascular stents, followed by coil embolization of the pseudoaneurysm, can preserve the arterial lumen while excluding the aneurysm from the circulation (Fig. 7). This technique can also be used in patients with VA dissection with a hypoplastic contralateral VA or in patients with bilateral VA dissections, to preserve adequate blood flow to the posterior circulation [38]. Surgical occlusion to treat a ruptured aneurysm on one side potentially leads to rupture of the contralateral lesion in cases involving dissecting aneurysms of the bilateral VAs [51].
Images from a 45-year-old woman with acute subarachnoid hemorrhage. a Left vertebral angiogram shows a wide-necked dissecting aneurysm (arrow) in the intracranial segment of the left vertebral artery. Note the left PICA (arrowhead) arising from the wall opposite the aneurysm. b Lateral left vertebral arteriogram obtained after stent-assisted coil occlusion (arrow) shows a widely patent lumen of the vertebral artery and preservation of blood flow in the left PICA. Arrowheads indicate the proximal and distal ends of the stent. The patient made an uneventful recovery within a week. She received daily doses of 325 mg of aspirin and 75 mg of clopidogrel for 3 months after the procedure
The use of stents in patients with ruptured dissecting aneurysm has several limitations. These “reconstructive” techniques may be associated with aneurysm recurrence and, thus, requires close clinical and angiographic follow-up (Fig. 8), [52]. Use of a stent in an acutely ruptured dissecting aneurysm might also lead to catastrophic complications, such as vessel rupture during placement of the stent or coils. Another problem is that acute thrombotic occlusion of the stent or thromboembolic complications might occur after stent placement, because adequate anticoagulation therapy and antiplatelet therapy are not feasible in the acute stage of SAH [43, 44].
Images from a 65-year-old woman with acute subarachnoid hemorrhage caused by a VA dissecting aneurysm. a Lateral left vertebral angiogram shows a dissecting aneurysm (arrows) in the intracranial segment of the left VA. The left PICA (arrowhead) arises from the proximal portion of the aneurysm. The contralateral VA was hypoplastic (not shown). b A curved, multiplanar, reformatted image of CT angiography obtained 2 days after placement of two overlapping stents shows the widely patent lumen of the VA and preserved blood flow in the left PICA (arrow). There is no evidence of extraluminal contrast material. c The patient complained of severe, sudden-onset headache 3 days after the initial endovascular procedure. Follow-up CT angiogram shows a newly developed aneurysm (arrow) in the medial aspect of the stented segment and active extravasation of contrast material (arrowheads). d Follow-up left vertebral angiogram shows a newly developed saccular pseudoaneurysm (arrows). The left PICA arises from the opposite wall. e An oblique image of the left vertebral arteriogram obtained after coil occlusion of the pseudoaneurysm (arrows) shows complete occlusion of the aneurysm, with good patency of the parent vessel. The patient experienced no further hemorrhagic episode after endovascular coil occlusion. On follow-up 3 months later, her score on the Glasgow outcome scale was 3 (severe disability)
Fusiform dissecting aneurysm can be treated with double-stent placement [53, 54]. Placement of overlapping stents theoretically decreases stent porosity, which may impede intra-aneurysmal blood flow considerably and thus accelerate intrasaccular thrombus formation [53]. Although there are successful case reports of fusiform dissecting aneurysms treated with double stents or stents and subsequent coiling of the aneurysm, this therapy has not been established for larger patient series and must therefore be considered as experimental in nature.
Posterior circulation ischemic symptoms
The goal of treatment in patients with posterior circulation ischemic symptoms caused by intracranial VA dissection is to prevent further thromboembolic events. Although no randomized controlled trials have been performed to determine optimal drug therapy, anticoagulation with intravenously administered heparin followed by oral administration of warfarin is considered the treatment of choice for patients with posterior circulation ischemic symptoms. Anticoagulation with a target international normalized ratio of 2.0–3.0 is generally used for the first 3–6 months [1].
Endovascular treatment has a limited role in the treatment of patients with posterior fossa ischemic symptoms [55, 56]. Endovascular coil occlusion should be considered in patients with ischemic symptoms who have a dissecting aneurysm of “pearl and string” appearance on angiographic images, because VA dissection with this aneurysmal morphology has the potential to grow and subsequently rupture, regardless of initial presentation (Fig. 9) [57]. Stent-assisted angioplasty may be performed in patients who have recurrent symptoms of ischemia despite adequate anticoagulation or in those in whom anticoagulation is contraindicated [56, 58]. Stents allow apposition of the dissected segment to the vessel wall and obliteration of the false lumen and thus can limit the occurrence of further embolic events [59]. It is believed that the risk of vessel rupture is higher than in intracranial atherosclerosis if stent-assisted angioplasty is performed in patients with VA dissection. Thus, extremely slow inflation with a screw-type inflation device is needed to prevent arterial rupture when the balloon is inflated.
Images from a 40-year-old woman with recurrent ischemic symptoms caused by VA dissection. a Axial T2-weighted magnetic resonance image shows a high-signal-intensity lesion (arrow) in the dorsolateral portion of the left medulla, indicating acute infarction. b Lateral left vertebral angiogram shows a dissecting aneurysm (arrows) in the intracranial portion of the left VA. c Lateral left vertebral arteriogram after embolization with platinum coils shows complete occlusion of the dissecting aneurysm (arrows). The patient achieved neurologic stability after coil embolization
Posterior fossa mass effect
A chronic dissecting aneurysm causing mass effect on the brain stem or cranial nerves can be successfully managed by endovascular trapping of the aneurysm (Fig. 10). Complete occlusion of the parent VA proximal and distal to the aneurysm should also be performed, to prevent recanalization of the dissecting aneurysm. Improvement of symptoms related to mass effect after endovascular aneurysm occlusion has been reported in the literature. The mechanism for symptomatic improvement could be the elimination of the persistent pulsation of the aneurysm in close contact with the brain stem, although the mass effect by the coil mass itself remains [60].
Images from a 45-year-old man with progressive myelopathy. a Axial T2-weighted image shows a lobulated mixed-stage hematoma (arrows) compressing the medulla oblongata (arrowhead) at the level of the foramen magnum. b Lateral right vertebral angiogram shows a dissecting aneurysm with a pseudolumen (arrows) in the intracranial portion of the right VA. c Anteroposterior right vertebral arteriogram shows complete occlusion of the right VA and a coil mass (arrows) filling the dissecting aneurysm. Note the preservation of the right posterior inferior cerebellar artery (arrowheads). Neurologic symptoms gradually improved after endovascular treatment. On follow-up 1 year later, the patient remained asymptomatic
It is noteworthy that there are occasional case reports in which chronic, dissecting, thrombosed, giant, aneurysms continued to enlarge, despite the complete thrombosis of the aneurysm [61–64]. The suggested mechanism to account for continued growth after complete endovascular occlusion is the adventitial neovascularization by the vasa vasorum on the occluded VA. Thus, it is recommended that surgical clip placement and resection of the aneurysm should be considered in such cases that are refractory to endovascular treatment [64].
Summary
Imaging findings of intracranial VA dissections vary according to the specific clinical presentation. MR imaging and MDCT angiography, non-invasive imaging techniques, are replacing catheter angiography in the diagnosis of intracranial VA dissection. Endovascular treatment is now considered a major therapeutic option for patients with a ruptured dissecting aneurysm or a chronic dissecting aneurysm. Anticoagulation therapy is currently considered the initial treatment of choice in patients with posterior circulation ischemic symptoms. Endovascular treatment, such as stent-assisted angioplasty or coil occlusion at the dissection site, can be performed in selected patients with posterior fossa ischemic symptoms.
References
Schievink WI (2001) Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med 344:898–906
Tay KY, U-King-Im JM, Trivedi RA et al (2005) Imaging the vertebral artery. Eur Radiol 15:1329–1343
Standring S, Crossman AR (eds) (2005) Neuroanatomy. Gray’s anatomy: the anatomical basis of clinical practice, 39th edn. Elsevier, London, pp 298–300
Wilkinson IM (1972) The vertebral artery: extracranial and intracranial structure. Arch Neurol 27:392–396
Mokri B, Houser OW, Sandok BA, Piepgras DG (1988) Spontaneous dissections of the vertebral arteries. Neurology 38:880–885
Hasan I, Wapnick S, Tenner MS, Couldwell WT (2002) Vertebral artery dissection in children: a comprehensive review. Pediatr Neurosurg 37:168–177
Pelkonen O, Tikkakoski T, Leinonen S et al (2003) Extracranial internal carotid and vertebral artery dissections: angiographic spectrum, course and prognosis. Neuroradiology 45:71–77
Brandt T, Grond-Ginsbach C (2002) Spontaneous cervical artery dissection: from risk factors toward pathogenesis. Stroke 33:657–658
Thanvi B, Munshi SK, Dawson SL, Robinson TG (2005) Carotid and vertebral artery dissection syndromes. Postgrad Med J 81:383–388
Grau AJ, Brandt T, Buggle F et al (1999) Association of cervical artery dissection with recent infection. Arch Neurol 56:851–856
Schievink WI, Wijdicks EF, Kuiper JD (1998) Seasonal pattern of spontaneous cervical artery dissection. J Neurosurg 89:101–103
Mizutani T, Miki Y, Kojima H, Suzuki H (1999) Proposed classification of nonatherosclerotic cerebral fusiform and dissecting aneurysms. Neurosurgery 45:253–260
Mizutani T, Kojima H, Asamoto S, Miki Y (2001) Pathological mechanism and three-dimensional structure of cerebral dissecting aneurysms. J Neurosurg 94:712–717
Yamada M, Miyasaka Y, Yagishita S, Fujii K (2003) Dissecting aneurysm of the intracranial vertebral artery associated with proximal focal degeneration of the elastica: a comparative pathological study of the vertebral artery in patients with and without aneurysms. Surg Neurol 60:431–437
Yonas H, Agamanolis D, Takaoka Y, White RJ (1977) Dissecting intracranial aneurysms. Surg Neurol 8:407–415
Endo S, Nishijima M, Nomura H, Takaku A, Okada E (1993) A pathological study of intracranial posterior circulation dissecting aneurysms with subarachnoid hemorrhage: report of three autopsied cases and review of the literature. Neurosurgery 33:732–738
Caplan LR, Baquis GD, Pessin MS et al (1988) Dissection of the intracranial vertebral artery. Neurology 38:868–877
Caplan LR, Biousse V (2004) Cervicocranial arterial dissections. J Neuroophthalmol 24:299–305
Krings T, Piske RL, Lasjaunias PL (2005) Intracranial arterial aneurysm vasculopathies: targeting the outer vessel wall. Neuroradiology 47:931–937
Schubiger O, Valavanis A, Hayek J (1980) Computed tomography in cerebral aneurysms with special emphasis on giant intracranial aneurysms. J Comput Assist Tomograph 4:24–32
Schubiger O, Valavanis A, Wichmann W (1987) Growth-mechanism of giant intracranial aneurysms: demonstration by CT and MR imaging. Neuroradiology 29:266–271
de Bray JM, Penisson-Besnier I, Dubas F, Emile J (1997) Extracranial and intracranial vertebrobasilar dissections: diagnosis and prognosis. J Neurol Neurosurg Psychiatry 63:46–51
Beletsky V, Nadareishvili Z, Lynch J et al (2003) Cervical artery dissection: time for a therapeutic trial? Stroke 34:2856–2860
Koch S, Air M, Rabinstein A, Reyes-Iglesias Y, Romano JG, Forteza A (2005) Diffusion-weighted magnetic resonance imaging in symptomatic vertebrobasilar atherosclerosis and dissection. Arch Neurol 62:1228–1231
Hoyosa T, Adachi M, Yamaguchi K, Haku T, Kayama T, Kato T (1999) Clinical and neuroradiological features of intracranial vertebrobasilar artery dissection. Stroke 30:1083–1090
Kameda W, Kawanami T, Kurita K et al (2004) Lateral and medial medullary infarction: a comparative study analysis of 214 patients. Stroke 35:694–699
Yoshimoto Y, Wakai S (1997) Unruptured intracranial vertebral artery dissection. Stroke 28:370–374
Nakagawa K, Touho H, Morisako T et al (2000) Long-term follow-up study of unruptured vertebral artery dissection: clinical outcomes and serial angiographic findings. J Neurosurg 93:19–25
Yamada M, Kitahara T, Kurata A, Fujii K, Miyasaka Y (2004) Intracranial vertebral artery dissection with subarachnoid hemorrhage: clinical characteristics and outcomes in conservatively treated patients. J Neurosurg 101:25–30
Mizutani T, Aruga T, Kirino T, Miki Y, Saito I, Tsuchida T (1995) Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery 36:905–913
Shah GV, Quint DJ, Trobe JD (2004) Magnetic resonance imaging of suspected cervicocranial arterial dissections. J Neuroophthalmol 24:315–318
Mascalchi M, Bianchi MC, Mangiafico S, et al (1997) MRI and MR angiography of vertebral artery dissection. Neuroradiology 39:329–340
Gelal FM, Kitis O, Calli C, Yunten N, Vidinli BD, Uygur M (2004) Craniocervical artery dissection: diagnosis and follow-up with MR imaging and MR angiography. Med Sci Monit 10:MT109–MT116
Auer A, Felber S, Schmidauer C, Waldenberger P, Aichner F (1998) Magnetic resonance angiographic and clinical features of extracranial vertebral artery dissection. J Neurol Neurosurg Psychiatry 64:474–481
Okumura A, Araki Y, Nishimura Y et al (2001) The clinical utility of contrast-enhanced 3D MR angiography for cerebrovascular disease. Neurol Res 23:767–771
Chen CJ, Tseng YC, Lee TH, Hsu HL, See LC (2004) Multisection CT angiography compared with catheter angiography in diagnosing vertebral artery dissection. AJNR Am J Neuroradiol 25:769–774
Kitanaka C, Sasaki T, Eguchi T, Teraoka A, Nakane M, Hoya K (1994) Intracranial vertebral artery dissections: clinical, radiological features, and surgical considerations. Neurosurgery 34:620–627
Kurata A, Ohmono T, Miyasaka Y, Fujii, K, Kan S, Kitahara T (2001) Coil embolization for the treatment of ruptured dissecting vertebral aneurysms. AJNR Am J Neuroradiol 22:11–18
Inamasu J, Nakamura Y, Saito R et al (2003) Endovascular treatment of ruptured vertebral artery dissection in the acute stage. Cerebrovasc Dis 16:306–308
Rabinov JD, Hellinger FR, Morris PP, Ogilvy CS, Putman CM (2003) Endovascular management of vertebrobasilar dissecting aneurysms. AJNR Am J Neuroradiol 24:1421–1428
Albuquerque FC, Fiorella DJ, Han PP, Deshmukh VR, Kim LJ, McDougall CG (2005) Endovascular management of intracranial vertebral artery dissecting aneurysms. Neurosurg Focus 18:E3
Ramgren B, Cronqvist M, Rommer B, Brandt L, Holtas S, Larsson EM (2005) Vertebrobasilar dissection with subarachnoid hemorrhage: a retrospective study of 29 patients. Neuroradiology 47:97–104
Sugiu K, Tokunaga K, Watanabe K et al (2005) Emergent endovascular treatment of ruptured vertebral artery dissecting aneurysms. Neuroradiology 47:158–164
Yuki I, Murayama Y, Vinuela F (2005) Endovascular management of dissecting vertebrobasilar artery aneurysms in patients presenting with acute subarachnoid hemorrhage. J Neurosurg 103:649–655
Halbach VV, Higashida RT, Dowd CF et al (1993) Endovascular treatment of vertebral artery dissections and pseudoaneurysms. J Neurosurg 79:183–191
Tsukahara T, Wada H, Satake K, Yaoita H, Takahashi A (1995) Proximal balloon occlusion for dissecting vertebral aneurysms accompanied by subarachnoid hemorrhage. Neurosurgery 36:914–920
Leibowitz R, Do HM, Marcellus ML, Chang SD, Steinberg GK, Marks MP (2003) Parent vessel occlusion for vertebrobasilar fusiform and dissecting aneurysms. AJNR Am J Neuroradiol 24:902–907
Yamaura I, Tani E, Yokota M et al (1999) Endovascular treatment of ruptured dissecting aneurysms aimed at occlusion of the dissected site by using Guglielmi detachable coils. J Neurosurg 90:853–856
Iihara K, Saka N, Murao K et al (2002) Dissecting aneurysms of the vertebral artery: a management strategy. J Neurosurg 97:259–267
Hamada JI, Kai Y, Morioka M, Yano S, Todaka T, Ushio Y (2003) Multimodal treatment of ruptured dissecting aneurysms of the vertebral artery during the acute stage. J Neurosurg 99:960–966
Otawara Y, Ogasawara K, Ogawa A, Kogure T (2002) Dissecting aneurysms of the bilateral vertebral arteries with subarachnoid hemorrhage: report of three cases. Neurosurgery 50:1372–1375
Mackay CI, Han PP, Albuquerque FC, McDougall CG (2003) Recurrence of a vertebral artery dissecting pseudoaneurysm after successful stent-supported coil embolization: case report. Neurosurgery 53:754–761
Benndorf G, Herbon U, Sollmann WP, Campi A (2001) Treatment of a ruptured dissecting vertebral artery aneurysm with double stent placement: case report. AJNR Am J Neuroradiol 22:1844–1848
Mehta B, Burke T, Kole M, Bydon A, Seyfried D, Malik G (2003) Stent-within-a-stent technique for the treatment of dissecting vertebral artery aneurysms. AJNR Am J Neuroradiol 24:1814–1818
Willing SJ, Skidmore F, Donaldson J, Nobo UL, Chernukha K (2003) Treatment of acute intracranial vertebrobasilar dissection with angioplasty and stent placement: report of two cases. AJNR Am J Neuroradiol 24:985–989
Ahn JY, Chung SS, Lee BH et al (2005) Treatment of spontaneous arterial dissections with stent placement for preservation of the parent artery. Acta Neurochir (Wien) 147:265–273
Naito I, Iwai T, Sasaki T (2002) Management of intracranial vertebral artery dissections initially presenting without subarachnoid hemorrhage. Neurosurgery 51:930–938
Cohen JE, Gomori M, Umansky F (2003) Endovascular management of spontaneous bilateral symptomatic vertebral artery dissections. AJNR Am J Neuroradiol 24:2052–2056
Malek AM, Higashida RT, Phatouros CC et al (2000) Endovascular management of extracranial carotid artery dissection achieved using stent angioplasty. AJNR Am J Neuroradiol 21:1280–1292
Shin YS, Kim SY, Cho KH, Cho KG (2004) Treatment of vertebral artery dissecting aneurysms presenting with progressive myelopathy. J Clin Neurosci 11:896–898
Katayama Y, Tsubokawa T, Miyazaki S, Furuichi M, Hirayama T, Himi K (1991) Growth of totally thrombosed giant aneurysm within the posterior cranial fossa. Neuroradiology 33:168–170
Hecht ST, Horton JA, Yonas H (1991) Growth of a thrombosed giant vertebral artery aneurysm after parent artery occlusion. AJNR Am J Neuroradiol 12:449–451
Nagahiro S, Takada A, Goto S, Kai Y, Ushio Y. (1995) Thrombosed growing giant aneurysms of the vertebral artery: growth mechanism and management. J Neurosurg 82:796–801
Iihara K, Murao K, Sakai N et al (2003) Continued growth of and increased symptoms from a thrombosed giant aneurysm of the vertebral artery after complete endovascular occlusion and trapping: the role of vasa vasorum. J Neurosurg 98:407–413
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoon, W., Seo, J.J., Kim, T.S. et al. Dissection of the V4 segment of the vertebral artery: clinicoradiologic manifestations and endovascular treatment. Eur Radiol 17, 983–993 (2007). https://doi.org/10.1007/s00330-006-0272-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-006-0272-8