Abstract
The pathogenesis of intracranial arterial aneurysms (AA) remains unclear, despite their clinical importance. An improved understanding of this disease is important in choosing therapeutic options. In addition to the “classical” berry-type aneurysm, there are various other types of intracranial AA such as infectious, dissecting or giant, partially-thrombosed aneurysms. From the clinician’s perspective, the hypothesis that some of these intracranial AA might be due to abluminal factors has been proposed for several years. Indeed, this hypothesis and the empirical use of anti-inflammatory drugs in giant intracranial aneurysms have been confirmed by recent studies reporting that an enzyme involved in the inflammatory cascade (5-lipoxygenase or 5-LO) promotes the pathogenesis of specific aneurysms in humans. 5-LO generates different forms of leukotrienes which are potent mediators of inflammation. Adventitial inflammation leads to a weakening of the media from the abluminal part of the vessel wall due to the release of proinflammatory factors that invade the media, thereby degrading the extracellular matrix, the elastic lamina of the vascular wall, and, finally, the integrity of the vessel lumen. This in turn results in a dilation of the vessel and aneurysm formation. Moreover, neoangiogenesis of vasa vasorum is found in close proximity to 5-LO activated macrophages. In addition to this biological cascade, we argue that repeated subadventitial haemorrhages from the new vasa vasorum play an important role in aneurysm pathogenesis, due to a progressive increase in size mediated by the apposition of new layers of intramural haematoma within the vessel wall. Intracranial giant AA can therefore be regarded as a proliferative disease of the vessel wall induced by extravascular activity. Considering certain aneurysmal vasculopathies as an abluminal disease might alter current therapeutic strategies. Therapy should not only be aimed at the intraluminal repair of the artery, but also cross the vessel wall to reach the vasa vasorum. Drug-eluting stents placed proximal to the lesion and targeted to the origin of the vasa vasorum could be considered as a potential future option. “Intelligent” MRI contrast agents (i.e., macrophage marking) could be used to detect vasa vasorum proliferation and weakening of the vessel wall in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pathogenesis of intracranial arterial aneurysms (AA) is complex and still not fully understood, despite its importance to the choice of treatment for this disease. Various shear stresses (flow, turbulences, jet effects and others) are known to produce AA—these represent “luminal” aneurysmal vasculopathies in which it is postulated that these stresses induce pathogenetic changes in normal vessel walls. In contrast, structural vessel-wall diseases (inflammatory, infectious, collagen diseases and others) are “abluminal” aneurysmal vasculopathies. In these, there is a primary abnormality of the vessel wall which is potentially aggravated by shear stresses [1–4]. A mechanistic viewpoint held by clinicians is that the different types of aneurysm share a common final pathophysiology, and should therefore share the same treatment [5]. As a result, therapies aimed at vessel-wall repair have been given less prominence than those aimed at repair of the luminal part of the aneurysm. Flow-related AA associated with brain arterio-venous malformations are treated by correction of the shunt and thus of the abnormal stresses. This is an example of pathophysiologic management of a specific group of AA. Similarly, infectious AA treated with antibiotics and the spontaneous repair of some dissecting AA further point to the role played by the vessel wall and its capacities for repair.
The specific group of “partially-thrombosed AA” constitutes a particular type of AA encountered in neurological practice. Clinicians have hypothesised since 1980 that such giant or partially-thrombosed aneurysms might be due to abluminal factors [6, 7]. However, this hypothesis was not confirmed until recently, when a study conducted by Zhao et al. “biologically” confirmed these early hypotheses. They concluded that partially-thrombosed aneurysms are the result of a process that is mediated from the “outside of the vessel” by the vasa vasorum of the affected vessels [8].
The role of vaso vasorum and the 5-lipoxgenase pathway
Vasa vasorum or vasa nutricia constitute a normal arterial network within the adventitia that is important for supplying nutrients and oxygen to both the adventitia and media. Whereas the presence of vasa vasorum within the proximal segments of the intracranial internal and the vertebral artery piercing the dura is confirmed [9], reports on intracranial arteries are contradictory [10, 11]. A growing body of evidence suggests that in intracranial atherosclerosis, vasa vasorum are present more distally on the carotid and vertebrobasilar systems. Furthermore, the absence of vasa vasorum in intracranial arteries in neonates and children, as well as the increased density of vasa vasorum in proximal segments of atherosclerotic intracranial arteries, indicate that vasa vasorum in proximal segments of intracranial arteries in adults are acquired and reactive in nature, and express an angiogenetic potential [11]. The supply to vasa vasorum is not known in detail, but some clinical situations suggest that the network receives contributions from branches arising from the main trunk itself [9].
In their recent work [8], Zhao and colleagues defined one potential mechanism responsible for the formation of arterial aneurysms, namely 5-lipoxygenase (5-LO), which is a key enzyme in leukotriene production that is expressed in leucocytes, macrophages and mast cells [12]. Once these cells are activated, 5-LO generates different forms of leukotrienes that are potent mediators of inflammation by further activating macrophages and recruiting monocytes and T-cells [13]. One of the leukotrienes, LTD4, binds to endothelial cells of the vasa vasorum, which leads to an increase of leucocyte extravasation. This adventitial inflammation leads to a weakening of the media by release of proinflammatory factors that invade the media and lead to a dilation that subsequently results in aneurysm formation [8]. It is important to note that the weakening of the artery does not occur from the inside of the vessel, rather it is caused from the outside, i.e., by abluminal factors, namely the 5-LO pathway.
The role of the vasa vasorum in the formation of aneurysms is clearly emphasised by Zhao et al. [8] in their study. Leukotrienes stimulate the production of macrophage inflammatory proteins (MIPs) that participate in the recruitment of leucocytes and the release of factors such as proteases that degrade the extracellular matrix, the elastic lamina of the vascular wall, and, finally, the integrity of the vessel lumen [14–16]. In addition, neoangiogenesis of vasa vasorum is encountered in close proximity to 5-LO activated macrophages, indicating that the inflammatory process of the adventitia might lead to a vicious circle by which an increase of vasa vasorum in turn facilitates the supply of inflammatory cells to the vessel wall [8, 17]. Activation of the proinflammatory 5-LO pathway in combination with hypercholesterolaemia has also been linked to the formation of atherosclerosis as a chronic inflammatory disorder of the vessel wall [13, 18]. These data therefore link the hyperlipidaemia-dependent vessel-wall inflammation of atherosclerosis to the formation of AA via the same pathogenetic route in which the vasa vasorum play a crucial role [19] (Fig. 1). The possibility of a subadventitial, i.e., intramural haematoma resulting from these vasa vasorum is implicit from these observations.
Model of the vasa vasorum and the 5-LO pathway participation in leucocyte recruitment, arterial remodelling and intracerebral giant arterial aneurysm formation. Macrophages reach the adventitia via vasa vasorum that arise from the parent vessels themselves (see also Fig. 4). These adventitial macrophages express 5-LO and subsequently generate leukotrienes which in turn activate (1) T cells, (2) other macrophages, (3) proliferation of vasa vasorum, and (4) monocytes, leading to an increased extravasation of leucocytes from the vasa vasorum to the adventita. These activated leucocytes release proinflammatory factors (such as metalloproteinases) that damage the media by degradation of the extracellular matrix (ECM) and the elastic lamina that lead to a focally-weakened parent-vessel wall from which subsequently the aneurismal lumen may form (5) [8]. We complement this biological cascade with the traditional clinical observation of subadventitial haematomas in so-called “partially-thrombosed aneurysms”. Repeated subadventitial bleeding from the vasa vasorum (6) leads to an onion-shaped intramural haematoma of different ages (see also Fig. 2)
The pathogenesis of giant aneurysms
From a clinical perspective, there are several intracranial pathological conditions which could now be explained by these findings. Amongst those entities are so-called giant or “partially-thrombosed” AA. In 1980, Schubiger et al. suggested that formation of intracranial giant artery aneurysms could be due to a chronic dissection process associated with recurrent subadventitial haemorrhage from the vasa vasorum [6]. Yasargil reported that on surgical exploration of giant intracranial aneurysms a tremendous network of fine vessels covering the aneurysm could be seen [20]. They argued that these small vessels might represent the increased amount of vasa vasorum at the aneurysmal wall level, and that they may be responsible for the curvilinear enhancement noted on CT after contrast injection. Subsequently, it was noted that subarachnoid haemorrhage in partially-thrombosed AA did not occur from the aneurysm itself, but instead from the vessel wall with formation of fresh clot both inside and outside the vessel wall [7] (Fig. 2). Postmortem microscopic studies of 18 cases with giant intracranial aneurysms demonstrated that the wall of such AA consisted of fibrous tissue (often with calcifications), loss of the elastic lamina and muscularis, and a large number of small vessels within the wall corresponding to the vasa vasorum [9]. In a more recent report, Iihara et al. noted growth of a giant partially-thrombosed aneurysm after complete occlusion of the parent vessel in a patient [21]. Although there was no angiographically demonstrated evidence of filling of a lumen, the aneurysm continued to enlarge, requiring surgery. During the operation, the presence of markedly developed vasa vasorum was recognised on the parent artery and neck of the aneurysm [21]. The common finding of these studies is that recurrent bleeding from the vasa vasorum can result in an increase in size of the aneurysm and in further proliferation of neomembranes and new vessels. The increase in size of a giant aneurysm is therefore not due to intraluminal factors (i.e., change in haemodynamics, weak vessel wall leading to centrifugal growth of the aneurysm) but due to the apposition of new layers of thrombus at the periphery. Similarly, thrombosis of the so-called partially-thrombosed aneurysm seen on imaging studies does not correspond to intraluminal thrombus, but more likely refers to recurrent intramural haematoma similar to intramural haematoma seen on dissections of large arteries [22]. Intracranial giant AA can therefore be regarded as a proliferative disease of the vessel wall induced by extravascular activity [23, 24].
Example of a giant aneurysm of the basilar tip in a 60-year-old patient who presented with a partial third cranial nerve palsy along with a contralateral moderate hemiparesis. Frames a and b were taken on admission and show the “partially-thrombosed” aneurysm with “clots” of different ages represented by crescent-shaped signal alterations in the vessel wall. Frame c was taken 1 year later and demonstrates a new crescent-shaped peripheral haematoma in the outer vessel wall (arrow). Digital subtraction angiography (DSA) in 3D (frame d) shows the lumen of the aneurysm; however, the greatest part (i.e., the subadventitial haematoma) of the aneurysm is not visualised, since DSA can only depict intraluminal portions of the vessels. Frames e and f schematically explain the increase in size as being due to recurrent haemorrhages into the aneurysm vessel wall from the vasa vasorum, leading to onion-shaped thrombus formations within the adventita [7]. (BA basilar artery, PCA posterior cerebral artery, SCA superior cerebellar artery)
The primary cause for the dissection is unknown. However, one might presume that atherosclerosis can—via a chronic inflammatory response—lead to a thinning of the media and cause vascular rupture within the adventitia. The haemorrhage stops the further dissection of the media; otherwise, an acute transmural dissection with subarachnoid haemorrhage occurs [15, 25, 26]. The increase in number and size of the vasa vasorum fits well with this concept. Since the biological phenomenon is persisting, the effects may be still active, which explains why some cases of dissection continue to evolve even after therapeutic occlusion of the vessel (Fig. 3).
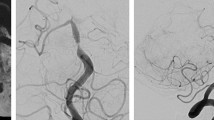
Example of vasa vasorum around a giant, partially-calcified vertebral artery aneurysm in a 25-year-old patient. Twelve years prior to admission, a giant fusiform aneurysm of the right vertebral artery was found and surgically treated by proximal exclusion (“clipping”) of the parent vessel. On admission, the patient’s CT demonstrated a partially-calcified aneurysm (asterisk) with peripheral haemorrhage (arrowhead). Angiography (right vertebral artery, lateral projection) indicates that the aneurysm is completely excluded. However, there are many small vessels seen surrounding the aneurysm (arrows) that represent the vasa vasorum. This case illustrates that despite exclusion of the parent artery and the aneurismal lumen, the vessel-wall disease is still active
These hypotheses do not however apply to all intracranial aneurysms. The most frequently encountered intracranial aneurysm, namely the berry type, cannot be regarded as the same disease as the giant chronic or acute dissecting aneurysms [27]. The latter often demonstrate a multifocal character, suggesting non-haemodynamic triggering factors. Giant aneurysms also behave differently over time, with about 50% thrombosing, whereas only 2% of berry aneurysms show complete spontaneous thrombosis in relation to focal vascular exclusion of the vasa vasorum system. The topography and localisation of these aneurysms is different, as is their epidemiology and association with certain genetic or familial diseases [9]. Furthermore, these comments apply only to adults—in children the mechanism of vessel wall dissection is likely to be different.
Conclusion
Adventitial inflammatory reactions and vasa vasorum appear to play an important role in intracranial giant aneurysm formation. This biological explanation retrospectively confirms the empirical use of steroids and other anti-inflammatory drugs in acutely symptomatic so-called “partially-thrombosed” aneurysms [28]. This understanding of the pathophysiology has important therapeutic implications, since therapy should not be aimed solely at the intraluminal repair of the artery. One should also consider a treatment regimen that is able to cross the vessel wall to reach the vasa vasorum. Drug-eluting stents placed proximal to the lesion and targeted to the origin of the vasa vasorum could be considered as a potential future option (Fig. 4). “Intelligent” MRI contrast agents (i.e., macrophage marking) could be used to detect vasa vasorum proliferation and weakening of the vessel wall in vivo [29–32].
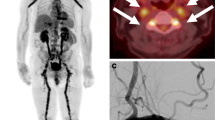
Example of enlarged vasa vasorum arising from the parent vessel itself. This 48-year-old patient presented with a paraganglioma (asterisk in a) which was supplied from the ascending pharyngeal artery and an artery arising from the proximal intrapetrous segment of the internal carotid artery (ICA) (arrow in c). The artery ran retrograde along with the ICA within the carotid canal (arrows in a and b, arrowhead in c) on the outer surface of the ICA, and represented enlarged vasa vasorum. Such enlargement is commonly observed in this disease and is believed to be related to the angiogenetic activity of the tumour
This conjunction of biological research and clinical experience may in the future give rise to treatments aimed at the cause, rather than the morphology, of the aneurysmal vasculopathy.
References
Inci S, Spetzler RF (2000) Intracranial aneurysms and arterial hypertension: a review and hypothesis. Surg Neurol 53:530–540 (discussion 540–532)
Ferguson GG (1972) Physical factors in the initiation, growth, and rupture of human intracranial saccular aneurysms. J Neurosurg 37:666–677
Stehbens WE (1989) Etiology of intracranial berry aneurysms. J Neurosurg 70:823–831
Stehbens WE (1990) Pathology and pathogenesis of intracranial berry aneurysms. Neurol Res 12:29–34
Lasjaunias P (2000) From aneurysm to aneurysmal vasculopathies. Oper Tech Neurosurg 3:160–165
Schubiger O, Valavanis A, Hayek J (1980) Computed tomography in cerebral aneurysms with special emphasis on giant intracranial aneurysms. J Comput Assist Tomogr 4:24–32
Schubiger O, Valavanis A, Wichmann W (1987) Growth-mechanism of giant intracranial aneurysms; demonstration by CT and MR imaging. Neuroradiology 29:266–271
Zhao L, Moos MP, Grabner R et al (2004) The 5-lipoxygenase pathway promotes pathogenesis of hyperlipidemia-dependent aortic aneurysm. Nat Med 10:966–973
Berenstein A, Lasjaunias P, TerBrugge KG (2004) Surgical neuroangiography, vol 2.1. Springer, Berlin Heidelberg New York
Aydin F (1998) Do human intracranial arteries lack vasa vasorum? A comparative immunohistochemical study of intracranial and systemic arteries. Acta Neuropathol (Berl) 96:22–28
Connolly ES Jr, Huang J, Goldman JE, Holtzman RN (1996) Immunohistochemical detection of intracranial vasa vasorum: a human autopsy study. Neurosurgery 38:789–793
Samuelsson B (1983) Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science 220:568–575
Spanbroek R, Grabner R, Lotzer K et al (2003) Expanding expression of the 5-lipoxygenase pathway within the arterial wall during human atherogenesis. Proc Natl Acad Sci USA 100:1238–1243
Sinha S, Frishman WH (1998) Matrix metalloproteinases and abdominal aortic aneurysms: a potential therapeutic target. J Clin Pharmacol 38:1077–1088
Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT (2002) Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest 110:625–632
Daugherty A, Cassis LA (2002) Mechanisms of abdominal aortic aneurysm formation. Curr Atheroscler Rep 4:222–227
Kumamoto M, Nakashima Y, Sueishi K (1995) Intimal neovascularization in human coronary atherosclerosis: its origin and pathophysiological significance. Hum Pathol 26:450–456
Lotzer K, Spanbroek R, Hildner M et al (2003) Differential leukotriene receptor expression and calcium responses in endothelial cells and macrophages indicate 5-lipoxygenase-dependent circuits of inflammation and atherogenesis. Arterioscler Thromb Vasc Biol 23:e32–e36
Palinski W (2004) Aneurysms: leukotrienes weaken aorta from the outside. Nat Med 10:896–898
Yasargil MG (1984) Clinical considerations, surgery of the intracranial aneurysms and results. Microneurosurgery, vols 1 and 2. Thieme, Stuttgart
Iihara K, Murao K, Sakai N et al (2003) Continued growth of and increased symptoms from a thrombosed giant aneurysm of the vertebral artery after complete endovascular occlusion and trapping: the role of vasa vasorum. Case report. J Neurosurg 98:407–413
Yonas H, Agamanolis D, Takaoka Y, White RJ (1977) Dissecting intracranial aneurysms. Surg Neurol 8:407–415
Stehbens WE (1998) Apoptosis and matrix vesicles in the genesis of arterial aneurysms of cerebral arteries. Stroke 29:1478–1480
Endo S, Nishijima M, Nomura H, Takaku A, Okada E (1993) A pathological study of intracranial posterior circulation dissecting aneurysms with subarachnoid hemorrhage: report of three autopsied cases and review of the literature. Neurosurgery 33:732–738
Dollery CM, Owen CA, Sukhova GK, Krettek A, Shapiro SD, Libby P (2003) Neutrophil elastase in human atherosclerotic plaques: production by macrophages. Circulation 107:2829–2836
Ross R (1999) Atherosclerosis—an inflammatory disease. N Engl J Med 340:115–126
Stehbens WE (1999) Misconceptions of the pathology of intracranial arterial aneurysms. J Clin Pathol 52:708
Cyrus T, Sung S, Zhao L, Funk CD, Tang S, Pratico D (2002) Effect of low-dose aspirin on vascular inflammation, plaque stability, and atherogenesis in low-density lipoprotein receptor-deficient mice. Circulation 106:1282–1287
Blankenberg FG, Mari C, Strauss HW (2002) Development of radiocontrast agents for vascular imaging: progress to date. Am J Cardiovasc Drugs 2:357–365
Trivedi RA, JM UK-I, Graves MJ et al (2004) In vivo detection of macrophages in human carotid atheroma: temporal dependence of ultrasmall superparamagnetic particles of iron oxide-enhanced MRI. Stroke 35:1631–1635
Corot C, Petry KG, Trivedi R et al (2004) Macrophage imaging in central nervous system and in carotid atherosclerotic plaque using ultrasmall superparamagnetic iron oxide in magnetic resonance imaging. Invest Radiol 39:619–625
Litovsky S, Madjid M, Zarrabi A, Casscells SW, Willerson JT, Naghavi M (2003) Superparamagnetic iron oxide-based method for quantifying recruitment of monocytes to mouse atherosclerotic lesions in vivo: enhancement by tissue necrosis factor-alpha, interleukin-1beta, and interferon-gamma. Circulation 107:1545–1549
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krings, T., Piske, R.L. & Lasjaunias, P.L. Intracranial arterial aneurysm vasculopathies: targeting the outer vessel wall. Neuroradiology 47, 931–937 (2005). https://doi.org/10.1007/s00234-005-1438-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-005-1438-9