Abstract
Standard CT dose measurements were performed on a Siemens Sensation 16 scanner. CT dose indices, free-in-air (CTDIF) and weighted (CTDIW), were measured in all available axial and helical beam collimations of the head and body scanning modes. The effect of tube current, high voltage, rotation time, beam collimation and pitch on the CT doses was investigated. CT doses increased as a power function of high voltage. The kVp exponent n varied with beam collimation from 2.7 to 3.1 for CTDIW, and from 2.4 to 2.6 for CTDIF. Automatic change of the focal spot size increased radiation doses up to a factor of 1.18. Measured small-focus CTDIW values differed from those displayed at the console from –24 to 14%. Peripheral doses in the head phantom were higher compared to the body phantom by a factor of 1.5 to 2. Central doses are 2.7 to 4.1 times higher. Differences in beam collimation resulted in 50% variation in the CTDIW in the body phantom and 60% in the head phantom. In conclusion, our study has confirmed the great impact of technique factors and acquisition parameters on CT doses. The provided comprehensive dosimetric data will facilitate the dose-effective use of the scanner studied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The markedly reduced acquisition times and the isotropic resolution achieved with the advent of multislice spiral computed tomography (CT) scanners have broadened the clinical applications of CT [1–3]. In recent years, the wide availability of new technology scanners combined with the new clinical indications has led to a notable increase in the annual rate of CT examinations [4–6]. However, CT is a high radiation imaging modality compared to radiography. As a major contributor to the collective effective dose to the general population, the use of CT has raised concern in the national and international radiation protection agencies [7–11]. Apart from the clinical benefits, the widespread and expanding use of multislice scanners also bears the potential of causing a higher radiation burden to patients compared to conventional CT [12, 13]. Therefore, the need for increased awareness regarding multi-detector scanner radiation issues is imperative [14–16].
The aim of the present study was to provide a comprehensive evaluation of the dosimetric characteristics of a 16-slice scanner. A thorough investigation of the impact of the imaging parameters on the administered doses will facilitate the acquisition optimization toward the minimization of radiation doses. Specifically, we have performed an extensive series of standardized CT measurements and analyzed the effect of technique factors on fundamental CT radiation dose parameters. Derived data elucidate the relative effects of scanning parameters on administered doses and enable the estimation of the patient-effective dose as well as the comparison of CT scanners and scanning protocols.
Materials and methods
CT scanner characteristics
The dosimetric characteristics of a third generation CT scanner, the SOMATOM Sensation 16 multi-slice spiral CT (Siemens AG, Forchheim, Germany), were evaluated in the current study. The scanner employs a large scanning field of view (body mode) and a small scanning field of view (head mode). The two modes differ in filtration and selectable high voltage settings. The head mode operates at 80 and 120 kVp, whereas the body mode employs increased filtration, and the selectable high voltage values are 80, 120 and 140 kVp. The X-ray tube is equipped with a small and a large filament that corresponds to the small and large focal spot, respectively. The change between focal spots occurs automatically depending on the heat deposition on the anode.
The available beam collimations, i.e., the nominal beam widths at the isocenter of the scanner, are determined by the N×h product, where N is the number of slices acquired simultaneously and h the active detector width. For helical scans of the body, these are 24 mm (16×1.5 mm) and 12 mm (16×0.75 mm), whereas axial scans can be performed with collimations of 24, 18 (12×1.5 mm), 10 (2×5 mm), 9 (12×0.75 mm) and 2 (2×1 mm) mm. Helical scans of the head may be obtained at 24, 12, 9 and 1.2 (2×0.6 mm) mm. Axial operation allows collimations of 18, 10, 9, 2 and 1.2 mm. Apart from the collimation, field of view, scan length and kVp, other selectable parameters are the reconstructed slice width, the mAs per rotation, the rotation time (0.5, 0.75, 1 and 1.5 s) and the table feed per rotation. Another indirectly determined parameter is the spiral scan pitch (P) [17]:
The directly selectable parameters involved allow the continuous variation of pitch from 0.5 to 1.5. Moreover, in the specific scanner, instead of the conventional tube current-rotation time product (mAs), a quantity termed the effective mAs is introduced for the helical scans. According to the manufacturer, the effective mAs is defined as:
As a result, the actual tube current is also an indirectly determined quantity, since it is a function of the effective mAs, rotation time, table feed and beam collimation.
Expressing the radiation dose in CT
Absorbed doses at CT are commonly expressed in terms of the computed tomography dose index (CTDI). The CTDI is the integral of the absorbed dose to air from a single rotation (Kair) along a line that is parallel to the axis of rotation, restricted to a length of 50 mm on either side of the irradiated slice, divided by the slice collimation N×h [17]:
CTDI can be measured free-in-air on the axis of rotation (CTDIF) or in dedicated CT phantoms. Measurements at the periphery (CTDIP) and center (CTDIC) of the phantoms are combined to yield the weighted CTDI (CTDIW) [17]:
The corresponding dosimetric quantities measured for a scan series are termed the multiple scan average dose (MSAD) and volume CTDI (CTDIV) and are related to CTDIF and CTDIW according to the following equations [17]:
and
A further dosimetric quantity of interest, applicable to any specific examination, is the scan dose length product (DLP). The DLP is defined as the product of CTDIV and total scan length [17]:
DLP can be used for the estimation of the patient-effective dose from examinations at CT, according to the following equation [11]:
EDLPs are body region-specific conversion coefficients that have been released by the European Commission and range from 0.0023 to 0.019 mSv mGy−1 cm−1 [11].
Phantoms and measurement methods
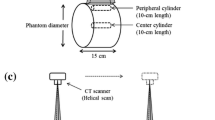
The fundamental CT dose quantities examined in the present study were the CT dose index 100 free-in-air (CTDIF) and the weighted CT dose index (CTDIW). The CTDI measurements were performed using a pencil-shaped ionization chamber with a 10-cm active length (MDH Industries, Monrovia, CA). CTDIF was measured at the center of rotation, while two polymethylmethacrylate (PMMA) CT phantoms, a 32-cm diameter body phantom and a 16-cm diameter head phantom, were used for evaluating CTDIW. For the head and body phantoms, measurements were performed at the center of the phantom and at four positions at 1 cm depth from the surface. The central (CTDIc) and the average value of the peripheral measurements (CTDIp) were used for the calculation of CTDIw according to Eq. (4).
For beam collimations available in both the axial and helical mode, CTDIF and CTDIW were measured by exposing the ionization chamber during a single axial tube rotation. For beam collimations available only in the helical mode, full-chamber scans free-in-air and full-chamber scans in the center and the periphery of the phantoms were performed at a pitch value of 1, for the determination of the multiple scan average dose (MSAD) and volume CTDI (CTDIV). The measured MSAD and CTDIV values correspond to the required CTDIF and CTDIW values for the beam collimations in question (Eqs. 5 and 6).
Phantom measurements
Central and peripheral doses
The mAs impact on CTDIW was evaluated for head and body scans for all available beam collimations. For each collimation and high-voltage setting, peripheral and central doses were measured in the head and the body phantoms over the range of selectable mAs values with an increment of 10 mAs, and the corresponding CTDIW values were calculated. The rotation time was 0.5 s for body phantom scans and 0.75 s for head phantom scans. These rotation times are commonly employed in clinical protocols at our institution. Peripheral and central doses were compared for the head and the body phantom at all available beam collimations and high voltage settings.
CTDIw, focal spot change and console-displayed values
Plotting CTDIW against the tube current (mAs/rotation time) allowed the determination of the point of the focal spot change. Linear regression analysis of the measured CTDIW against tube load (mAs) was also performed. Normalized CTDIW values (nCTDIW) expressed in mGy/100 mAs were derived for all collimations and both the small and large focus by separate linear regression over the small and large focus measurements, respectively. The experimentally determined CTDIW values were compared to those provided by the manufacturer.
Phantom size and pitch effect on CTDIw
In order to investigate the effect of phantom size on CTDI doses, the head and the body phantoms were axially scanned at 140 kVp and 18-, 10- and 9-mm beam collimation. This high-energy beam is available only in the heavily filtered body mode and was expected to yield a pronounced expression of the phantom effect on CT doses. Finally, the pitch effect on CT doses was investigated by MSAD and CTDIV measurements at 120 kVp and 12-mm beam collimation for head and body scans. The scans were performed at a fixed tube load value of 200 mAs effective.
Free-in-air measurements
Free-in-air measurements were performed for the determination of normalized CTDIF values (nCTDIF) for all collimations of the head and body modes and both foci. Rotation times were similar to those adopted for the phantom measurements. The mAs values were also varied at a 10-mAs increment, and linear regression analysis was used for the determination of small and large focus nCTDIF. CTDIW over CTDIF quotients were calculated from the measured data.
The rotation time effect on CT doses was separately investigated by measuring CTDIF for axial head and body scanning modes at rotation times of 0.5, 0.75, 1 and 1.5 s with a constant tube current value (120 mA), which corresponds to the small focus of the X-ray tube. This was accomplished by varying the mAs value appropriately.
CT doses and high voltage
The effect of tube voltage on CT doses was investigated by measuring CTDIF and CTDIW for all the available beam collimations and kVp settings for the body scan modes. All measurements were performed at 100 mAs and a rotation time of 0.5 s (small focal spot).
Results
CTDIw increases linearly with mAs, and since the rotation time was held constant, the actual relationship examined was CTDIw versus tube current. Table 1 presents the linear regression data. The coefficient of determination was always better than 0.989, and the slopes of the best-fit lines correspond to the average normalized CTDIw over the mAs settings. However, there is a discontinuity in nCTDIW in both the head and body mode due to the automatic change from the small to large focal spot. As illustrated in Fig. 1a, for body scans transition occurs above 280 mA at 80 and 120 kVp and above 240 mA at 140 kVp. In the head mode the large focal spot operates above 213 mA at 80 and 120 kVp (Fig. 1b). Figure 2 shows the large-over-small focus CTDIW ratio for body and head acquisition modes, respectively. The increase in the administered dose ranges from 4 to 18% for body scans and from 6 to 11% for head scans. No focus change was observed for a beam collimation of 10 mm and collimations of 2 and 1.2 mm for head scans. Measured normalized values for the small focus are provided in Table 2 and compared with the values provided by the manufacturer and displayed on the scanner console. Console readings were always proportional to the tube current-rotation time product of the scan, which means that a single nCTDIW value is adopted by the manufacturer, irrespective of the focal spot size. The discrepancies between the measured CTDI values and the values displayed on the console are notable. Measured small-focus CTDIW values differed from those displayed at the console from –24 to 14% for the body phantom and from –4 to 14% for the head phantom. Maximum differences are increased to 32 for the 32-cm phantom and to 22% for the 16-cm phantom if the displayed values are compared to the large focal spot CTDIW. It is evident that an informed and dose-effective use of any specific scanner can only be established with on-site measurements of its dosimetric characteristics.
The effect of focal spot change tube current on normalized CTDIW for the (a) body and (b) head acquisition modes. Transition from the small to the large filament, results in a discontinuity in nCTDIW values. In the body mode, this occurs above 280 mA at 80 and 120 kVp, and above 240 mA at 140 kVp. In the head mode, the large focal spot operates above 213 mA at 80 and 120 kVp
Table 3 shows the dependency of CTDIF on rotation time for axial scanning modes. The mAs and rotation time setting were such that a constant tube current of 120 mA was maintained, which corresponds to the small focus of the X-ray tube. The coefficient of determination of the linear relationship between CTDIF and rotation time was better than 0.998.
CT doses increase as a power function of high voltage (kVp):
Table 4 presents the parameters of the best-fit results. The kVp exponent n varies with beam collimation from 2.7 to 3.1 for CTDIW and from to 2.4 to 2.6 for CTDIF.
The ratios of peripheral to central dose depend on the beam collimation and decrease with high voltage. For the body phantom, ratios range from 1.84 to 2.72 (Fig. 3a), whereas for the head phantom a less wide distribution of values between 1.14 and 1.39 was observed due to the better penetration of radiation (Fig. 3b).
The CTDIF over CTDIW ratio for the body phantom decreases with high voltage and ranges from 2.19 to 3.07 (Table 5). For the head phantom, the quotients range from 1.07 to 1.45, but increase with high voltage. This is probably attributable to the decreased degree of scattering in the head phantom and the increasing penetrating ability of radiation with high voltage (harder beam).
Radiation doses at the center of a 16-cm-diameter phantom are three times higher than at the center of a 32-cm-diameter phantom, and these ratios are practically independent of beam collimation (Fig. 4). Similarly, peripheral doses are higher by a factor of 1.7. As a result, the weighted CTDI values measured at the head phantom are two times higher than those measured at the body phantom for the same CTDI free-in-air value.
The effect of pitch variation on CT doses is illustrated in Fig. 5. It is interesting to note that when the pitch value is increased at a constant effective mAs setting, not only do the CT doses not decrease, but also there is a potential of increase due to the automatic focus change resulting from the effective mAs definition.
Our measurements have confirmed the strong dependence of CTDIW on beam collimation. The maximum differences in the body phantom were 37, 25 and 30% at 80, 120 and 140 kVp, respectively. In the head phantom, the maximum deviations were 35% at 80 kVp and 60% at 120 kVp. However, no monotonous pattern on the beam collimation effect was observed (Fig. 6). In the body phantom, the CTDIW values for the 24- and 18-mm beam width are comparable. An increase follows when collimation decreases to 12 mm. CTDI decreases again at 10 mm, rises at 9 mm and declines at 2 mm. In the head phantom, starting from the 24-mm collimation, CTDI values exhibit a successive decrease-increase behavior. The most dose-effective beam collimations are the 24 and 10 mm for the helical and axial modes, respectively. The most burdening acquisition modes are the 9-mm beam width for the body phantom and the 1.2-mm collimation for the head phantom.
Discussion
In the present study, a dosimetric evaluation of a 16-slice scanner was conducted with respect to its CT dose indices (CTDI and CTDI). The dependency of CT doses on technique factors and acquisition parameters was evaluated. The provided data, such as the influence of the focus change on the CTDIW and the measured CTDIW values, which differ significantly from the console displayed values, will facilitate optimal dose usage for the specific scanner. Moreover, they allow the user of the scanner to calculate CTDIW values from simple free-in-air measurements. CTDIW values in turn allow a broad estimate of the patient-effective dose (ED) from any specific examination to be made on the basis of the scan dose length product (DLP) using Eq. (8).
Experimental determination of CTDI
The experimentally verified good linearity of CT doses with tube current and rotation time indicates that dosimetric evaluation does not necessitate a set of measurements spanning the entire possible mAs and rotation time settings for the available scanner modes. However, a series of mAs measurements is required at the available kVp settings for a single-beam collimation in order to determine the point of focus change, preferably at the highest rotation speed, at the head and body acquisition mode. Then, for each kVp and beam collimation, the small and large focus normalized CTDI values should be determined.
CTDIW and beam collimation
The values of CTDIW depend on the fractional contribution of the penumbral region on the total administered dose (the integral of beam profile) [5, 12]. The penumbra width at both ends of the beam is independent of the total beam width. For the same number of detectors, the penumbral contribution decreases with the total width beam. Hence, the total collimation of 24 mm (16×1.5 mm) is more dose effective than 12 (16×0.75 mm). Similarly, for the 12-slice mode, a beam collimation of 18 (12×1.5 mm) yields a lower CTDIW compared to a collimation of 9 (12×0.75 mm) (Fig. 6).
However, radiation should be uniformly distributed over the detector bank. Therefore, as the number of active channels increases (i.e., the number of simultaneously acquired slices), the beam profile should be wider to exclude the edge detectors from the penumbral region. For a given slice width, increasing the number of acquired slices increases the CTDIW. This over-beaming effect results in increased CT doses compared to single- and dual-slice scanners and is more pronounced in quad-slice CT systems [5, 12]. For the scanner considered, the 9-mm collimation is slightly more dose efficient than the 12 mm, although less wide, because of the reduced number of active detectors (12 versus 16).
Collimations that are dual slice are expected to be more efficient than the 16- or 12-slice modes, since the beam may be closely collimated to its nominal value. Each penumbral tail falls into a detector channel, and the need for dose uniformity over the detectors is always fulfilled. The fraction of the beam profile being utilized is now determined by the physical or electronic configuration of the detectors, i.e., the imaged slice width just as in a single-slice scanner. For the scanner studied, beam utilization is highest for the 10-mm width followed by the 2-mm collimation. At a collimation of 1.2 mm, the beam width becomes comparable to the penumbral region and presents its peak value compared to all available beam widths.
The over-beaming effect is reasonably expected to depend also on the focal spot size. Specifically, it should be amplified when the large focal spot operates due to an increased contribution of the penumbral region. The relative contribution of the penumbra, and as a result the degree of amplification, will also depend on the beam collimation. The large focus CTDIW over small focus CTDIW ratio variation with beam collimation (Fig. 2) reflects the increase of over-beaming with focal spot size and can be compared to the small focus CTDIW versus the beam collimation graph (Fig. 6). Similar patterns are observed on the two graphs, with a continuous increase from 18 to 9 mm. Although focal spot change takes place at 10-mm collimation as well, it cannot be dosimetrically determined because it is the best collimated beam width.
The high voltage and phantom size effect
The power relationship of CTDIW and high voltage (CTDIw=C (kVp)n) allows the user to modify the mAs value appropriately when the kVp setting is altered in scanning protocols. The provided data show that the exponent values n depend on beam collimation, hence a complete evaluation over the available beam widths is required (Table 4). Note that the kVp exponents n for the CTDIw differ from those for CTDIF. This is an expected result due to the increased amount of scattering material. The change of CTDIF with high voltage reflects only the change in photon and energy fluence generated by the X-ray tube, whereas CTDIw also incorporates the variations of radiation transport and absorption in the measurement phantoms. However, reported experience as to whether image quality is maintained or degraded when high voltage is reduced in favor of patient dose is controversial, and further investigation is required [18–21].
The distribution of radiation within the scanned volume is not only size dependent, but also varies with slice collimation and tube voltage. The ratios of central to peripheral doses decrease with kVp and follow an erratic behavior with beam width. The ratios are higher for the 32-mm phantom compared to the 16-mm phantom, and this is attributable to the difference in the thickness of the intervening medium between the center of the phantom and the position of peripheral measurement (Fig. 3). As the mean photon energy increases with high voltage, the penetrating ability of radiation increases as well, which leads to a decline of the dose ratios.
The effect of phantom size on radiation doses is also illustrated by the CTDIF/CTDIW ratios provided in Table 5. The average dose ratio was 2.48 for the body phantom and 1.24 for the head phantom, which is attributable to the attenuation properties of the two phantoms. However, the ratios for the two phantoms show an inverse dependence on beam energy, which is probably the outcome of the variation in the beam penetrating ability with photon energy, inherent filtration and the average beam travel length from the focal spot to the phantom periphery. In accordance with Nickoloff et al. [22], we found that central and peripheral doses increase drastically as the phantom diameter decreases. A 50% reduction in diameter leads to a 200% increase in CTDIW values. This finding indicates that CT protocols should be individually tailored to the body habitus of the examinees. For this reason, pediatric CT and dosimetry make up a very large field of research, requiring thorough and specific investigations [20, 23–28]. Although previous studies have addressed the issue of adapting technique factors to patient size, there are no data pertaining to the scanner model of our study, and further research is necessary [18, 20–24].
Rotation time and focal spot change
Our study has shown that automatic focus change may lead to an up to 18% increase in administered doses (Fig. 2). Hence, avoidance of tube currents corresponding to the large focal spot is an advisable policy when selecting the mAs setting. Since the parameters that can be selected are effective mAs, rotation time (RT) and pitch (P) (by varying the table feed) for helical scans, and mAs per rotation and rotation time RT for axial scans, the tube current I is determined by:
and
Hence, selection of the small focal spot can be accomplished by increasing the rotation time when a high mAs value is required. This behavior contrasts the common knowledge originating from scanners for which the rotation time and tube current are both independent selectable parameters [15].
The pitch effect
Increasing the pitch value of helical scans while maintaining the same effective mAs setting may even lead to an increase in patient doses (Fig. 5). This is attributable to the effective mAs definition adopted by the manufacturer. According to Eq. 2, the actual tube load value increases with pitch in order to maintain comparable noise levels in the reconstructed images. This in turn, depending on the rotation time setting, may trigger the large focal spot operation and increase the radiation dose (Eq. 10, Fig. 1). Mahesh et al. [29] first observed that for a Siemens Somatom Plus Volume Zoom scanner, an increase in pitch does not produce a radiation dose reduction. However, they did not quote the effective mAs concept, but interpreted this behavior as an idiosyncrasy of the scanner; they also did not comment on the adverse effect of focal spot change. Therefore, the users of a specific scanner should be aware of the effective mAs feature when designing helical protocols with a pitch >1. Similarly, caution must be exercised when making effective dose calculations on the basis of CTDIV derived from nCTDIW. Since the pitch effect is taken into account in the effective mAs value, no further correction is required, and the following equation applies:
Dosimetric comparison of scanners
Upon comparing scanners, users should be aware that manufacturers tend to use different definitions concerning the mAs value shown in the control panel. Comparison of the dosimetric characteristics of different scanner types should be made on the basis of the actual tube current-time product value. A General Electric 16-slice scanner operating at 140 kVp and 150 mAs with a pitch value of 1.5 (termed the high-speed mode) delivers 11.6, 11.5 and 10.2 mGy for a beam collimation of 10, 15 and 30 mm, respectively [15]. The above tube load corresponds to a value of 100 mAs, which was effective for the scanner used in our study (Eq. 2), and as seen in Table 2, the doses delivered by helical scans of equivalent technique factors are 12.7 mGy for a beam collimation of 12 and 11.6 mGy for a beam collimation of 24 mm. Had we erroneously used an mAs value of 150, it would have resulted in a 50% overestimation of doses and rendered the comparison misleading. However, a thorough comparison of scanners and scanning protocols should be based not only on the dose deposition, but also on the evaluation of the resulting image quality [30, 31].
Comparison with a previous study
Hamberg et al. presented a dosimetric study of a General Electric 16-slice scanner exclusively in terms of CTDIW [15]. The scanner studied allows only two values of helical pitch (0.75 and 1) and five helical collimations (5, 10, 15 and 20 mm), which can also be employed in axial scans. They evaluated the dose increase due to focal spot change (10%) and the difference between measured and displayed values (10% higher in the head mode and 13% lower in the body mode) for a single collimation setting (5 mm). Similarly, they evaluated the dose increase with high voltage (kVp to the power of 2.5 in the head phantom and 2.8 in the body phantom) only for the 5-mm collimation. CTDIW values were found to decrease continuously with collimation, probably due to a different configuration of the number of active detectors N and detector widths h, determining the beam collimation (N×h), compared to the present study scanner. CT doses decreased with increasing pitch when a constant mAs value was maintained, since tube load is expressed in actual mAs per rotation. Finally, they did not provide air-to-phantom dose conversion coefficients, as no free-in-air measurements were performed.
Conclusions
The varying degrees of the over-beaming effect between available beam collimations and the automatic focal spot change result in wide variations in CT doses with acquisition parameters. The provided dosimetric data will facilitate the dose-effective use of the scanner studied. For some specific settings, the penalty for speed may be an increase in the administered dose: fast rotations at high tube load may trigger the large focus and increase the radiation dose. The same effect may occur when the pitch increases from 1 at a constant effective mAs value. Therefore, when a high tube load is required, an increased rotation time should be preferred in order to avoid the automatic selection of the large focal spot. Narrow collimations should be avoided as they are less dose effective, unless their use is dictated by the clinical need for thin reconstructed slices. Helical scans at 24-mm collimation or axial scans at 10-mm are to be preferred on the basis of protecting the patient from radiation.
References
Fuchs T, Kachelriess M, Kalender WA (2000) Technical advances in multi-slice spiral CT. Eur J Radiol 36:69–73
Hara K, Johnson CD, MacCarty RL, Welch TJ, McCollough CH, Harmsen WS (2001) CT colonography: single- versus multi-detector row imaging. Radiology 219:461–465
Ohnesorge B, Flohr T, Becker C, Kopp AF, Schoepf UJ, Baum U, Knez A, Klingenbeck-Regn K, Reiser MF (2000) Cardiac imaging by means of electrocardiographically gated multisection spiral CT: initial experience. Radiology 217:564–571
Mettler FA, Wiest PW, Locken JA, Kelsey CA (2000) CT scanning: patterns of use and dose. J Radiol Prot 20:353–359
Brix G, Nagel HD, Stamm G, Veit R, Lechel U, Griebel J, Galanski M (2003) Radiation exposure in multi-slice versus single-slice spiral CT: results of a nationwide survey. Eur Radiol 13:1979–1991
Nickoloff EL, Alderson PO (2001) Radiation exposures to patients from CT: reality, public perception, and policy. AJR Am J Roentgenol 177:285–287
Shrimpton PC, Hart D, Hillier MC, Wall BF, Faulkner K (1991) Survey of CT practice in the UK. I. Aspects of examination frequency and quality assurance. National Radiological Protection Board publication no. NRPB-R248. Her Majesty’s Stationery Office, Chilton, England
Shrimpton PC, Jones DG, Hillier MC, Wall BF, Le Heron JC, Faulkner K (1991) Survey of CT practice in the UK. II. Dosimetric aspects. National Radiological Protection Board publication no. NRPB-R249. Her Majesty’s Stationery Office, Chilton, England
United Nations Scientific Committee on the Effects of Atomic Radiation. 2000 report to the General Assembly, Annex D: medical radiation exposures. New York, NY: United Nations, 2000
International Commission on Radiological Protection (2000) Managing patient dose in computed tomography. Oxford: Pergamon Press; ICRP Publication 87, Ann ICRP 2000; Vol 30, Iss 4
European Commission’s Study Group (1998) Quality criteria for computed tomography. Working Document. EUR 16262
McCollough CH, Zink FE (1999) Performance evaluation of a multi-slice CT system. Med Phys 26:2223–2230
Thomton FJ, Paulson EK, Yoshizumi TT, Frush DP, Nelson RC (2003) Single versus multi-detector row CT: comparison of radiation doses and dose profiles. Acad Radiol 10:379–385
Golding SJ, Shrimpton PC (2002) Radiation dose in CT: are we meeting the challenge? Br J Radiol 75:1–4
Hamberg LM, Rhea JT, Hunter GJ, Thrall JH (2003) Multi-detector row CT: radiation dose characteristics. Radiology 226:762–772
Brix G, Lechel U, Veit R, Truckenbrodt R, Stamm G, Coppenrath EM, Griebel J, Nagel HD (2004) Assessment of a theoretical formalism for dose estimation in CT: an anthropomorphic phantom study. Eur Radiol 14:1275–1284
Nagel HD (ed) (2000) Radiation exposure in computed tomography. Fundamentals, influencing parameters, dose assessment, optimisation, scanner data, terminology, 2nd edn. COCIR European Coordination Committee of the Radiological and Electromedical Industries, Frankfurt
Huda W, Scalzetti EM, Levin G (2000) Technique factors and image quality as functions of patient weight at abdominal CT. Radiology 217:430–435
Gupta AK, Nelson RC, Johnson GA, Paulson EK, Delong DM, Yoshizumi TT (2003) Optimization of eight-element multi-detector row helical CT technology for evaluation of the abdomen. Radiology 227:739–745
Cody DD, Moxley DM, Krugh KT, O’Daniel JC, Wagner LK, Eftekhari F (2004) Strategies for formulating appropriate MDCT techniques when imaging the chest, abdomen, and pelvis in pediatric patients. AJR Am J Roentgenol 182:849–859
Huda W, Lieberman KA, Chang J, Roskopf ML (2004) Patient size and X-ray technique factors in head computed tomography examinations. II. Image quality. Med Phys 31:595–601
Nickoloff EL, Dutta AK, Lu ZF (2003) Influence of phantom diameter, kVp and scan mode upon computed tomography dose index. Med Phys 30:395–402
Siegel MJ, Schmidt B, Bradley D, Suess C, Hildebolt C (2004) Radiation dose and image quality in pediatric CT: effect of technical factors and phantom size and shape. Radiology 233:515–522
Huda W, Lieberman KA, Chang J, Roskopf ML (2004) Patient size and X-ray technique factors in head computed tomography examinations. I. Radiation doses. Med Phys 31:588–594
Greess H, Lutze J, Nomayr A, Wolf H, Hothorn T, Kalendar WA, Bautz W (2004) Dose reduction in subsecond multislice spiral CT examination of children by online tube current modulation. Eur Radiol 14:995–999
Verdun FR, Lepori D, Monnin P, Valley JF, Schnyder P, Gudinchet F (2004) Management of patient dose and image noise in routine pediatric CT abdominalexaminations. Eur Radiol 14:835–841
Vock P (2005) CT dose reduction in children. Eur Radiol 15:2330–2340
Boone JM, Geraghty EM, Seibert JA, Wootton-Gorges SL (2003) Dose reduction in pediatric CT: a rational approach. Radiology 228:352–360
Mahesh M, Scatarige JC, Cooper J, Fishman EK (2001) Dose and pitch relationship for a particular multislice CT scanner. AJR Am J Roentgenol 177:1273–1275
Gurung J, Khan MF, Maataoui A, Herzog C, Bux R, Bratzke H, Ackermann H, Vogl TJ (2005) Multislice CT of the pelvis: dose reduction with regard to image quality using 16-row CT. Eur Radiol 15:1898–1905
Wormanns D, Ludwig K, Beyer F, Heindel W, Diederich S (2005) Detection of pulmonary nodules at multirow-detector CT: effectiveness of double reading to improve sensitivity at standard-dose and low-dose chest CT. Eur Radiol 15:14–22
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Theocharopoulos, N., Perisinakis, K., Damilakis, J. et al. Dosimetric characteristics of a 16-slice computed tomography scanner. Eur Radiol 16, 2575–2585 (2006). https://doi.org/10.1007/s00330-006-0251-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-006-0251-0