Abstract
Today, MR is the only method needed for the morphological investigation of endocrine-active pituitary adenomas. In acromegaly and Cushing’s syndrome, the therapeutic attitude is directly dictated by MR data. We present the MR aspect of pituitary adenomas according to size, sex, age, endocrine activity and a few particular conditions such as hemorrhagic pituitary adenomas, pituitary adenomas during pregnancy, cavernous sinus invasion and postsurgical changes. When an intrasellar mass extending out of the sella turcica is detected, the goal of the MR examination is to indicate precisely the origin of the tumor, its extension in relation to the various surrounding structures, its structure and its enhancement in order to help in the differential diagnosis. Demonstration of very small pituitary adenomas remains a challenge. When SE T1- and Turbo SE T2-weighted sequences are non-diagnostic, enhanced imaging becomes mandatory; half-dose gadolinium injection, delayed sequence, dynamic imaging can be of some help.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pituitary adenomas are by far the most common pathology encountered in the sellar area. MR is usually the only method needed for the morphological investigation of endocrine-active pituitary adenomas. In acromegaly and Cushing’s syndrome, therapeutic attitude is directly dictated by MR data. CT should only be carried out on second intention and should remain exceptional to complement the MR images in case of osseous malformations, anatomic variants and calcification. We describe the MR aspect of pituitary adenomas according to size, sex, age, endocrine activity and a few particular conditions.
Usual MR findings in pituitary adenomas
Classically, pituitary adenomas are divided into two categories: pituitary microadenomas less than 10 mm in diameter and pituitary macroadenomas over 10 mm in diameter. We use the term “picoadenomas” to describe lesions smaller than 3 mm, posing diagnostic problems because of their small size.
Pituitary microadenomas and picoadenomas
Their location is almost systematically intrasellar; thus, they rarely cause visual defects. However, exceptionally a 10-mm pituitary adenoma may be very near the optic chiasm if the sella turcica is small or flat. The revealing symptom of pituitary microadenomas is usually endocrine dysfunction; they may rarely be a serendipitous discovery. Modifications of the sellar floor are more difficult to distinguish in MR imaging than on a CT scan, and although depressions, slopes and angulations of the sellar floor may be visualized through MR imaging, basic cortical thinning cannot.
On T1-weighted spin echo images, pituitary microadenomas are usually evidenced as rounded or oval, sometimes flattened or triangular intrasellar lesions, with loss of signal intensity compared to the unaffected anterior pituitary gland. In approximately 25% of cases, however, the more heavily T1-weighted signal of the pituitary adenoma is very similar to that of the unaffected pituitary gland, and complementary investigations are necessary. Pituitary microadenomas can also cause high signal intensity on the T1-weighted image, probably because of internal hemorrhagic transformation of all or parts of the adenoma, a rather frequent phenomenon in prolactinomas.
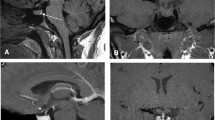
On T2-weighted MR images, signal intensity of pituitary adenomas typically resembles that of the temporal cortex, slightly hyperintense to that of the normal anterior lobe, which is very close to the white matter (Fig. 1).
However, on T2-weighted fast-spin echo MR images, the aspect of pituitary microadenomas fluctuates, in particular with the type of endocrine activity. The diagnosis of microadenomas is simple when they demonstrate high intensity in the T2-weighted image, although this signal may only be represent a part of the pituitary adenoma. Increased intensity on T2-weighted images is found in over 80% of microprolactinomas. Conversely, isosignal or hypointensity on T2-weighted images of lesions occurs in 2/3 of all growth hormone-secreting microadenomas (Fig. 2). Fast spin echo T2-weighted images are particularly helpful when looking for pituitary picoadenomas smaller than 3 mm in diameter for which T1-weighted images, and even gadolinium-enhanced sequences, are negative.
When both the T1-weighted and fast T2-weighted images corroborate the diagnosis (for example, low intensity T1-weighted signal and high intensity T2-weighted signal), which is the most usual case when investigating prolactinomas, gadolinium enhancement is unnecessary. On the contrary, when the diagnosis has not been clearly established, enhanced imaging becomes mandatory. A half-dose of gadolinium, i.e., 0.05 mmol/kg, and sometimes less (0.03 mmol/Kg), is injected into the veinous system, and T1 spin echo coronal views are obtained. Contrast-enhanced images typically show a hypointense lesion surrounded by the intense enhancement of the normal pituitary gland. The contrast-enhanced images may be negative if the tumor is extremely small, the dose of gadolinium is too high or the visualization window is too large.
Other techniques are also available: delayed sequences taken 30–40 min after injection of contrast may show late enhancement of the adenoma itself (Fig. 3). Correlation between the hypersignal in fast T2 and adenoma enhancement is good; surgery shows that these lesions are soft. However hypointense adenomas on T2, which are ordinarily firmer, and often secrete more, do not enhance at a distance from the gadolinium injection. Dynamic imaging can be applied systematically in the diagnosis of ACTH adenomas, or used as a complementary investigation when clinical signs are strongly evocative of a pituitary adenoma, but conventional MR images are not convincing. In such cases, dynamic imaging can evidence a lack or a temporary delay of enhancement in the pituitary adenoma relative to the normal pituitary gland.
Pituitary macroadenomas
Pituitary macroadenomas are intrasellar masses with extra-sellar extension, usually upwards into the suprasellar cistern, or still higher, compressing the third ventricle and sometimes the foramen of Monro. The tumoral mass can also extend downwards into the sphenoidal sinus or laterally into the cavernous sinus. What is asked of MR imaging differs for pituitary microadenomas and macroadenomas. When an intrasellar mass extending out of the sella is detected, one should seek the origin of the tumor (pituitary or not), its extension in relation to the various anatomical surrounding structures, its structure (firm, cystic, necrotic or hemorragic) and its enhancement. What we are asking of MR imaging, then, is not so much a positive diagnosis, but rather an assessment of the extension and a differential diagnosis.
Pituitary macroadenomas are centered on the sella turcica, which usually appears modified. An optimized localiser image may reveal a remodeled and enlarged sella, with a missing portion of the dorsum. If other morphological details are required, a localiser image CT or a standard X-ray film will provide more information, particularly regarding a modified sellar cortex. Nowadays, this is rarely necessary. Pituitary adenomas with suprasellar extensions are often polycyclical in shape, with one or two extensions into the suprasellar cistern. Their spontaneous signal fluctuates, but it is usually brighter on the T1-weighted spin echo images than that of pituitary microadenomas. The aspect of macroadenomas is often clearly inhomogenous, particularly so on the fast T2-weighted images, with disseminated areas of hypersignal reflecting cystic or necrotic portions of the adenoma. The adenomatous tissue usually enhances slightly after contrast media injection, but the object of enhanced imaging is to visualize normal pituitary tissue. It usually forms a strongly enhancing pseudocapsule around the adenoma: above it, behind it, rarely below or in front of it, and usually unilaterally. The coronal section of the enhanced T1-weighted image generally reveals a unilateral layer of normal pituitary tissue located between the adenoma and the elements of the cavernous sinus, of crucial importance to neurosurgeons (Fig. 4). The hyperintense posterior lobe is modified: it appears either flattened or displaced and is well seen on the axial sections, or an ectopic collection of antidiuretic hormone is located within the pituitary stalk, which is compressed by the superior pole of the macroadenoma. The pituitary stalk is strongly tipped laterally. The relative positions of the optic chiasm and the pituitary macroadenoma are best studied on the coronal sections rather than on the sagittal views. When the suprasellar extension is large, the chiasm itself may be difficult to identify. In this case fast spin T2-weighted coronal sections should help because the optic chiasm is clearly hypointense (Fig. 5).
After gadolinium injection, discrete meningeal enhancement is usually noticeable near the area where the meninges are in contact with the adenoma, and particularly so in the anterior cranial fossa, along with a possible dural tail, which has previously been described with meningiomas of the sellar area as well as with hypophysitis. In our experience, the enhanced nearby dura matter has no specificity whatsoever.
MR aspects according to sex and age
Prolactin-secreting microadenomas are very common in young women. Some may spontaneously remain dormant over very long periods. They do not develop after menopause. When prolactin-secreting adenomas are discovered in male patients, they have usually reached the stage of macroadenomas. This is probably due in part to the fact that clinical signs are less obvious than in women, and in part to the fact that their potential development is probably different. Cavernous sinus involvement is far from being exceptional.
Pediatric pituitary adenomas are not only exceptional, but also potentially very active. Prolactin-secreting adenomas can be responsible for late puberty, while ACTH-secreting adenomas can cause Cushing’s disease.
MR aspects according to types of secretion
Prolactinomas are usually discovered at the stage of microadenomas owing to distinctive clinical signs found in young women, including amenorrhea, galactorrhea and hyperprolactinemia (over 30 or 40 μg/l). Most of the time, the prolactinoma is hypointense on the T1-weighted image, while it is hyperintense on the T2-weighted image in four cases out of five. This hypersignal may only be exhibited by a portion of the adenoma. Correlation between prolactin rates and adenoma size is usually good. However, given two prolactinomas of equal size, the hypointense tumor on the T2-weighted image secretes more than its countpart. Medical treatment based on bromocriptine and analogs decreases adenoma volume drastically. As a result, diagnosis becomes difficult or impossible. We strongly recommend MRI documentation before instituting the medical treatment. In some cases, when prolactinomas are imaged at a distance from the medical treatment with bromocriptine, peculiar scarred tissue can be seen, which is evocative of a former pituitary adenoma: it is a very local remodeling of the pituitary gland, forming a V on its superior aspect (Fig. 6).
While prolactinomas and GH-secreting adenomas are usually located laterally in the sella turcica, ACTH-secreting adenomas in Cushing’s disease, ordinarily smaller in size, are more often located along the median line. Because of the severe prognosis of the syndrome and the surgical possibilities, repeated sequences and MR investigations should be carried out without hesitation.
Growth hormone-secreting adenomas have the unique characteristic of exhibiting a hyposignal on the T2-weighted image in 2/3 of the cases. Spontaneous infarctus or necrosis of GH-secreting adenomas is far from being exceptional: certain acromegalies that were detected late in the course of the disease exhibit an enlarged, partially empty sella turcica, lined with adenomatous tissue that proves difficult to analyze. Medical treatment based on octreotride analogs (somatostatin) decreases the size of the adenoma by an average of 35% and brings the level of somatomedin C back to normal in 50% of cases. It proves very useful before surgery.
Macroadenomas can be nonfunctioning, but they can also be prolactin-secreting adenomas, gonadotrope adenomas and growth hormone-secreting adenomas. The greater their size, the more heterogenous they are, as areas of cystic necrosis are caused by poor tumoral blood supply. Gonadotrope adenomas are often massive and have a strong tendency to recur.
Particular aspects
Hemorragic pituitary adenomas
Hemorrhage occurs in all or parts of 20% of all pituitary adenomas, but it is usually occult. Pituitary apoplexy, with the usual headache, pseudomeningeal syndrome, cranial nerve paralysis and severe hypopituitarism is generally caused by massive hemorrhage within a pituitary macroadenoma. Smaller scale hemorrhage occurs much more often and can be seen within pituitary adenomas. Bromocriptin is held responsible for a certain degree of intratumoral hemorrhages in prolactinomas, although the phenomenon is sometimes revealed on MR images before the treatment has been instituted. Recurrent hemorrhage is possible and can cause repeated headaches. Intratumoral hemorrhages are revealed by spontaneous hypersignals on the T1-weighted images. A level line can sometimes be seen within the hemorrhage. This is due to sedimented blood cell membranes and hemoglobin residues (deoxyhemoglobin, methemoglobin). Although there is no blood-brain barrier within the pituitary parenchyma, small linear or curved hyposignals can sometimes be found after intratumoral hemorrhage: these are caused by hemosiderin deposits.
Pituitary adenomas and pregnancy
Normal pituitary tissue increases in height during pregnancy (0.08 mm per week, i.e., almost 3 mm during the whole pregnancy), while pituitary adenomas increase in volume. The normal pituitary tissue has a longer T1 during pregnancy. The increased volume of the prolactinoma is especially visible when medical treatment has been interrupted. Vision should be closely monitored during this period, and a follow-up MRI can be discussed.
Involvement of the cavernous sinus
Involvement of the cavernous sinus can modify the prognosis, but compression and involvement remain difficult to differentiate. The best sign of involvement remains complete encircling of the intracavernous carotid by the tumor. The diagnosis can practically be eliminated if it can be demonstrated that a strip of normal pituitary tissue lies between the tumor and the cavernous sinus. Large pituitary adenomas can apply pressure onto the cavernous sinus and cause convex deformation of its external wall without necessarily involving it. Finally, in case of massive involvement, the cavernous sinus intensity is identical to that of the intrasellar tumor on the T1-weighted images, before and after gadolinium injection, and on the T2-weighted images.
Aspects after surgery
The surgical cavity is often filled with packing material after transphenoidal resection of a pituitary adenoma. Gelfoam is frequently used and is impregnated with blood and secretions. The presence of gelfoam, of secretions and perhaps of periadenomatous adherences usually keeps the cavity from collapsing in the days and weeks following surgery. Blood, secretions and packing material slowly involute over the following 2 or 3 months. At this time, a few fragments of blood-impregnated gelfoam can still be found in the surgical cavity. If the diaphragm of the sella turcica was torn in the course of surgery, fat or muscle implants are inserted by the surgeon to prevent the occurrence of a cerebrospinal fluid fistula. Their resorption is much longer: implanted fat involutes slowly and may exhibit a hypersignal on the T1-weighted image up to 2–3 years after surgery.
A postoperative follow-up MRI 2 or 3 months after surgery is very useful to monitor further development of a resected adenoma. An earlier MRI, made 48 h after surgery, checks for potential complications and may visualize residual tumor, i.e., a mass of identical intensity as the adenoma before surgery that commonly occupies a peripheral portion of the adenoma. This early investigation also proved extremely helpful to interpret the follow-up MRI. At this stage the remaining normal pituitary tissue can be characterized: it is usually asymmetrical, and a hypersignal is frequently observed at the base of the deviated hypophyseal stalk due to an ectopic collection of antidiuretic hormone.
The second month follow-up MRI is essential to check for potential recurrence, which usually appears as a rounded or very convex mass that is isointense with the initial tumor.
References
Ahmadi J, North CM, Segall HD, Zee CS, Weiss MH (1986) Cavernous sinus invasion by pituitary adenomas. Am J Roentgenol 146:257–262
Bonneville JF, Cattin F, Gorczyca W, Hardy J (1993) Pituitary microadenomas: early enhancement with dynamic CT-implications of arterial blood supply and potential importance. Radiology 187:857–861
Brismon MH (1996) Symptoms of pituitary apoplexy rapidly reversed with bromocriptine. Case report. J Neurosurg 85:1153–1155
Colombo N, Loli P, Vignati F, Scialfa G (1994) MR of corticotropin-secreting pituitary microadenomas. Am J Neuroradiol 15:1591–1595
Davis PC, Hoffman JC Jr, Spencer T et al (1987) MR Imaging of pituitary adenoma: CT, clinical and surgical correlation. Am J Roentgenol 148:797–802
Davis PC, Hoffman JC Jr, Malko JA et al (1987) Gadolinium-DTPA and MR imaging of pituitary adenoma: a preliminary report. Am J Neuroradiol 8:817–823
Davis PC, Gokhale KA, Josep GJ (1991) Pituitary adenoma: correlation of half dose gadolinium enhanced MRI with surgical findings in 26 patients. Radiology 180:779–784
Davis PC, Hoffman JC Jr, Spencer T, Tindall GT, Braun IF (1987) MR Imaging of pituitary adenoma: CT, clinical, and surgical correlation. Am J Roentgenol 148:797–802
Dietemann JL, Portha C, Cattin F, Mollet E, Bonneville JF (1983) CT Follow-up of microprolactinomas during bromocriptine-induced pregnancy. Neuroradiology 25:133–138
Doppman JL, Frank JA, Dwyer AJ et al (1988) Gadolinium DTPA enhanced MR imaging of ACTH-secreting microadenomas of the pituitary gland. J Comput Assist Tomogr 12:728–735
El Gammal T, Brooks BS (1989) MR imaging of the ectopic bright signal of posterior pituitary regeneration. Am J Neuroradiol 10:323–328
Elster AD (1993) Modern imaging of pituitary. Radiology 187:1–14
Knosp E, Kitz K, Steiner E, Matula CH (1991) Pituitary adenomas with parasellar invasion. Acta Neurochir 53:65–71
Kucharczyk W, Davis DO, Kelly WM, Sze G, Norman D, Newton TH (1986) Pituitary adenomas: high-resolution MR imaging at 1.5 T. Radiology 161:761–765
Kucharczyk W, Bishop JE, Plewes DB, Keller MA, George S (1994) Detection of pituitary microadenomas: comparison of dynamic keyhole fast spin-echo, unenhanced, and conventional contrast-enhanced MR imaging. Am J Neuroradiol 15:671–679
Lundin P, Bergström K, Nyman R, Lundberg PO, Muhr C (1992) Macroprolactinomas: serial MR imaging in long term bromocriptine therapy. Am J Neuroradiol 13:1279–1291
Lundin P (1997) Long-term octreotide therapy in growth hormone-secreting pituitary adenomas: evaluation with serial MR. Am J Neuroradiol 18:765–772
Scotti G, Yu CY, Dillon WP et al (1988) MR imaging of cavernous sinus involvement by pituitary adenomas. Am J Neuroradiol 9:657–664
Steiner E, Knosp E, Herold CJ et al (1992) Pituitary adenomas: findings of postoperative MR imaging. Radiology 185:521–527
Teng MM, Huang C, Chang T (1988) The pituitary mass after transsphenoidal hypophysectomy. Am J Neuroradiol 9:23–26
Youssem DM, Arrington JA, Zinreich SJ, Kumar AJ, Bryan RN (1989) Pituitary adenomas: possible role of bromocriptine in intratumoral hemorrhage. Radiology 170:239–243
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonneville, JF., Bonneville, F. & Cattin, F. Magnetic resonance imaging of pituitary adenomas. Eur Radiol 15, 543–548 (2005). https://doi.org/10.1007/s00330-004-2531-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-004-2531-x