Abstract
The aim of this study is to evaluate the clinical significance of 124I positron emission tomography (PET) using a combined PET/CT tomograph in patients with differentiated thyroid carcinoma and to compare the PET/CT results with 131I whole-body scintigraphy (WBS), dedicated PET and CT alone. Twelve thyroid cancer patients were referred for diagnostic workup and entered complete clinical evaluation, including histology, cytology, thyroglobulin level, ultrasonography, fluorine-18 fluorodeoxyglucose (FDG)-PET, FDG-PET/CT and CT. Lesion-based evaluation showed a lesion delectability of 56, 87 and 100% for CT, 124I-PET, and combined 124I-PET/CT imaging, respectively. Lesion delectability of 131I-WBS was 83%. We conclude that 124I-PET/CT imaging is a promising technique to improve treatment planning in thyroid cancer. It is particularly valuable in patients suffering from advanced differentiated thyroid cancer prior to radio-iodine therapy and in patients with suspected recurrence and potential metastatic disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Patients with well-differentiated thyroid cancer (DTC) generally have a good prognosis. However, widespread metastatic disease or tumor recurrence can be associated with significant morbidity, and even mortality. Proper staging is crucial for appropriate therapy planning. Treatment options involve radio-iodine therapy and, in advanced disease, external beam radiotherapy and chemotherapy [1].
In addition, thyreoglobulin (TG), 131I-WBS, ultrasonography (US), US-guided fine-needle biopsy and FDG-PET are useful diagnostic tools in the follow-up of thyroidectomized patients with DTC [1–5], of which 131I-WBS was shown to be most sensitive procedure in follow-up of DTC [6]. Recent studies showed that FDG-PET results lead to changes in approaches to surgical treatment plans in a significant number of patients, especially in cases with poor tumor differentiation, where reduced or lost iodine-accumulating ability leads to false-negative 131I-scanning results [1–3].
In the majority of patients, however, iodine uptake in the tumor is adequate for scintigraphy and radio-iodine therapy. Therefore, patients with DTC may benefit from technical improvements in tumor scintigraphy with iodine.
Iodine-124, an isotope with a half-life of 4.2 days, is suitable for PET imaging and has already been used for dosimetry [7–13]. Although PET is a superior imaging system with physical advantages compared to planar gamma cameras and SPECT, it has not been established for routine imaging of thyroid cancer with iodine for several reasons: first, because of the complex decay scheme of 124I that includes several high-energy gamma rays and makes 124I imaging a challenge for a gamma camera. In addition, the limited availability of 124I, which needs a high-MeV cyclotron for production, together with the somewhat limited availability of PET scanners further limit the widespread acceptance of 124I imaging. However, upon availability measurements with 124I made under realistic scan conditions using different PET scanner models have shown that satisfactory imaging results can be achieved [12]. However, 124I will only be made available for satellite PET sites if it can be established as a superior radiopharmaceutical for thyroid cancer imaging.
Second, a major challenge when interpreting PET or SPECT images with highly specific tracers such as 124I is the lack of identifiable anatomical structures, thus making an accurate localization of foci of tracer uptake highly problematic [14]. By correlating the PET information with the available anatomical background information such as obtained from a dual-modality PET/CT exam, the spatial localization of lesions may be much improved. By acquiring both PET and CT data sets in a single scan session using a combined PET/CT tomograph, the PET and CT data can be acquired in a single session and the resulting imaging sets are intrinsically co-registrated [14, 15]. Until now, only a few case-reports concerning 124I-PET/CT have been published showing the potential of this new and innovative imaging tool [16–18].
The purpose of this study was for the first time to evaluate 124I-PET/CT scanning in patients with differentiated thyroid carcinoma by comparing diagnostic imaging results of established imaging procedures with the results of 124I-PET and CT alone.
Material and methods
Patients
Twelve patients (five female and seven male, age: 31–76 years, mean 59 years) with DTC who where admitted to the Department of Nuclear Medicine at the University Hospital, Essen, for radio-iodine therapy were included in this study. All patients gave informed consent. Eleven patients were examined during the postoperative phase after thyroidectomy under maximal thyroid-stimulating hormone (TSH) stimulus (52±21 mU/l). One patient with known pulmonary metastasis was examined following an exogenous TSH stimulation by recombinant human TSH (rhTSH). Iodine excretion in the urine was within physiological limits in all patients (128±50 μg/l), excluding an iodine contamination. The tumor classification in these patients according to the Union Internationale Contre le Cancer (AJCC) system [19] is shown in Table 1. Six patients presented with elevated TG, and no patient had pathologically elevated TG antibodies (Table 2). Surgical intervention was performed in three patients after completion of the diagnostic imaging; two patients received external radiation beam therapy in addition to radio-iodine therapy. During routine clinical staging, computed tomographic imaging (CT), 124I-PET, combined 124I-PET/CT, US and separate FDG-PET were performed. All imaging procedures were completed within 2 weeks.
Methods
Radiopharmaceutical preparation of 124I
[124I] Iodide was produced using the CV28 cyclotron by means of a [124Te] Tellurium (d, 2n) [124I] Iodine reaction. A platinum-iridium target plated with 99.8% [124Te] Telluriumdioxide was irradiated with 14 MeV deuterons at 15 μA. Subsequently, the [124I] Iodine was distilled at 745°C in an quartz apparatus and the [124I] Iodide was inserted in 0.2 ml of 0.01 N NaOH. [20] About 100 MBq (70–90 μl) of this solution was applied to a commercially available capsule (Amersham, Brauschweig, Germany) just before administration to the patient.
124I-PET/CT data acquisition
Data acquisition was performed using a combined PET/CT system (Biograph, Siemens Medical Solutions Hoffman Estates, USA manufactured by CPS, Knoxville, TN). The biograph consists of a single-slice spiral CT (Somaton Emotion) and a dedicated PET scanner based on ECAT EXACT HR+.
PET/CT imaging commenced 24 h postoral administration of 84±15 MBq 124I. The patient was positioned on the table and a topogram (scout scan) was used to define the axial imaging range. Typically, patients were scanned from the head to the abdomen, thus yielding four to seven contiguous bed positions. First, a single-spiral CT scan was acquired covering the entire axial imaging range. All CT scans were acquired with a tube voltage of 130 kVp, 160 mAs, a slice width of 5 mm and a pitch of 1.6. As all patients were scheduled for radio-iodine-therapy, no intravenous CT contrast agent was applied due to the potential iodine blockade. The CT data were used for PET attenuation correction [21]. After completion of the CT scan, the patient was advanced automatically to the PET (to the rear of the combined gantry), and emission scanning started. Whole-body emission data were acquired in 3D mode for 5 min per bed position. Image reconstruction of the corrected emission data was performed after Fourier rebinning (AW-OSEM at two iterations and eight subsets with a 5 mm post-reconstruction Gaussian filter). Total scan time was 25–40 min depending on the number of beds scanned.
Interpretation of the 124I-PET examinations
Validation of 124I-PET findings was not possible in all lesions since most lesions were not biopsied. Therefore, we introduced the following diagnostic criteria for reading the 124I-PET images:
-
(1)
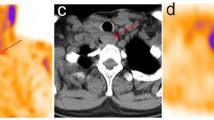
Intense focal tracer accumulation exceeding normal regional tracer uptake by at least a factor of five was rated as tumor or thyroid remnant, depending on the location and morphology (Fig. 1 shows the weakest positive lesion interpreted as tumor, with a tumor/background ration of 6.2).
-
(2)
Focal tracer accumulation in the pelvis of the kidney or bladder was considered to be physiologic.
-
(3)
Non-focal linear uptake following the intestine was rated as non-specific, non-pathological uptake.
These criteria were confirmed in 6 lesions (of 69 total lesions) by histology.
I-131 WBS
High-dose WBS was performed 5–8 days after oral administration of 3,000 MBq 131I using a dual-head gamma camera (BodyScan, Siemens, Erlangen, Germany) with a high-energy collimator.
Ultrasound
Lymph node staging was performed in all 12 patients by Ultrasound (Sonoline Elegra, Siemens, Erlangen, Germany) as part of morphological diagnostic imaging. Lymph nodes were called metastatic according to the criteria of Görges et al. that include the Solbiati-Index (ratio of largest to smallest diameter), internal structure and vascular pattern yielding a sensitivity of 90% and specificity of 82% [22].
Data analysis
Two experienced radiologists (GA, JFD) assessed the results of anatomical imaging procedures. Two experienced nuclear medicine specialists (LSF, RG), without knowledge of the CT findings, or clinical data, evaluated the PET images. Finally the PET/CT-images were read by two nuclear medicine specialists (LSF, RG) and two radiologists in consensus (GA, JFD). Except for six cases where histological results were available, a consensus reading based on the sum of all clinical data and imaging procedures was established by a committee consisting of the two radiologists and the two nuclear medicine specialists. A consensual verdict was reached for each patient with respect to the presence or absence of disease and the number and localization of malignant lesions. This consensus, combined with results of US and 131I-WBS, served as the standard of reference and reflected the final clinical diagnosis.
This consensus procedure resulted in a set of data for each patient with respect to primary tumor/local recurrence, lymph node status and metastasis. Lesion delectability of the individual procedures was calculated from these data.
Results
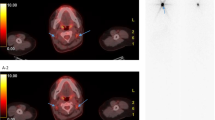
The summarized findings of the individual diagnostic techniques are shown in Table 2. 131I-WBS did not allow any differentiation of multiple foci in thyroid remnants in all patients as shown in Fig. 2. Besides evaluation of thyroid remnants the clinical readings from 124I-PET and 131I-WBS agreed in 10/12 (83%) patients. The discrepancies in three patients can be described as follows.
-
(1)
A cervical lymph node, which was close to thyroid remnant metastasis was not detected in 131I-WBS (patient 4, see Figs. 3, 4).
Fig. 3 Fig. 4 131I-WBS compared to 124I-PET/CT (patient 4, see Fig. 3) does not allow differentiation between thyroid remnant and cervical lymph node metastasis
-
(2)
131I-WBS missed two small bone metastasis (patient 11, see Fig. 1).
In CT alone, especially primary malignancies, lymph node metastasis and small metastases involving the bone were not detected, yielding in a lesion delectability of 35, 50 and 65%, respectively. On the other hand, CT showed iodine-negative pulmonary metastases in 4/12 (33%) patients [16].
Compared to the combination of separate 124I-PET and CT readings, the combined assessment of fused PET and CT images in a fusion display did not reveal additional tumor manifestations. However, the accurate topographic localization of the tumor resulted in a change of staging in two patients and a change in management in one patient who underwent re-surgery due to a lymph node metastasis (patient 4). Table 3 summarizes the results of the different imaging procedures including 131I-WBS and ultrasound (cervical and abdominal).
As can be seen from Tables 2, 3, the morphologic and metabolic imaging procedures showed considerable variation in results. The sensitivities of each modality for primary tumor, lymph node staging and organ metastases are shown Table 3. The overall lesion delectability in 124I-PET, CT, 124I-PET/CT and 131I-WBS were 87, 56, 100 and 83%, respectively.
Discussion
In CT alone, especially primary malignancies, lymph node metastasis [17, 18] and small metastases involving the bone were not detected. On the other hand, CT showed iodine-negative pulmonary metastases in 4/12 (33%) patients [16] and thus was highly accurate in the diagnosis of organ metastases. These findings exclude curative 131I therapy and convey important prognostic information. Therefore, the combination of 124I-PET and CT appears to be synergistic.
Compared to the combination of separate 124I-PET and CT readings, the combined assessment of fused PET and CT resulted in a change of staging in two patients and a change in management in one patient. From these considerations, it is clear that 124I-PET/CT may provide incremental diagnostic value over the individual imaging modalities.
To date, there have been no studies examining the impact of 124I-PET on the management of DTC, although 124I-PET has been shown to be a useful imaging technique for the diagnosis and management of thyroid diseases [12]. However, interpreting PET scans with highly specific tracers such as 124I is challenged by the lack of identifiable anatomical structures in the PET imaging. This shortcoming of diagnostic procedures using radioactive iodine is subdued by PET/CT as we firstly could show in this study. This data confirms the promising results of first case studies from our group [16–18].
Moreover, several other aspects favor 124I-PET and 124I-PET/CT imaging. High-dose WBS is typically performed 3–8 days after the administration of 131I. Our data indicate that 124I-PET can be performed as early as 24 h after the administration of the radiotracer without sacrifice in diagnostic accuracy compared to high-dose WBS. As a consequence, faster treatment stratification is possible, e.g., initiation of surgery for the removal of easily accessible tumor manifestations or external beam radiation for metastases with insufficient uptake for radio-iodine therapy. Finally, a radiation exposure of 5–10 mSv from the administration of 50–100 MBq 124I compares favorably with 60 mSv from 1,000 MBq 131I [23]. Our data prove the superiority of 124I-PET over planar 131I-WBS even at lower 124I activities and therefore at considerably lower radiation exposure. Thus, 124I-PET is an alternative for high-dose diagnostic 131I-WBS [24] in follow-up of DTC, which is less time-consuming and more convenient for the patient.
Limitations of the present study are the small number of patients, which results from the rarity of the disease, and also the fact that histological confirmation of the findings was possible in only some of the cases, since the other patients were treated by radio-iodine and in some cases by external radiotherapy or were classified as inoperable.
Conclusions
Using a combined PET/CT system, we were firstly able to show that 124I is an efficient diagnostic tool in DTC. With an overall lesion delectability of 97%, 124I-PET/CT is a promising approach in patients suffering from advanced DTC before radio-iodine therapy and patients with suspected recurrence and/or metastases. In addition to the synergistic effects of combining morphologic imaging with highly specific functional imaging, 124I-PET/CT represents a innovative, suitable, low-dose alternative to the clinical standard of high-dose 131I-WBS in follow-up of patients with DTC.
The diagnostic and logistic advantages of 124I-PET and 124I-PET/CT can only be utilized clinically if 124I becomes more widely available. If a PET/CT scanner is available, it should be the preferred imaging technique for DTC patients.
References
Frilling A, Görges R, Tecklenborg K et al (2000) Value of preoperative diagnostic modalities in patients with recurrent thyroid carcinoma. Surgery 28:1067–1074
Alnafisi NS, Driedger AA, Coates G, Moote DJ, Raphael SJ (2000) FDG PET of recurrent or metastatic 131I-negative papillary thyroid carcinoma. J Nucl Med 41:1010–1015
Chung JK, So Y, Lee JS et al (1999) Value of FDG PET in papillary thyroid carcinoma with negative 131I whole-body scan. J Nucl Med 40:986–992
Grünwald F, Kalicke T, Feine U et al (1999) Fluorine-18 fluorodeoxyglucose positron emission tomography in thyroid cancer: results of a multicentre study. Eur J Nucl Med 26:1547–1552
Hooft L, Hoekstra OS, Deville W et al (2001) Diagnostic accuracy of 18F-fluorodeoxyglucose positron emission tomography in the follow-up of papillary or follicular thyroid cancer. J Clin Endocrinol Metab 86:3779–3786
Filesi M, Signore A, Ventroni G, Melacrinis FF, Ronga G (1998) Role of initial iodine-131 whole-body scan and serum thyroglobulin in differentiated thyroid carcinoma metastases. J Nucl Med 39:1542–1546
Frey P, Townsend D, Flattet A et al (1986) Tomographic imaging of the human thyroid using 124I. J Clin Endocrinol Metab 63:918–927
Lambrecht RM, Woodhouse N, Phillips R et al (1988) Investigational study of iodine-124 with a positron camera. Am J Physiol Imaging 3:197–200
Crawford DC, Flower MA, Pratt BE et al (1997) Thyroid volume measurement in thyrotoxic patients: comparison between ultrasonography and iodine-124 positron emission tomography. Eur J Nucl Med 24:1470–1478
Erdi YE, Macapinlac H, Larson SM et al (1999) Radiation dose assessment for I-131 therapy of thyroid cancer using I-124 PET imaging. Clin Positron Imaging 2:41–46
Frey P, Townsend D, Jeavons A, Donath A (1985) In vivo imaging of the human thyroid with a positron camera using 124I. Eur J Nucl Med 10:472–476
Pentlow KS, Graham MC, Lambrecht RM et al (1996) Quantitative imaging of iodine-124 with PET. J Nucl Med 37:1557–1562
Eschmann SM, Reischl G, Bilger K et al (2000) Evaluation of dosimetry of radioiodine therapy in benign and malignant thyroid disorders by means of iodine-124 and PET. Eur J Nucl Med 29:760–767
Beyer T, Townsend DW, Brun, T et al (2000) A combined PET/CT scanner for clinical oncology. J Nucl Med 41:1369–1379
Townsend DW, Cherry SR (2001) Combined anatomy and function: the path of true image fusion. Eur Radiol 11:1968–1974
Freudenberg LS, Antoch G, Görges R et al (2002) Combined PET/CT with Iodine-124 in diagnosis of mediastinal micrometastases in thyroid carcinoma. Internet J Radiol 2:2
Freudenberg LS, Antoch G, Gorges R et al (2003) Combined PET/CT with iodine-124 in diagnosis of spread metastatic thyroid carcinoma: a case report. Eur Radiol 13 [Suppl 4]:19–23
Freudenberg LS, Antoch G, Görges R et al (2002) 124I-PET/CT in metastatic follicular thyroid carcinoma. Eur J Nucl Med 29:1106
Fleming ID, Cooper JS, Henson DE (eds) (1997) AJCC cancer staging manual. 5th edn. American Joint Committee on Cancer. Lippincott-Raven, Philadelphia
Knust EJ, Dutschka K, Weinreich R (2000) Preparation of 124I solutions after thermodistillation of irradiated 124TeO2 targets. Appl Radiat Isot 52:181–184
Kinahan PE, Townsend DW, Beyer T et al (1998) Attenuation correction for a combined 3D PET/CT scanner. Med Phys 25:2046–2053
Görges R, Fotescu D, Renzing-Köhler K et al (2003) Diagnostic value of high-resolution B-mode and power-mode sonography in the follow up of thyroid cancer. Eur J Ultrasound 16:191–206
International Commission on Radiological Protection (eds) (1998) ICRP publication 80; Addendum to publication 53—radiation dose to patients from radiopharmaceuticals. Ann ICRP 28:3
Shapiro B, Gross MD (2001) Follow-up of patients with well-differentiated thyroid cancer. In: Biersack HJ, Grünwald F (eds) Thyroid cancer. Spinger, Berlin Heidelberg New York
Acknowledgements
The authors thank G. Hüdepohl for producing the radioisotopes and radiopharmaceuticals. Further, we are indebted to S. Pabst, B. Terschüren, A. Colakovic and S. Heistrüvers for their assistance with the data acquisition.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Freudenberg, L.S., Antoch, G., Jentzen, W. et al. Value of 124I-PET/CT in staging of patients with differentiated thyroid cancer. Eur Radiol 14, 2092–2098 (2004). https://doi.org/10.1007/s00330-004-2350-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-004-2350-0