Abstract
The objective of this chapter is to present an overview of the following topics: PET physics and scanners, radiopharmaceutical for PET and indications, imaging technique, normal findings and variations, interpretation of PET-CT scans, false-positive findings, 18F-FDG PET-CT and PET scanning in differentiated thyroid cancer (DTC), role in thyroglobulin (Tg)-positive and iodine-negative patients, prognostic value of 18F-FDG PET scans, and the role of TSH in 18F-FDG PET scanning. The chapter concludes with a brief introduction to 124I PET scanning and other PET-CT radiopharmaceuticals.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- 18F-FDG PET scans
- Positron emission tomography
- Computed tomography
- PET
- PET-CT
- Thyroid cancer
- Radiopharmaceuticals
- False positive
- Tg positive
- Iodine negative
Introduction
Positron emission tomography-computed tomography (PET-CT) is a diagnostic technique that has become very important in oncology [1]. The objective of this chapter is to present an overview of the following topics: PET physics and scanners, radiopharmaceutical for PET and indications, imaging technique, normal findings and variations, interpretation of PET-CT scans, false-positive findings, 18F-FDG PET-CT and PET scanning in differentiated thyroid cancer (DTC), role in thyroglobulin (Tg)-positive and iodine-negative patients, prognostic value of 18F-FDG PET scans, and the role of TSH in 18F-FDG PET scanning. The chapter concludes with a brief introduction to 124I PET scanning and other PET-CT radiopharmaceuticals.
PET Physics and Scaners
The basis for a PET scan is the injection of a positron-emitting radiopharmaceutical into the patient that localizes in cancers and can be imaged. A positron is a positive electron, and when emitted from the radioisotope, it travels up to a few millimeters before coming in contact with an electron that has a negative charge. These particles of equal mass and opposite charges annihilate one another. The annihilation of the masses of the positive and negative electrons produces two photons, each with an energy of 511 keV. This is derived from the equation e = mc 2. The photons travel in opposite directions with an angle of 180°. A positron camera usually consists of a ring of detectors designed to identify photons interacting at precisely the same time on opposite positions on the ring (180°). These are called coincident events. Millions of coincident events can be reconstructed into images of the distribution of the positron-emitting radiopharmaceutical within the patient.
However, an important problem for imaging positron-emitting radiopharmaceuticals is the correction of attenuation of those photons by the tissue. Specifically, coincident photons arising from different parts of the patient travel through different lengths of the body before reaching the ring of detectors. Thus, when a positron is emitted from a lesion in the skin on a shoulder, one photon travels through millimeters of tissue and then strikes the detector whereas the other photon has to travel through the width of the patient before interacting with the opposing detector. However, more of the photons that have to travel the longer distance through tissue will be absorbed (thus more attenuation) than those that have to travel the shorter distance—attenuation. In order to take this into account, the attenuation of the photons by the tissues of the body must be corrected. Historically, this has been obtained by repeating the scan using a source of radiation such as germanium (68Ge) outside the patient, which in turn allows a measure of the different amounts of attenuation present. Over the past several years, improved technology has resulted in a PET scanner that has been combined with a CT scanner (PET-CT) [2]. The CT images are now used routinely for attenuation correction and for providing an anatomic correlation with the functional PET images. Currently, most companies manufacture predominately hybrid PET-CT scanners, and now, the first clinical PET-MR hybrid scanner has been installed. Because the positron detector is about 15 cm long, the detector must be moved to six to seven positions, called bed positions, in order to image the entire head, neck, and trunk areas. A PET scan takes approximately 30 min to complete. The extrinsic attenuation correction by the older technique (i.e., 68Ge) required a further 20–30 min, but this is reduced to about 1–2 min for combined PET-CT. Therefore, the combined PET-CT scan provides better attenuation correction, additional anatomic information, and faster throughput of patients.
Prior to the widespread sale and installation of hybrid PET-CT scanners, several companies produced scintigraphic gamma cameras. These were conventional whole-body gamma cameras with two detectors whose primary functions were to produce anterior and whole-body scintiscans or single-photon tomographic images. The two heads could also be used for detecting coincident gamma rays, and therefore, they could be used for PET imaging [3–5]. Unfortunately, these cameras had several disadvantages, the most important of which was the reduced counting ability of the two detectors versus the ring format of a dedicated PET instrument. Secondly, these cameras also had significantly reduced resolution, whereas one of the important benefits of a PET scanner is the excellent resolution. The future will be in the dedicated PET scanners combined with CT.
Radiopharmaceuticals for PET and Indications
In oncology, the PET radiopharmaceutical that is most frequently employed is 18F-fluorodeoxyglucose (18F-FDG). 18F-FDG is taken into cells by Glut transporters where it is acted on by hexokinase. However, thereafter, it is not metabolized as rapidly as glucose and remains within the cells. Cancer cells have an increase in Glut and hexokinase. Images are obtained 1–2 h after intravenous injection of 18F-FDG and demonstrate cells that have taken up and retained this radio-agent.
The main role of PET using 18F-FDG is in areas of oncology distinct from thyroid cancer, and these include differentiating a benign from malignant lung nodule, in staging and evaluating the response to therapy of non-small cell lung cancer, lymphoma, head and neck cancer, esophageal and recurrent colorectal cancer, and melanoma or breast cancer [6–10]. PET-CT imaging of the brain is also important not only for cancers but in diagnosis of degenerative diseases such as Alzheimer’s, multi-infarct dementia, and Parkinson’s disease. PET-CT also has a role in defining whether or not ischemic myocardium is viable. In the management of patients with differentiated thyroid cancer, its main indication is the evaluation of patients who have an elevated thyroglobulin (Tg) blood level and a negative whole-body 131I and/or 123I diagnostic scan and/or 131I post-therapy scan. 18F-FDG is approved for this purpose in the United States. It is also valuable in the patient who has a positive radioiodine scan but where the thyroglobulin is disproportionally high (e.g., a small remnant with uptake <1 % seen on scintiscan associated with a Tg >1000 ng/ml). Another use, albeit a minor one, is to differentiate a benign from a malignant thyroid nodule, but the reliability and cost-effectiveness argue against this approach (see Chap. 25). 18F-FDG PET-CT has a limited role in pretreatment staging of patient with newly diagnosed cancer, but it is useful for such in patients who have primary lymphoma of the thyroid or anaplastic thyroid cancer and is discussed in the relevant chapters (see Chaps. 43, 76, 87, 93, and 97).
In the case of thyroid cancer, there is a second positron emitter, 124I, that has valuable properties and will have an increasing role in the management of differentiated thyroid cancer. 120I and 122I are also positron emitters but have not been introduced into clinical practice [11]. Table 43.1 provides the physical characteristics of these positron emitters, and 124I is briefly discussed near the end of this chapter as well as in Chap. 103.
Imaging Technique
18F-FDG is injected intravenously, and imaging is initiated approximately 1 h later. The patient should fast for at least 6 h before the injection, and the patient should typically be NPO after midnight. The patient should not exercise vigorously for 24 h prior to the study. In the United States, most authorities recommend measuring serum glucose prior to the injection of 18F-FDG because high levels alter the distribution of the radiopharmaceutical and lower the sensitivity of the scan. In countries where the incidence of latent and prediabetic patients is low, this is not necessary. When the glucose is greater than 200 mg/dl, we advise canceling the procedure until the blood glucose is normal. 18F-FDG PET imaging in diabetics requires the involvement of the patient, referring physician, and nuclear medicine technologist and physician in achieving safely an appropriate level of glucose.
After injection of 18F-FDG, the patient should rest in a quiet, warm, and comfortable room for the hour between the injection of 18F-FDG and imaging. The patient should not talk or chew. The reason for these precautions is that active muscles take up 18F-FDG, and the uptake can cause difficulty in interpretation, which may result in false-positive findings (see below). In some patients, uptake of 18F-FDG in brown fat in the neck can be misinterpreted as metastases to nodes. However, as reported by Garcia et al. [12], brown fat uptake can be essentially eliminated by keeping the patient warm several hours before the scan, and we and others have found that a single dose of propranolol 1 h before injection of 18F-FDG may also help [12]. Some authorities delay scanning for 90–120 min. The extra time allows more of the background activity to be excreted through the kidneys thus making the lesions easier to detect. For thyroid cancer, this is seldom necessary, and for a steady throughput of patients, the delay of 1 h is the best compromise for accuracy and efficiency.
Normal Findings and Variations
The brain highly concentrates 18F-FDG because the brain depends on a significant amount of glucose for function. 18F-FDG is excreted through the kidneys and the bladder, and these areas may appear to have significant uptake. There is varying uptake in muscles and myocardium, but in a patient who has fasted and been inactive and has a normal fasting glucose, these structures should have modest uptake and allow anatomic correlation without interfering with the interpretation. 18F-FDG may concentrate diffusely in the liver.
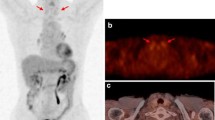
Although the thyroid is a metabolically active gland, somewhat surprisingly, the normal thyroid is typically not observed or is only faintly observed on 18F-FDG scan 1 h after injection (see also Chap. 25). PET-CT allows the thyroid anatomy to be defined by the CT and frequently in patients being scanned for non-thyroidal cancer, the normal thyroid cannot be identified on 18F-FDG PET (Fig. 43.1) When there is diffusely increased uptake in the thyroid, the patient usually has autoimmune thyroid disease, most often chronic lymphocytic thyroiditis, and less frequently Graves’ hyperthyroidism (Fig. 43.2) [14–17]. In the occasional patient with autoimmune thyroid disease, the uptake of 18F-FDG can be focal and misinterpreted as a malignancy in the thyroid [16].
A coronal PET scan (left). The image on the right is the transaxial images of the PET scan, CT, and combined PET-CT, as oriented in Fig. 43.1 and at the thyroid level. The thyroid has intense uptake of FDG. The patient had Hashimoto’s thyroiditis
Focal uptake of 18F-FDG in the thyroid in a patient being evaluated for a non-thyroidal disease has about 10–50 % chance of being a thyroid cancer. The wide range depends on patient selection for a pathological diagnosis [18]. Cancer detected this way is usually a differentiated primary thyroid lesion, but metastasis from the non-thyroidal cancer can also occur. The latter happens most frequently in patients with melanoma and lung, renal cell, breast, or bowel cancer. In an analysis of more than 4500 18F-FDG PET scans, 2.3 % showed some abnormality in the thyroid [13]. In 87 patients, this was not pursued because of the severity of their primary cancer. However, 15 patients had a biopsy and 7 (47 %) of these were thyroid cancer. Kang et al. also reported that 2.2 % of scans showed a thyroid “incidentaloma” [14]. Some of these were diffuse and consistent with thyroiditis, but 4 of 15 (27 %) focal lesions were malignant. Several reports indicated a risk of cancer of 50 % or greater [15]. In an unpublished interinstitutional investigation, we and other colleagues identified 15 focally “hot” lesions. Nine patients were referred for operation and eight cancers were diagnosed histologically (Fig. 43.3). Ho et al. found a prevalence of thyroid uptake on 18F-FDG PET-CT of 3.7 % in their retrospective review of 5877 subjects with no previous history of thyroid malignancy. Of patients who had verification by cytology or histology, 14 % (8/55) were found to have thyroid malignancies [16]. In a separate study, focal thyroidal uptake was identified in 76 of 6241 (1.2 %) [17]. Only 14 patients (18 %) had a biopsy and 4 (28.6 %) had papillary thyroid carcinoma. This begs the question of how many of the remaining 62 had cancer. Are et al. evaluated 16,300 PET scans in 8800 patients [18]. They found 263 thyroidal abnormalities (1.6 % of scans, 2.9 % of patients). Fifty-seven of the patients had fine needle aspiration (FNA), and 24 cancers were found (42 %). The incidence of thyroid cancer was 72 % in 27 patients who had thyroidectomy, and 19/20 patients had papillary cancer with the remaining patient having a lymphoma.
The term incidentaloma has been challenged for two reasons. As noted, a meaningful proportion is cancer and the lesions are functioning; hence, they prefer the term “metaboloma.”
Some investigators note the degree of uptake in a metaboloma does not predict histology and underpins the importance of further investigations, in particular FNA, to exclude thyroid cancer. Others suggest that the intensity of 18F-FDG uptake and the CT attenuation pattern significantly improve the accuracy of PET-CT for differentiating benign from malignant focal thyroid lesions. Benign nodules had a mean standardized uptake value (SUV) of 6.7± 5.5 whereas malignant ones had a mean value of 10.7 ± 7.8 (P < 0.05) [19]. SUV is a quantitative measurement of the glucose uptake in regions of interest that can be selected on the image of the patient when viewed on the console of a computer. Researchers also evaluated 18F-FDG PET performed for screening in healthy subjects. Chen et al. reported that thyroid metabolomas were identified in 1.2 % (60/4803) of patients, and that of those who underwent FNA and surgery, 14 % (7/50) metaboloma were found to be malignant [20].
Because undiagnosed thyroid cancer can appear as focally “hot” on 18F-FDG scan, there was hope that 18F-FDG PET would differentiate a malignant from a benign nodule. The technology is expensive and even if perfect would not replace FNA. In a study of nine patients, three cancers were PET positive but four of six benign lesions showed focal uptake [21]. Uematsu et al. using an SUV of five were able to separate four cancerous nodules from six benign ones but a patient with thyroiditis had an SUV of 6.3 [22]. In a small series of 15 patients with indeterminate cytopathology, 18F-FDG PET-CT was positive in eight and negative in seven [23]. Of the eight positive cases, four were cancer, whereas three of the seven patients with negative 18F-FDG PET-CT scans did subsequently have cancer confirmed. These results are equivalent to tossing a coin.
Therefore, not all focal 18F-FDG PET-positive lesions in the thyroid are cancerous and an FNA, or ultrasound (US)-guided FNA, is appropriate to establish the diagnosis in most patients [24, 25]. Theoretically, 18F-FDG PET might have a role in the indeterminate microfollicular lesion where the a priori likelihood of cancer is greater than in nodules in general. A different group of investigators conducted 18F-FDG PET-CT in 88 patients with nondiagnostic cytology [26]. Every patient had surgery and pathological confirmation and none of 41 patients (46 %) with a negative 18F-FDG PET-CT had cancer. Thus, a negative 18F-FDG PET could be more helpful than a positive one. In summary, the degree of focal 18F-FDG uptake in a metaboloma does not predict histology and underpins the importance of further investigations—in particular FNA—to exclude thyroid cancer, and even in the indeterminate case, a focal “hot” spot on 18F-FDG PET is not necessarily a cancer. However, a negative scan appears to exclude that diagnosis [27]. Again, further reading is available in Chap. 25.
Interpretation of PET-CT Scan
Regions of abnormal uptake of 18F-FDG in recurrent or metastatic cancer are usually easy to identify. Combined PET-CT allows the exact anatomic site to be defined. Many authorities simply report on the findings on the scan. The optimal results are obtained by interpreting the results while sitting at a computer terminal and viewing systematically the coronal, sagittal, and transaxial projections. It is helpful to scroll through images of regions where an abnormality is identified or suspected. Some experts use a quantitative numeric result, which determines the uptake in the lesions compared to the amount of radiopharmaceutical injected. This is called the standardized uptake value (SUV). In general, a lesion with an SUV >2.5 is frequently accepted as being due to cancer; however, in one study discussed previously, the cutoff between malignant and benign thyroid nodule was five. In practice, the SUV is not very helpful to establish whether or not cancer is present in the thyroid, and it has also been referred to as the “silly uptake value” [28]. Locoregional and distant thyroid metastases are usually recognized visually, and the SUV is important as a baseline for comparison with subsequent scans to judge the response to treatment. SUVs may also have prognostic value as discussed below. Other investigators use the ratio of uptake in the cancer to background activity instead of the SUV. When a comparison of quantitative measurements is made between two studies, it is important to ensure they were conducted under identical conditions and that the SUV is not interpreted with an uptake ratio.
False-Positive Findings
18F-FDG uptake on a PET-CT scan is not specific for thyroid cancer, and thus, 18F-FDG PET-CT scans performed to evaluate for thyroid cancer may have false-positive uptake due to other malignant or active cells [29, 30]. Accordingly, it would be for a patient with thyroid cancer to also have another cancer. As discussed above, about 1–3 % of PET or PET-CT scans conducted in patients with non-thyroidal cancers show a focal thyroidal abnormality of which somewhere between 10 and 50 % are cancers. The percentage of patients with thyroid cancer who are found to have a non-thyroidal cancer diagnosed by PET-CT is not known with certainty. The distribution of any abnormal uptake should be carefully evaluated to make sure that it is consistent with the expected spread of thyroid cancer. The common sites of residual cancer would be the thyroid bed and for metastases to the regional lymph nodes in the neck and mediastinum, and distant metastases to the lungs and skeleton. Inflammatory conditions including tuberculosis and sarcoidosis show abnormal uptake, but the distribution is not likely to be confused with thyroid cancer.
There are additional specific conditions that are important in the interpretation of PET scans conducted for thyroid cancer. The first is uptake of 18F-FDG in active neck muscles. Although the muscles are linear structures, the muscle uptake can result in what appears to be globular uptake similar to a lymph node. This is due to tomographic slices across muscle bellies. The finding is more common in patients who are nervous and trembling or shivering. This was first identified by Barrington et al. and they advised diazepam prior to injection of the radiopharmaceutical [31]. This requires knowledge a priori of the patients who would likely be stressed. In the United States, the physician prescribing the tranquilizer is responsible for the well-being of the patient. When the physician conducting the scan prescribes the drug, he/she must have all the requirements for conscious sedation in place. As a result, he/she is usually reluctant to take this step. Not all physicians have found that medications to reduce anxiety help reduce the false positives [38]. The poor response to anxiolytic medication most likely results from a different but more common cause of a false-positive uptake of 18F-FDG in brown fat (Fig. 43.4) [32–35]. Superficially, this looks similar to the muscle uptake, but the distribution follows the shape of the neck being narrower at the level of the chin and wider at the thoracic inlet. In contrast, the muscle uptake follows the contour of the sternocleidomastoid and is therefore narrower at the manubrium. Uptake in brown fat is thought to be increased in cold temperature [33]. Our experience is that it is more common in thin women and in winter. A recent laboratory study in rats confirms that the 18F-FDG uptake is increased by cold temperature, and it could be reduced by propranolol, and in one study, 90 % of the patients showed a reduction after 40 mg of propranolol orally 1 h prior to injection of 18F-FDG [12, 36]. Garcia et al. demonstrated that virtually all 18F-FDG uptake in brown fat can be eliminated with control of the ambient temperature around the patient prior to injection of the 18F-FDG [12]. Brown fat is also localized along the vertebrae and around the diaphragm, and it can also take up on 18F-FDG PET. The third abnormality that can be incorrectly diagnosed as cancer is asymmetric uptake in the functional muscles of one vocal cord when the contralateral nerve was damaged during thyroidectomy [37, 38]. A similar finding has been reported in a granulomatous foreign body reaction around a Teflon® implant to improve a paralyzed vocal cord [39]. Uptake of 18F-FDG in the thymus has also been reported [40–45]. This is more common in younger patients and in those who have recently been treated with chemotherapy and perhaps after therapeutic 131I. Although the bilobed shape is characteristic, this can be misinterpreted as mediastinal metastases. Combining PET-CT usually allows the differentiation of these false positives from metastatic cancer considerably easier.
18F-FDG PET-CT and PET Scanning in Differentiated Thyroid Cancer: Role in Tg-Positive and Iodine-Negative Patients
The consensus for the optimal treatment for differentiated thyroid cancer is total or near total thyroidectomy and 131I therapy in selected patients excluding those with small primary cancers [46]. The combination of surgery and 131I therapy removes all functioning thyroid and allows for follow-up using measurement of serum Tg and in selected patients whole-body scan with 123I. When the therapy has been successful, both follow-up studies are negative. When there is residual or recurrent disease, both scans are typically abnormal and allow a decision to be made about retreating with 131I. However, in 15–30 % of patients with residual or recurrent disease, there is a discrepancy between the scans usually with Tg being measurable and the diagnostic radioiodine scan being negative. The reasons for this could be that there are too few cells to be identified on scan or a defect in the function or location of the sodium-iodide symporter (NIS) . This is a difficult and trying problem for the patient and physician, and it has led to differences in philosophies about management (see Chap. 47). Some take the position that a therapeutic administration of 131I will deliver sufficient radiation to seek out, localize in, and kill the cells producing the Tg [47, 48]. But not all physicians caring for these patients accept this approach [49–52]. An alternative approach is to employ imaging scans that are not dependent on the NIS for identifying the thyroid tissue. Imaging could include US of the neck, CT, MRI, and a range of nuclear medicine procedures (see Chap. 44). Taking the nuclear medicine scans in their historical sequence, thallium 201 (201Tl) is a large atom that is taken up by the sodium potassium channel of cells. It is valuable for evaluating myocardial perfusion. Thallium is concentrated by some cancers including thyroid cancer and became popular for imaging patients with thyroid cancer [53–56]. Because of the low energy of the emitted photons and the high absorbed radiation, the small injected activity results in 201Tl images that are poor in comparison to those made with radiopharmaceuticals containing technetium 99m (99mTc). As a result, 201Tl is presently not used in patients with thyroid cancer. When 99mTc-labeled radiopharmaceuticals were developed for studying myocardial perfusion, they were investigated in oncology and found to be valuable in identifying sites of metastatic thyroid cancer. The images are superior to 201Tl because the emitted photons have a higher energy that is suited for current gamma cameras. In addition, a larger quantity of radioactivity can be injected. The two radiopharmaceuticals are sestamibi and tetrofosmin [57]. In one report of 110 scans in 99 patients, 99mTc sestamibi and Tg agreed in 96 % [58]. However, in most of the patients, both scans were negative. There were only 16 patients with abnormal results who had a whole-body radioiodine scan. In four (25 %) of the patients, the 99mTc sestamibi scan demonstrated a lesion not seen on 131I images. Similarly, 99mTc tetrofosmin has been demonstrated to identify iodine-negative cancer. Gallowitsch et al. identified 39 of 44 lesions on 99mTc tetrofosmin scans compared with 19 on whole-body 131I scans [59]. Subsequent publications from these and other investigators confirm these findings [60–63]. Finally, thyroid cancer may have somatostatin receptors [64], and the somatostatin receptor imaging agent, 111In pentetreotide, has also been used, but the sensitivity does not make it a powerful scan [65, 66]. This has been discussed further in Chap. 44. It is surprising to report that the first publication of 18F-FDG PET scan for thyroid cancer dates from as early as 1987 [67]. Then, there was a delay of almost a decade after which several subsequent reports appeared. Presently, many articles have been published addressing the role and value of 18F-FDG PET scan in adult patients who have measurable Tg but negative radioiodine studies [68–77]. In addition, several editorials and reviews have been published attesting to the value of 18F-FDG PET [78–82]. We and other authors have found this also to be of value in children [83]. The images are superior to scans from all the other radiopharmaceuticals discussed briefly above, and the combination of PET with CT provides anatomic information that increases both sensitivity and specificity. In general, the interpretation can be reached with confidence.
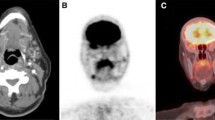
It has become apparent that the less differentiated the thyroid cancer is, the more likely the 18F-FDG PET scan will be positive. Very well-differentiated cancers show less or even no uptake of 18F-FDG while having excellent 131I uptake. This has been called a “flip/flop” phenomenon [84, 85]. Evidence for this is the comparison of 18F-FDG and 131I in 47 lesions [86]. Thirty-three lesions were iodine-positive but 20 (61 %) of these were 18F-FDG PET negative. Conversely, there were 9 18F-FDG PET positive lesions from 14 (64 %) iodine-negative lesions. Figure 43.5 shows an abnormal 18F-FDG PET-CT scan.
The left panel shows an 18F-FDG PET scan in a patient who has had thyroidectomy and 131I therapy. The arrowhead shows a small area of 18F-FDG uptake in the region of residual left lobe. Several other areas of uptake in the neck are metastases in lymph nodes. The right panel shows transaxial images at the level of the thyroid with a PET image at the top, CT image in the middle, and fused PET-CT image at the bottom. The arrowhead shows the same spot and the arrow shows one of the nodal metastasis
The original studies evaluated 18F-FDG PET in a spectrum of patients some who had a remnant after surgery, some who had metastases that could be imaged with radioiodine, and some who had iodine-negative scans but measurable Tg. These investigations confirmed that the overall sensitivity of 18F-FDG PET was low and the scan was not useful for “all-comers” [87]. However, when the Tg-positive/iodine-negative patients were analyzed separately, the sensitivity increased and the specificity remained powerful. Table 43.2 provides data from several publications including recent ones using 18F-FDG PET-CT. It is apparent that the sensitivity varies from about 60 % to as high as 100 %. The high sensitivities are most likely due to the small number of patients being studied who had extensive disease. As the scan has become more widely used, patients with smaller volumes of cancer are being studied, and it is not surprising that the sensitivity has fallen. To determine specificity, it is not possible in many of these reports because all the patients studied had an elevated Tg; in other words, there were no patients who could be defined as being free of disease. In many reports, there is a relationship to the level of Tg, but there is no cutoff above which all 18F-FDG PET scans are positive or below which they are all negative. In the United States, the current approved indication for PET is a Tg value of >10 ng/ml in a patient who has been treated by thyroidectomy and 131I and who has a negative radioiodine scan. Although the Tg value of >10 ng/ml is arbitrary, publications do support that patients with Tg >10 ng/ml are more likely to have positive 18F-FDG PET scans. Bertagna et al. in a study of 52 patients found there was a significant positive correlation between 18F-FDG PET-CT-positive results and Tg levels [88]. They did not note any statistically significant correlation between 18F-FDG PET-CT results and TSH levels. They found the highest accuracy was achieved when Tg was above 21 ng/ml. 18F-FDG PET or 18F-FDG PET-CT is not usually positive in patients with Tg levels below 2 ng/ml; however, there is usually no indication for the scan in that setting [89]. One benefit of 18F-FDG PET is to identify surgically resectable sites of disease when clinical information or other imaging scans show one site of disease and 18F-FDG PET shows several [90]. This prevents performing surgery only to be followed by identification of “new” recurrence.
In regard to 18F-FDG PET-CT vs. 18F-FDG PET alone, the former is more accurate than the latter. Palmedo et al. found that 18F-FDG PET-CT has a diagnostic accuracy of 93 % and an improvement of 15 % over 18F-FDG PET alone [91]. Mirallie et al. showed that 18F-FDG PET-CT increased the diagnostic performance considerably over that of 18F-FDG PET or CT alone with a sensitivity of 96 % for 18F-FDG PET-CT, 82 % for 18F-FDG PET, and 73 % for CT [92].
In a study with 50 patients with differentiated thyroid cancer, Esteva et al. found that the size of the cancer (p < 0.05) and thyroid capsular invasion (p < 0.05) were significantly associated with positive 18F-FDG PET studies [93]. Choi et al. concluded that the absence of perithyroidal and lymphovascular invasion was an independent variable for false-negative findings on initial 18F-FDG PET-CT in patients with papillary thyroid cancer [94].
Our group reviewed 98 18F-FDG PET-CTs in 76 patients and determined sensitivities per patient and per lesion of 87 % and 89 %, respectively, in patients with differentiated thyroid cancer [95]. We also reported that metastases in small cervical nodes might be missed due to the limitations of spatial resolution of PET. The most frequent sites of recurrent differentiated thyroid cancer are lymph nodes (53 %) and the thyroid bed (28 %). A study adding a higher resolution delayed dedicated neck 18F-FDG PET-CT acquisition showed an increase number of abnormal foci in 29 % of the studies [96]. This increase was statistically significant. Kaneko et al. compared 18F-FDG PET-CT and 131I in nine patients with 33 lesions [97]. They identified all lesions by 18F-FDG PET-CT but only 58 % with radioiodine. They confirm the SUV was statistically higher in lesions that did not take up iodine (6.6 ± 2.8 vs. 4.2 ± 1.8; P = 0.007), and as expected, Tg values were significantly higher in patients with metastases to lymph nodes.
Choi et al. investigated 18F-FDG PET-CTs for the initial staging of patients with papillary thyroid cancer and concluded that it did not provide additional information compared to neck sonography [98]. 18F-FDG PET-CT showed lower sensitivity and higher specificity than sonography for detection of cervical node metastasis; however, no statistically significant difference was noted in their study (p > 0.99). Even when the radioactive scans show iodine-avid disease, 18F-FDG PET-CT may alter the management of some patients. Piccardo et al. showed that 18F-FDG PET-CT detects more metastases in 45 % of patients with advanced differentiated thyroid cancer and a rising Tg in comparison with whole-body radioiodine and bone scans [99].
False-negative findings occur in well-differentiated cancer that retains ability to take up iodine avidly and in lesions that are too small to be resolved with modern dedicated 18F-FDG PET-CT scanners that are less than about 6–8 mm. It should be emphasized that the PET and CT and combined PET-CT images should be studied since occasionally an abnormality can be identified on the CT images but not the 18F-FDG PET.
Meta-analyses confirm the value of 18F-FDG PET and 18F-FDG PET-CT in differentiated thyroid cancer where radioiodine images are negative in particular when Tg is elevated [100–102]. In one analysis of 18F-FDG PET and 18F-FDG PET-CT for only papillary cancer, sensitivities calculated by two methods were 77 % and 84 % and specificities 85 % and 84 %, respectively [101]. In another, Dong et al. reviewed 549 abstracts from which they selected 195 publications, and after determining which ones met criteria of careful design, defined patient population, indications for 18F-FDG PET or 18F-FDG PET-CT, and confirmation of diagnosis, they were left with 25 articles, 6 of which dealt with F-FDG PET-CT [102]. Overall, the sensitivity in patients with measurable Tg and a negative 131I scan was 0.885 (95 % CI: 0.828–0.929) and specificity 0.847 (95 % CI: 0.715–0.934). In the six investigations dealing with 18F-FDG PET-CT, the sensitivity and specificity were 0.935 (95 % CI: 0.870–0.973) and 0.839 (95 % CI: 0.723–0.920), respectively.
Prognostic Value of 18F-FDG PET Scans
A very abnormal 18F-FDG PET scan with lesions showing a high SUV is a harbinger of a bad outcome [103]. In a study of 125 patients followed for 41 months, 14 patients died from thyroid cancer. The most important predictors were high SUV and a volume of cancer greater than 125 ml. In those with smaller tumor volumes, the 3-year survival was 96 %, and even patients with distant metastases who were 18F-FDG PET negative all survived. This again reflects the “flip/flop” phenomenon. Well-differentiated cancer cells take up iodine and are amenable to 131I therapy; less well-differentiated cancer cells do not take up iodine but do concentrate 18F-FDG. In contrast, patients who are iodine negative as well as 18F-FDG PET negative appear to have a good prognosis. In our experience, no patient has died and the recurrence rate is low.
As already discussed above, a positive 18F-FDG PET scan can alter the management. Alnafisi et al. found that PET changed management in 7 of 11 patients, and Goshen et al. reported change in management in 6 of 20 [70, 104]. For example, when the radioiodine scan was negative, identification of a focal 18F-FDG PET lesion can make surgery an option. A lesion in a vertebral body can be considered for surgical or external beam radiation. Our approach for lesions in the neck that are identified using 18F-FDG PET is to obtain an US for precise anatomic localization and for US-guided FNA. The patient is operated on using intraoperative US to re-identify the site and to ensure it is excised [105]. All patients had a decrease in Tg and 64 % achieved undetectable values.
Ota et al. explored in a pilot study the use of 18F-FDG PET-CT for detection of bone metastases from differentiated thyroid carcinoma. The sensitivities of 18F-fluoride PET-CT and 99mTc bone scintigraphy were significantly higher than that of 18F-FDG PET-CT in their report [106]. Nakajo et al. conducted a comparison of 18F-FDG PET-CT vs. 18F-FLT PET-CT in 20 patients with postoperative differentiated thyroid cancer, evaluating the images of lymph node and distant metastases. In a per-patient-based analysis, the sensitivity, specificity, and accuracy were 92 %, 86 %, and 90 %, respectively, for 18F-FDG PET-CT and 69 %, 29 %, and 55 %, respectively, for 18F-FLT PET-CT. The accuracy of 18F-FDG PET-CT was significantly better than that of 18F-FLT PET-CT (P = 0.023) [107].
The Role of TSH in 18F-FDG PET Scanning
The vast majority of 18F-FDG studies in oncology including patients with thyroid cancer are conducted when the patient is euthyroid or, in some patients with thyroid cancer, when they are biochemically slightly hyperthyroid because of a desire to completely suppress the patient’s TSH blood level. However, whether or not elevated TSH blood levels significantly improve detection of thyroid cancer on 18F-FDG PET scans is controversial. Anecdotal patients who were studied twice—once when euthyroid and once with an elevated TSH—showed no difference in detection of lesions in those case reports [108, 109]. In contrast, Sisson reported increased uptake under TSH stimulus [110]. Several publications confirm this finding. This is in keeping with in vitro studies showing that TSH increases glucose uptake into thyroid cells [111]. In a study of 17 patients imaged when TSH was <0.05 μU/ml and again when the TSH was >22 μU/ml (on average 42 days later), the lesion-to-background ratio increased by an average of 63.1 % [112]. In three out of ten patients, new lesions were identified. In a similar experiment, 30 patients who had negative or equivocal 131I scans and abnormal or equivocal Tg were studied before and after injection of recombinant human thyrotropin (rhTSH) [113]. When the TSH was low, a total of 45 lesions were seen in nine patients, and after administration of rhTSH, 78 lesions were seen in 19 patients. Therefore, many more lesions were diagnosed and some 18F-FDG PET-negative patients became 18F-FDG PET positive. These investigators did calculate an increase in SUV or lesion-to-background ratio. In a smaller study of seven patients, only one patient changed from 18F-FDG PET negative to positive after injection of rhTSH [114].
In a study with 76 patients, we concluded that TSH levels at the time of 18F-FDG PET-CT did not appear to impact the ability to detect disease or the 18F-FDG uptake [95]. In a meta-analysis study, Ma et al. found statistically significant patient-based and lesion-based differences of 18F-FDG uptake under TSH stimulation with enhanced tumor-to-background ratios compared with studies conducted when TSH was suppressed [115]. Management was altered in 9 % of patients. They also reported that the clinical significance of TSH-stimulated 18F-FDG PET also depended on the knowledge of the range of possible treatments as well as cost and side effects. Of note, no end points such as survival and mortality were reported. A study comparing 18F-FDG PET-CT performed before and 24 h after rhTSH administration in 63 patients indicated that rhTSH-stimulated 18F-FDG PET-CT was significantly more sensitive than non-stimulated PET-CT for the detection of lesions (95 % vs. 81 %; p = 0.001) and tended to be more sensitive for the detection of involved organs (94 % vs. 79 %; p = 0.054). However, the number of patients in whom any lesion was detected was not different between the two groups. Management was altered in 6 % of patients [116]. In a study with 44 patients, Vera et al. also concluded that rhTSH-stimulated 18F-FDG PET-CT has low sensitivity; however, the patients studied had relatively low Tg values [117]. They found no correlation between 18F-FDG PET-CT and Tg levels, and positive scans were found in 20 % of patients with Tg levels lower than 10 ng/ml.
These data present a problem. Should all patients be studied with an elevated TSH? If yes, then should they be hypothyroid or euthyroid and injected with rhTSH? RhTSH has not been approved for 18F-FDG PET scanning. One possible solution is to obtain a TSH-stimulated 18F-FDG PET scan when the patient receives his/her rhTSH for a diagnostic radioiodine scan. The rhTSH injections could be made on Monday and Tuesday. The 18F-FDG PET scan could then be obtained on Wednesday morning after a 6-h fast and then the tracer of radioiodine could be administered. Neither scan would interfere with the other. This 18F-FDG PET scan could be performed when a high index of suspicion for disease still exists after a negative standard 18F-FDG PET scan was performed with a low TSH.
124I PET Scanning (See Also Chap. 103)
124I has a half-life (T1/2) of 4.2 days. It can be employed for PET imaging analogous to the use of 123I and 131I for planar and single-photon tomographic imaging [118]. PET always provides tomographic information and the images have superior resolution, and they can be used to determine volumes with accuracy. The combination of PET-CT allows images to be obtained faster, produces excellent correction for attenuation of 511 keV photons, and provides anatomic correlation (Fig. 43.6). There were theoretical concerns that the high-energy positrons and complex decay scheme with high-energy gamma photons of 124I would not allow high-quality images to be obtained. Experimental studies with phantoms show that high-quality, well-resolved, images of 124I are possible using a dedicated PET camera. This radioisotope of iodine is not easy to produce and is not widely used.
(a) The upper panel on left shows anterior whole-body 124I scan in patient after thyroidectomy. Significant residual thyroid is present bilaterally (long black arrow). There is also uptake of 124I in the salivary glands (short black arrows) and stomach (arrowhead). (b) The upper panel on the right shows follow-up scan 1 year later with 131I ablation of thyroid. (c) The lower three images represent coronal tomographic images of the lower head, neck, and thyroid area, which were obtain at the same time as (a) above. The left, center, and right images are CT, 124I PET, and fused PET-CT, respectively
124I has been of value in benign conditions of the thyroid, for example, in obtaining an accurate measurement of the volume of tissue to allow calculation of a specific absorbed radiation dose when treating Graves’ disease [119–121]. One study showed an excellent correlation to the volume determined by US [122]. PET has the advantage of demonstrating functioning tissue as well as total volume. The role of 124I in management of thyroid cancer is in development, but 124I has significant potential because volumetric calculation of lesions can be obtained and the 4.2-day T1/2 allows measurements to be made over time to measure total body clearance and T1/2 in metastases. This allows more accurate dosimetry to be calculated, and quoting one group of investigators “is a useful procedure especially in advanced DTC, and allows the administration of safer and more effective radioiodine activities as well as earlier multimodal interventions compared to standard empirical protocols” [123]. This is expanded below and in Chap. 103.
In comparing uptake and clearance of 124I and 131I, Eschmann et al. [124] evaluated 3 patient and Atkins et al. [128] evaluated 25 patients, and both studies demonstrated good concordance.
Multiple studies have now been published comparing lesion detection of 124I and 131I, and this is discussed in more detail in Chap. 103. Freudenberg et al. [125, 126] reported twelve patients who were imaged 24 h after an average prescribed activity of 84 MBq (2.3 mCi) 124I. The patients had 124I PET scan, combined PET-CT, CT, and post-therapy 131I scans. The detectability of lesions was 87 %, 100 %, 56 %, and 83 %, respectively.
In a study with 69 patients, Capoccetti et al. compared total body 124I PET-CT and whole-body scintigraphy conducted before and after therapy with 131I [127]. The two scans matched in 86.6 % of the studies. 124I PET-CT detected more disease in 7 % of the patients, mostly in lymph nodes, and 131I whole-body scan detected more disease in 4.9 %, mostly in disseminated 131I-avid lung metastases. Another study involving 70 patients compared the ability of 124I PET-CT to detect iodine-avid lung metastases and found that only 1 of 7 patients with disseminated lung metastases on the 131I whole-body scan had uptake on the 124I PET-CT [128]. This issue is particularly concerning if one is to use 124I PET-CT for evaluation of extent of disease and prescribed activity calculation prior to 131I therapy.
Comparing 124I PET-CT with 131I planar whole-body scan for the detection of residual and metastatic well-differentiated thyroid cancer, Van Nostrand et al. found that 124I PET-CT identified 50 % more foci of radioiodine uptake suggestive of additional residual thyroid tissue and/or metastases in 32 % patients [129]. Freudenberg et al. compared 124I PET-CT and 18F-FDG PET-CT in the detection of recurrent differentiated thyroid carcinoma in patients with rising Tg values. They found sensitivities of 80 % and 70 % for 124I PET-CT and 18F-FDG PET-CT, respectively [130]. One third of the lesions demonstrated uptake with both tracers, while two thirds were positive on only one. Using both tracers, the sensitivity was 91 %.
124I has also been studied for dosimetric calculations. The advantage is that high-resolution three-dimensional (3D) images can be acquired and the uptake, residence time, and volume of distribution determined. Sgouros et al. imaged 15 patients at 4, 20, 44 h and 4–6 days after prescribed activities from 74 to 148 MBq (2-4 mCi) of 124I [131]. The results demonstrated that the method is feasible, and sequential PET scans can be used to obtain cumulated activity images for 3D dosimetry. Because the distribution of radioiodine within a lesion can be inhomogeneous, an accurate measurement of the radiation absorbed dose in different regions within cancer can be determined. The variability of the radiation absorbed dose is considerable and knowledge that regions of cancer are not going to receive an adequate dose would be the reason to consider additional treatment such as external radiation. It has been possible to obtain real-time three-dimensional radiobiological dosimetry using a software package, and although the researchers indicated this is unlikely to be used routinely, it certainly would be valuable in patients with extensive disease [132]. 124I has been used to monitor the response to the MAPK kinase (MEK) 1 and MEK2 inhibitor selumetinib and to evaluate if the drug could reverse refractoriness to radioiodine in patients with metastatic thyroid cancer. In their study of 20 participants, Ho et al. report that selumetinib produces clinically meaningful increases in iodine uptake and retention in a subgroup of patients with thyroid cancer that is refractory to radioiodine. [133].
It should be noted that historically 124I was used therapeutically [134]. This radioisotope deposits significant radiation; therefore, the diagnostic tracer could theoretically cause “stunning,” a controversial topic debated elsewhere in this textbook (Chaps. 16, 17, and 18). Researchers using 124I should study this possibility by comparing 124I and 131I post-therapy scans.
124I has been used to label peptides, antibodies, and other compounds and appears to be a suitable tracer for both quantitative and imaging pharmacological investigations [135].
Other PET-CT Radiopharmaceuticals (See Chap. 44)
Somatostatin receptor scintigraphy using predominantly 111In-labeled tracers has been used to evaluate differentiated thyroid cancers that have reduced or absent uptake of radioiodine. Somatostatin receptor analogues have also been labeled with positron emitters. 68Ga DOTATOC is a PET tracer that has a high affinity for somatostatin receptors 2 and 5. Middendorp et al. compared 68Ga DOTATOC and 18F-FDG PET-CT in patients with suspected recurrent DTC [136]. The data indicates that 18F-FDG PET-CT is better than 68Ga DOTATOC in the detection of recurrent disease that is radioiodine negative. Specifically, it is more accurate in detecting lung and bone metastases. In radioiodine-positive cancers, both tracers are equivalent.
Summary
PET-CT scanning with 18F-FDG is a very valuable scan for managing patients with well-differentiated thyroid cancer and specifically patients who have an elevated Tg and whose cancers cannot take up radioiodine. The sensitivity of the scan is high and the images identify sites of cancer that lead to a change in management in 20–60 %. Newer PET radionuclides such as 124I have potential for better lesion detection and more accurate dosimetry prior to therapy with 131I.
References
Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008;49:480–508.
Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000;41:1369–79.
Patton J, Turkington TG. Coincidence imaging with a dual-head scintillation camera. J Nucl Med. 1999;40:432–41.
Chowdhury FU, Scarsbrook AF. The role of hybrid SPECT-CT in oncology: current and emerging clinical applications. Clin Radiol. 2008;63:241–51.
Maurer AH. Combined imaging modalities: PET/CT and SPECT/CT. Health Phys. 2008;95:571–6.
Huebner RH, Park KC, Shepherd JE, et al. A meta-analysis of the literature for whole-body FDG PET detection of recurrent colorectal cancer. J Nucl Med. 2000;41:1177–89.
Weihrauch MR, Dietlein M, Schicha H, Diehl V, Tesch H. Prognostic significance of 18F-fluorodeoxyglucose positron emission tomography in lymphoma. Leuk Lymphoma. 2003;44:15–22.
Schiepers C, Filmont JE, Czernin J. PET for staging of Hodgkin’s disease and non-Hodgkin’s lymphoma. Eur J Nucl Med Mol Imaging. 2003;30 Suppl 1:S82–8.
Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–93.
Lin M. Molecular imaging using positron emission tomography in colorectal cancer. Discov Med. 2011;11:435–47.
Moerlein SM, Mathis CA, Brennan KM, Budinger TF. Synthesis and in vivo evaluation of 122I- and 131I-labelled iodoperidol, a potential agent for the tomographic assessment of cerebral perfusion. Int J Rad Appl Instrum B. 1987;14:91–8.
Agrawal A, Nair N, Baghel NS. A novel approach for reduction of brown fat uptake on FDG PET. Br J Radiol. 2009;82:626–31.
Cohen MS, Arslan N, Dehdashti F, et al. Risk of malignancy in thyroid incidentalomas identified by fluorodeoxyglucose-positron emission tomography. Surgery. 2001;130:941–6.
Kang KW, Kim SK, Kang HS, et al. Prevalence and risk of cancer of focal thyroid incidentaloma identified by 18F-fluorodeoxyglucose positron emission tomography for metastasis evaluation and cancer screening in healthy subjects. J Clin Endocrinol Metab. 2003;88:4100–4.
Ramos CD, Chisin R, Yeung HW, Larson SM, Macapinlac HA. Incidental focal thyroid uptake on FDG positron emission tomographic scans may represent a second primary tumor. Clin Nucl Med. 2001;26:193–7.
Ho TY, Liou MJ, Lin KJ, Yen TC. Prevalence and significance of thyroid uptake detected by 18F-FDG PET. Endocrine. 2011;40:297–302.
Chu QD, Connor MS, Lilien DL, Johnson LW, Turnage RH, Li BD. Positron emission tomography (PET) positive thyroid incidentaloma: the risk of malignancy observed in a tertiary referral center. Am Surg. 2006;72:272–5.
Are C, Hsu JF, Schoder H, Shah JP, Larson SM, Shaha AR. FDG-PET detected thyroid incidentalomas: need for further investigation? Ann Surg Oncol. 2007;14:239–47.
Choi JY, Lee KS, Kim HJ, Shim YM, Kwon OJ, Park K, Baek CH, et al. Focal thyroid lesions incidentally identified by integrated 18F-FDG PET/CT: clinical significance and improved characterization. J Nucl Med. 2006;47:609–15.
Chen YK, Ding HJ, Chen KT, Chen YL, Liao AC, Shen YY, et al. Prevalence and risk of cancer of focal thyroid incidentaloma identified by 18F-fluorodeoxyglucose positron emission tomography for cancer screening in healthy subjects. Anticancer Res. 2005;25:1421–6.
Adler LP, Bloom AD. Positron emission tomography of thyroid masses. Thyroid. 1993;3:195–200.
Uematsu H, Sadato N, Ohtsubo T, et al. Fluorine-18-fluorodeoxyglucose PET versus thallium-201 scintigraphy evaluation of thyroid tumors. J Nucl Med. 1998;39:453–9.
Hales NW, Krempl GA, Medina JE. Is there a role for fluorodeoxyglucose positron emission tomography/computed tomography in cytologically indeterminate thyroid nodules? Am J Otolaryngol. 2008;29:113–8.
Gianoukakis AG, Karam M, Cheema A, Cooper JA. Autonomous thyroid nodules visualized by positron emission tomography with 18F-fluorodeoxyglucose: a case report and review of the literature. Thyroid. 2003;13:395–9.
Park CH, Lee EJ, Kim JK, Joo HJ, Jang JS. Focal F-18 FDG uptake in a nontoxic autonomous thyroid nodule. Clin Nucl Med. 2002;27:136–7.
Giovanella L, Suriano S, Maffioli M, Ceriani L. 18FDG-positron emission tomography/computed tomography (PET/CT) scanning in thyroid nodules with nondiagnostic cytology. Clin Endocrinol (Oxf). 2011;74:644–8.
Wong C, Lin M, Chicco A, Benson R. The clinical significance and management of incidental focal FDG uptake in the thyroid gland on positron emission tomography/computed tomography (PET/CT) in patients with non-thyroidal malignancy. Acta Radiol. 2011;52:899–904.
Keyes Jr JW. SUV: standard uptake or silly useless value? J Nucl Med. 1995;36:1836–9.
Cook G, Maisey MN, Fogelman I. Normal variants, artefacts and interpretative pitfalls in PET with 18-fluoro-2-deoxyglucose and carbon-11 methionine. Normal variants, artefacts and interpretative pitfalls in PET with 18-fluoro-2-deoxyglucose and carbon-11 methionine. Eur J Nucl Med. 1999;26:1363–78.
Cook G, Wegner EA, Fogelman I. Pitfalls and artifacts in 18FDG PET and PET/CT oncologic imaging. Semin Nucl Med. 2004;XXXIV:122–33.
Barrington S, Maisey MN. Skeletal muscle uptake of fluorine-18 FDG: effect of oral diazepam. J Nucl Med. 1996;37:1127–9.
Hany TF, Gharehpapagh E, Kamel EM, Buck A, Himms-Hagen J, von Schulthess GK. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nucl Med Mol Imaging. 2002;29:1393–8.
Cohade C, Mourtzikos KA, Wahl RL. “USA-Fat”: prevalence is related to ambient outdoor temperature-evaluation with 18F-FDG PET/CT. J Nucl Med. 2003;44:1267–70.
Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/CT. J Nucl Med. 2003;44:170–6.
Yeung HW, Grewal RK, Gonen M, Schoder H, Larson SM. Patterns of 18F-FDG uptake in adipose tissue and muscle: a potential source of false-positives for PET. J Nucl Med. 2003;44:1789–96.
Tatsumi M, Engles JM, Ishimori T, Nicely O, Cohade C, Wahl RL. Intense 18F-FDG uptake in brown fat can be reduced pharmacologically. J Nucl Med. 2004;45:1189–93.
Igerc I, Kumnig G, Heinisch M, et al. Vocal cord muscle activity as a drawback to FDG-PET in the followup of differentiated thyroid cancer. Thyroid. 2002;12:87–9.
Zhu Z, Chou C, Yen TC, Cui R. Elevated F-18 FDG uptake in laryngeal muscles mimicking thyroid cancer metastases. Clin Nucl Med. 2001;26:689–91.
Yeretsian RA, Blodgett TM, Branstetter BF, Roberts MM, Meltzer CC. Teflon-induced granuloma: a false-positive finding with PET resolved with combined PET and CT. AJNR Am J Neuroradiol. 2003;24:1164–6.
Alibazoglu H, Alibazoglu B, Hollinger EF, et al. Normal thymic uptake of 2-deoxy-2[F-18]fluoro-D-glucose. Clin Nucl Med. 1999;24:597–600.
Kawano T, Suzuki A, Ishida A, et al. The clinical relevance of thymic fluorodeoxyglucose uptake in pediatric patients after chemotherapy. Eur J Nucl Med Mol Imaging. 2004;31:831–6.
Rini JN, Leonidas JC, Tomas MB, Karayalcin G, Tronco GG, Palestro CJ. FDG uptake in the anterior mediastinum. Physiologic thymic uptake or disease? Clin Positron Imaging. 1999;2:332.
Wittram C, Fischman AJ, Mark E, Ko J, Shepard JA. Thymic enlargement and FDG uptake in three patients: CT and FDG positron emission tomography correlated with pathology. AJR Am J Roentgenol. 2003;180:519–22.
Nakahara T, Fujii H, Ide M, et al. FDG uptake in the morphologically normal thymus: comparison of FDG positron emission tomography and CT. Br J Radiol. 2001;74:821–4.
Patel PM, Alibazoglu H, Ali A, Fordham E, LaMonica G. Normal thymic uptake of FDG on PET imaging. Clin Nucl Med. 1996;21:772–5.
Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214.
Pineda J, Lee T, Ain K, Reynolds JC, Robbins J. Iodine-131 therapy for thyroid cancer patients with elevated thyroglobulin and negative diagnostic scan. J Clin Endocrinol Metab. 1995;80:1488–92.
Schlumberger M, Mancusi F, Baudin E, Pacini F. 131I therapy for elevated thyroglobulin levels. Thyroid. 1997;7:273–6.
Wartofsky L. Management of scan negative thyroglobulin positive differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1998;83:4195–9.
McDougall I. 131I treatment of 131I negative whole body scan, and positive thyroglobulin in differentiated thyroid carcinoma: what is being treated? Thyroid. 1997;7:669–72.
Fatourechi V, Hay ID, Javedan H, Wiseman GA, Mullan BP, Gorman CA. Lack of impact of radioiodine therapy in tg-positive, diagnostic whole-body scan-negative patients with follicular cell-derived thyroid cancer. J Clin Endocrinol Metab. 2002;87:1521–6.
McDougall IR. Management of thyroglobulin positive/whole-body scan negative: is Tg positive/I therapy useful? J Endocrinol Invest. 2001;24:194–8.
Hoefnagel B, Delprat CC, Marcuse HR, Vijlder JJM. Role of thallium-201 total-body scintigraphy in follow-up of thyroid carcinoma. J Nucl Med. 1986;27:1854–7.
Iida Y, Hidaka A, Hatabu H, Kasagi K, Konishi J. Follow-up study of postoperative patients with thyroid cancer by thallium-201 scintigraphy and serum thyroglobulin measurement. J Nucl Med. 1991;32:2098–100.
Nakada K, Katoh C, Kanegae E, et al. Thallium-201 scintigraphy to predict therapeutic outcome of iodine-131 therapy of metastatic thyroid carcinoma. J Nucl Med. 1998;39:807–10.
Brandt-Mainz K, Muller SP, Reiners C, Bockisch A. Relationship between thyroglobulin and reliability of thallium 201 scintigraphy in differentiated thyroid cancer. Nuklearmedizin. 2000;39:20–5.
Yen TC, Lin HD, Lee CH, Chang SL, Yeh SH. The role of technetium-99m sestamibi whole-body scans in diagnosing metastatic Hurthle cell carcinoma of the thyroid gland after total thyroidectomy: a comparison with iodine-131 and thallium-201 whole-body scans. Eur J Nucl Med. 1994;21:980–3.
Almeida-Filho P, Ravizzini GC, Almeida C, Borges-Neto S. Whole-body Tc-99m sestamibi scintigraphy in the follow-up of differentiated thyroid carcinoma. Clin Nucl Med. 2000;25:443–6.
Gallowitsch H, Mikosch P, Kresnik E, Unterweger O, Gomez I, Lind P. Thyroglobulin and low-dose iodine-131 and technetium-99m-tetrofosmin whole-body scintigraphy in differentiated thyroid carcinoma. J Nucl Med. 1998;39:870–5.
Lind P, Gallowitsch HJ, Langsteger W, et al. Technetium-99m-tetrofosmin whole-body scintigraphy in the follow-up of differentiated thyroid carcinoma. J Nucl Med. 1997;38:348–52.
Lind P. Multi-tracer imaging of thyroid nodules: is there a role in the preoperative assessment of nodular goiter? Eur J Nucl Med. 1999;26:795–7.
Lind P, Gallowitsch HJ, Mikosch P, et al. Comparison of different tracers in the follow up of differentiated thyroid carcinoma. Acta Med Austriaca. 1999;26:115–7.
Drac-Kaniewska J, Kozlowicz-Gudzinska I, Tomaszewicz-Kubasik H, et al. 99mTc Tetrofosmin in diagnosis of distant metastases from differentiated thyroid cancer. Wiad Lek. 2001;54 Suppl 1:357–62.
Ahlman H, Tisell LE, Wangberg B, et al. The relevance of somatostatin receptors in thyroid neoplasia. Yale J Biol Med. 1997;70:523–33.
Sarlis NJ, Gourgiotis L, Guthrie LC, et al. In-111 DTPA-octreotide scintigraphy for disease detection in metastatic thyroid cancer: comparison with F-18 FDG positron emission tomography and extensive conventional radiographic imaging. Clin Nucl Med. 2003;28:208–17.
Baudin E, Schlumberger M, Lumbroso J, Travagli JP, Caillou B, Parmentier C. Octreotide scintigraphy in patients with differentiated thyroid carcinoma; contribution for patients with negative radioiodine scans. J Clin Endocrinol Metab. 1996;81:2541–4.
Joensuu H, Ahonen A. Imaging of metastases of thyroid carcinoma with fluorine-18 fluorodeoxyglucose. J Nucl Med. 1987;28:910–4.
Adams S, Baum RP, Hertel A, Schumm-Draeger PM, Usadel K-H, Hor G. Comparison of metabolic and receptor imaging in recurrent medullary thyroid carcinoma with histopathological findings. Eur J Nucl Med. 1998;25:1277–83.
Adams S, Baum R, Rink T, Schumm-Drager PM, Usadel KH, Hor G. Limited value of fluorine-18 fluorodeoxyglucose positron emission tomography for the imaging of neuroendocrine tumours. Eur J Nucl Med. 1998;25:79–83.
Alnafisi N, Driedger AA, Coates G, Moote DJ, Raphael SJ. FDG PET of recurrent or metastatic 131I-negative papillary thyroid carcinoma. J Nucl Med. 2000;41:1010–5.
Altenvoerde G, Lerch H, Kuwert T, Matheja P, Schafers M, Schober O. Positron emission tomography with F-18-deoxyglucose in patients with differentiated thyroid carcinoma, elevated thyroglobulin levels, and negative iodine scans. Langenbecks Arch Surg. 1998;383:160–3.
Berger F, Knesewitsch P, Tausig A, et al. [18F] Fluorodeoxyglucose hybrid PET in patients with differentiated thyroid cancer: comparison with dedicated PET. Eur Ass Nucl Med Congress Paris 2000. 2000.
Boer A, Szakall Jr S, Klein I, et al. FDG PET imaging in hereditary thyroid cancer. Eur J Surg Oncol. 2003;29:922–8.
Boerner AR, Petrich T, Weckesser E, et al. Monitoring isotretinoin therapy in thyroid cancer using 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2002;29:231–6.
Brandt-Mainz K, Muller SP, Gorges R, Saller B, Bockisch A. The value of fluorine-18 fluorodeoxyglucose PET in patients with medullary thyroid cancer. Eur J Nucl Med. 2000;27:490–6.
Riemann B, Uhrhan K, Dietlein M, et al. Diagnostic value and therapeutic impact of (18)F-FDG-PET/CT in differentiated thyroid cancer. Results of a German multicentre study. Nucl Med. 2013;52:1–6.
van Dijk D, Plukker JTM, Phan HTT, et al. 18-fluorodeoxyglucose positron emission tomography in the early diagnostic workup of differentiated thyroid cancer patients with a negative post-therapeutic iodine scan and detectable thyroglobulin. Thyroid. 2013;23:1003–9.
Macapinlac HA. Clinical usefulness of FDG PET in differentiated thyroid cancer. J Nucl Med. 2001;42:77–8.
Wong CO, Dworkin HJ. Role of FDG PET in metastatic thyroid cancer. J Nucl Med. 1999;40:993–4.
Khan N, Oriuchi N, Higuchi T, Zhang H, Endo K. PET in the follow-up of differentiated thyroid cancer. Br J Radiol. 2003;76:690–5.
Crippa F, Alessi A, Gerali A, Bombardieri E. FDG-PET in thyroid cancer. Tumori. 2003;89:540–3.
Ozkan E, Aras G, Kucuk NO. Correlation of 18F-FDG PET/CT findings with histopathological results in differentiated thyroid cancer patients who have increased thyroglobulin or antithyroglobulin antibody levels and negative 131I whole-body scan results. Clin Nucl Med. 2013;38:326–31.
Armstrong S, Worsley D, Blair GK. Pediatric surgical images: PET evaluation of papillary thyroid carcinoma recurrence. J Pediatr Surg. 2002;37:1648–9.
Joensuu H, Klemi PJ, Eerola E. Diagnostic value of flow cytometric DNA determination combined with fine needle aspiration biopsy in thyroid tumors. Anal Quant Cytol Histol. 1987;9:328–34.
Feine U, Lietzenmayer R, Hanke JP, Wohrle H, Muller-Schauenburg W. 18FDG whole-body PET in differentiated thyroid carcinoma. Flipflop in uptake patterns of 18FDG and 131I. Nuklearmedizin. 1995;34:127–34.
Shiga T, Tsukamoto E, Nakada K, et al. Comparison of 18F-FDG, 131I-Na, and 201Tl in diagnosis of recurrent or metastatic thyroid carcinoma. J Nucl Med. 2001;42:414–9.
Dietlein M, Moka D, Scheidhauer K, et al. Follow-up of differentiated thyroid cancer: comparison of multiple diagnostic tests. Nucl Med Commun. 2000;21:991–1000.
Bertagna F, Bosio G, Biasiotto G, Rodella C, Puta E, Gabanelli S, Lucchini S, et al. F-18 FDG-PET/CT evaluation of patients with differentiated thyroid cancer with negative I-131 total body scan and high thyroglobulin level. Clin Nucl Med. 2009;34:756–61.
Iagaru A, Masamed R, Singer PA, Conti PS. 2-Deoxy-2-[18F]fluoro-D-glucose-positron emission tomography and positron emission tomography/computed tomography diagnosis of patients with recurrent papillary thyroid cancer. Mol Imaging Biol. 2006;8:309–14.
Schluter B, Bohuslavizki KH, Beyer W, Plotkin M, Buchert R, Clausen M. Impact of FDG PET on patients with differentiated thyroid cancer who present with elevated thyroglobulin and negative 131I scan. J Nucl Med. 2001;42:71–6.
Palmedo H, Bucerius J, Joe A, Strunk H, Hortling N, Meyka S, Roedel R, et al. Integrated PET/CT in differentiated thyroid cancer: diagnostic accuracy and impact on patient management. J Nucl Med. 2006;47:616–24.
Mirallie E, Guiana T, Bridji B, Resche I, Rousseau C, Ansquer C, Bodet-Milin C, et al. Therapeutic impact of 18FDG-PET/CT in the management of iodine-negative recurrence of differentiated thyroid carcinoma. Surgery. 2007;142:952–8; discussion 952–8.
Esteva D, Muros MA, Llamas-Elvira JM, Jimenez Alonso J, Villar JM, Lopez de la Torre M, Muros T. Clinical and pathological factors related to 18F-FDG-PET positivity in the diagnosis of recurrence and/or metastasis in patients with differentiated thyroid cancer. Ann Surg Oncol. 2009;16:2006–13.
Choi JW, Yoon YH, Kim SM, Koo BS. Characteristics of primary papillary thyroid carcinoma with false-negative findings on initial 18F-FDG PET/CT. Ann Surg Oncol. 2011;18:1306–11.
Iagaru A, Kalinyak JE, McDougall IR. F-18 FDG PET/CT in the management of thyroid cancer. Clin Nucl Med. 2007;32:690–5.
Davison JM, Stocker DJ, Montilla-Soler JL, Jurgens JS, Allen TW, Holley TS, Stack AL. The added benefit of a dedicated neck F-18 FDG PET-CT imaging protocol in patients with suspected recurrent differentiated thyroid carcinoma. Clin Nucl Med. 2008;33:464–8.
Kaneko K, Abe K, Baba S, et al. Detection of residual lymph node metastases in high-risk papillary thyroid cancer patients receiving adjuvant I-131 therapy: the usefulness of F-18 FDG PET/CT. Clin Nucl Med. 2010;35:6–11.
Choi WH, Chung YA, Han EJ, Sohn HS, Lee SH. Clinical value of integrated [18F]fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in the preoperative assessment of papillary thyroid carcinoma: comparison with sonography. J Ultrasound Med. 2011;30:1267–73.
Piccardo A, Arecco F, Morbelli S, Bianchi P, Barbera F, Finessi M, Corvisieri S, et al. Low thyroglobulin concentrations after thyroidectomy increase the prognostic value of undetectable thyroglobulin levels on levo-thyroxine suppressive treatment in low-risk differentiated thyroid cancer. J Endocrinol Invest. 2010;33:83–7.
Stokkel MP, Duchateau CS, Dragoiescu C. The value of FDG-PET in the follow-up of differentiated thyroid cancer: a review of the literature. Q J Nucl Med Mol Imaging. 2006;50:78–87.
Miller ME, Chen Q, Elashoff D, Abemayor ES, John M. Positron emission tomography and positron emission tomography-CT evaluation for recurrent papillary thyroid carcinoma: meta-analysis and literature review. Head Neck. 2011;33:562–5.
Dong MJ, Liu ZF, Zhao K, Ruan LX, Wang GL, Yang SY, Sun F, Luo XG. Value of 18F-FDG-PET/PET-CT in differentiated thyroid carcinoma with radioiodine-negative whole-body scan: a meta-analysis. Nucl Med Commun. 2009;30:639–50.
Wang W, Larson SM, Fazzari M, et al. Prognostic value of [18F]fluorodeoxyglucose positron emission tomographic scanning in patients with thyroid cancer. J Clin Endocrinol Metab. 2000;85:1107–13.
Goshen E, Cohen O, Rotenberg G, Oksman Y, Karasik A, Zwas ST. The clinical impact of 18F-FDG gamma PET in patients with recurrent well differentiated thyroid carcinoma. Nucl Med Commun. 2003;24:959–61.
Karwowski J, Jeffrey RB, McDougall IR, Weigel RJ. Intraoperative ultrasonography improves identification of recurrent thyroid cancer. Surgery. 2002;132:924–8.
Ota N, Kato K, Iwano S, et al. Comparison of 18F-fluoride PET/CT, 18F-FDG PET/CT, and bone scintigraphy (planar and SPECT) in detection of bone metastases of differentiated thyroid cancer: a pilot study. Br J Radiol. 2014;87:20130444.
Nakajo M, Jinguji M, Tani A, et al. Diagnosis of metastases from postoperative differentiated thyroid cancer: comparison between FDG and FLT PET/CT studies. Radiology. 2013;267:891–901.
Wang W, Macapinlac H, Larson SM, Yeh SD, Akhurst T, Finn RD, Rosai J, Robbins RJ. [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography localizes residual thyroid cancer in patients with negative diagnostic (131)I whole body scans and elevated serum thyroglobulin levels. J Clin Endocrinol Metab. 1999;84:2291–302.
Grunwald F, Schomburg A, Bender H, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography in the follow-up of differentiated thyroid cancer. Eur J Nucl Med. 1996;23:312–9.
Sisson JC, Ackermann RJ, Meyer MA, Wahl RL. Uptake of 18-fluoro-2-deoxy-D-glucose by thyroid cancer: implications for diagnosis and therapy. J Clin Endocrinol Metab. 1993;77:1090–4.
Filetti S, Damante G, Foti D. Thyrotropin stimulates glucose transport in cultured rat thyroid cells. Endocrinology. 1987;120:2576–81.
Moog F, Linke R, Manthey N, et al. Influence of thyroid-stimulating hormone levels on uptake of FDG in recurrent and metastatic differentiated thyroid carcinoma. J Nucl Med. 2000;41:1989–95.
Petrich T, Borner AR, Otto D, Hofmann M, Knapp WH. Influence of rhTSH on [(18)F]fluorodeoxyglucose uptake by differentiated thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2002;29:641–7.
Chin BB, Patel P, Cohade C, Ewertz M, Wahl R, Ladenson P. Recombinant human thyrotropin stimulation of fluoro-D-glucose positron emission tomography uptake in well-differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2004;89:91–5.
Ma C, Xie J, Lou Y, Gao Y, Zuo S, Wang X. The role of TSH for 18F-FDG-PET in the diagnosis of recurrence and metastases of differentiated thyroid carcinoma with elevated thyroglobulin and negative scan: a meta-analysis. Eur J Endocrinol. 2010;163:177–83.
Leboulleux S, Schroeder PR, Busaidy NL, Auperin A, Corone C, Jacene HA, et al. Assessment of the incremental value of recombinant thyrotropin stimulation before 2-[18F]-Fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography imaging to localize residual differentiated thyroid cancer. J Clin Endocrinol Metab. 2009;94:1310–6.
Vera P, Kuhn-Lansoy C, Edet-Sanson A, Hapdey S, Modzelewski R, Hitzel A, et al. Does recombinant human thyrotropin-stimulated positron emission tomography with [18F]fluoro-2-deoxy-D-glucose improve detection of recurrence of well-differentiated thyroid carcinoma in patients with low serum thyroglobulin? Thyroid. 2010;20:15–23.
Lambrecht RM, Woodhouse N, Phillips R, et al. Investigational study of iodine-124 with a positron camera. Am J Physiol Imaging. 1988;3:197–200.
Flower M, Al-Saadi A, Harmer CL, et al. Dose response study on thyrotoxic patients undergoing positron emission tomography and radioiodine therapy. Eur J Nucl Med. 1994;21:531–6.
Frey P, Townsend D, Jeavons A, Donath A. In vivo imaging of the human thyroid with a positron camera using I-124. In vivo imaging of the human thyroid with a positron camera using I-124. Eur J Nucl Med. 1985;10:472–6.
Frey P, Townsend D, Flattet A, et al. Tomographic imaging of the human thyroid using 124I. J Clin Endocrinol Metab. 1986;63:918–27.
Crawford DC, Flower MA, Pratt BE, et al. Thyroid volume measurement in thyrotoxic patients: comparison between ultrasonography and iodine-124 positron emission tomography. Eur J Nucl Med. 1997;24:1470–8.
Freudenberg LS, Jentzen W, Stahl A, Bockisch A, Rosenbaum-Krumme SJ. Clinical applications of 124I-PET/CT in patients with differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2011;38 Suppl 1:S48–56.
Eschmann SM, Reischl G, Bilger K, et al. Evaluation of dosimetry of radioiodine therapy in benign and malignant thyroid disorders by means of iodine-124 and PET. Eur J Nucl Med Mol Imaging. 2002;29:760–7.
Freudenberg LS, Antoch G, Gorges R, et al. Combined PET/CT with iodine-124 in diagnosis of spread metastatic thyroid carcinoma: a case report. Eur Radiol. 2003;13 Suppl 4:L19–23.
Freudenberg LS, Antoch G, Jentzen W, et al. Value of 124I-PET/CT in staging of patients with differentiated thyroid cancer. Eur Radiol. 2004;14:2092–8.
Capoccetti F, Criscuoli B, Rossi G, Ferretti F, Manni C, Brianzoni E. The effectiveness of 124I PET/CT in patients with differentiated thyroid cancer. Q J Nucl Med Mol Imaging. 2009;53:536–45.
Freudenberg LS, Jentzen W, Muller SP, Bockisch A. Disseminated iodine-avid lung metastases in differentiated thyroid cancer: a challenge to 124I PET. Eur J Nucl Med Mol Imaging. 2008;35:502–8.
Van Nostrand D, Moreau S, Bandaru VV, Atkins F, Chennupati S, Mete M, Burman K, Wartofsky L. 124I positron emission tomography versus 131I planar imaging in the identification of residual thyroid tissue and/or metastasis in patients who have well-differentiated thyroid cancer. Thyroid. 2010;20:879–83.
Freudenberg LS, Antoch G, Frilling A, Jentzen W, Rosenbaum SJ, Kuhl H, Bockisch A, Gorges R. Combined metabolic and morphologic imaging in thyroid carcinoma patients with elevated serum thyroglobulin and negative cervical ultrasonography: role of 124I-PET/CT and FDG-PET. Eur J Nucl Med Mol Imaging. 2008;35:950–7.
Sgouros G, Kolbert KS, Sheikh A, Pentlow KS, Mun EF, Barth A, Robbins RJ, Larson SM. Patient-specific dosimetry for 131I thyroid cancer therapy using 124I PET and 3-dimensional-internal dosimetry (3D-ID) software. J Nucl Med. 2004;45:1366–72.
Sgouros G, Hobbs RF, Atkins FB, Van Nostrand D, Ladenson PW, Wahl RL. Three-dimensional radiobiological dosimetry (3D-RD) with 124I PET for 131I therapy of thyroid cancer. Eur J Nucl Med Mol Imaging. 2011;38 Suppl 1:S41–7.
Ho A, Grewal R, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368:623–32.
Dyson N, Francois PE. Some observations on the decay of iodine-124 and their implications in radioiodine therapy. Phys Med Biol. 1958;3:111.
Belov VV, Bonab AA, Fischman AJ, Heartlein M, Calias P, Papisov MI. Iodine-124 as a label for pharmacological PET imaging. Mol Pharm. 2011;8:736–47.
Middendorp M, Selkinski I, Happel C, Kranert WT, Grunwald F. Comparison of positron emission tomography with [18F]FDG and [68Ga]DOTATOC in recurrent differentiated thyroid cancer: preliminary data. Q J Nucl Med Mol Imaging. 2010;54:76–83.
Chung J-K, So Y, Lee JS, et al. Value of FDG PET in papillary thyroid carcinoma with negative 131I whole-body scan. J Nucl Med. 1999;40:486–92.
Dietlein M, Scheidhauer K, Voth E, Theissen P, Schicha H. Fluorine-18 fluorodeoxyglucose positron emission tomography and iodine-131 whole-body scintigraphy in the follow-up of differentiated thyroid cancer. Eur J Nucl Med. 1997;24:1342–8.
Grunwald F, Kalicke T, Feine U, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography in thyroid cancer: results of a multicentre study. Eur J Nucl Med. 1999;26:1547–52.
Stokkel MP, de Klerk JH, Zelissen PM, Koppeschaar HP, van Rijk PP. Fluorine-18 fluorodeoxyglucose dual-head positron emission tomography in the detection of recurrent differentiated thyroid cancer: preliminary results. Eur J Nucl Med. 1999;26:1606–9.
Helal BO, Merlet P, Toubert ME, Franc B, Schvartz C, Gauthier-Koelesnikov H, Prigent A, Syrota A. Clinical impact of 18F-FDG PET in thyroid carcinoma patients with elevated thyroglobulin levels and negative 131I scanning results after therapy. J Nucl Med. 2001;42:1464–9.
Freudenberg LS, Frilling A, Kuhl H, Muller SP, Jentzen W, Bockisch A, Antoch G. Dual-modality FDG-PET/CT in follow-up of patients with recurrent iodine-negative differentiated thyroid cancer. Eur Radiol. 2007;17:3139–47.
Ong SC, Ng DC, Sundram FX. Initial experience in use of fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography in thyroid carcinoma patients with elevated serum thyroglobulin but negative iodine-131 whole body scans. Singapore Med J. 2005;46:297–301.
Kim SJ, Lee TH, Kim IJ, Kim YK. Clinical implication of F-18 FDG PET/CT for differentiated thyroid cancer in patients with negative diagnostic iodine-123 scan and elevated thyroglobulin. Eur J Radiol. 2009;70:17–24.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this chapter
Cite this chapter
Iagaru, A., McDougall, I.R. (2016). Positron Emission Tomography-Computed Tomography (PET-CT and PET) in Well-Differentiated Thyroid Cancer. In: Wartofsky, L., Van Nostrand, D. (eds) Thyroid Cancer. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-3314-3_43
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3314-3_43
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-3312-9
Online ISBN: 978-1-4939-3314-3
eBook Packages: MedicineMedicine (R0)