Abstract
We report a new technique for ultrasound–anatomic correlations consisting of dissection of embalmed specimens during ultrasound examination. Our method consists of performing ultrasound during the different stages of dissection. The technique was developed by making observations of selected structures in two embalmed and two non-embalmed cadaver hands. The image quality was subjectively graded by consensus of two investigators, before and after denudation of the selected structures of the hand. As an example, the technique is demonstrated for the flexors at the metacarpophalangeal joint level, the extensor complex at the level of the proximal phalanx, and the dorsal hood of the second to fourth fingers. Before dissection the image quality in fresh specimens was graded moderate, and in embalmed specimens good. After dissection the image quality was good in fresh specimens and excellent in embalmed specimens. Our method is simple and does not require sophisticated material. Our results indicate that embalmed specimens could be better than non-embalmed specimens, because of the presence of artefacts in the non-embalmed specimens (gas deposits). The described methodology can yield excellent results regarding precise identification of different interfaces and structures, as observed at ultrasound.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In CT and MR imaging, correlations with anatomic dissection or anatomic slices have been widely performed, especially in certain subspecialty areas such as musculoskeletal imaging [1, 2, 3, 4, 5]. The use of such correlation methods is less well developed in the field of ultrasound. The most important problem is that the correlation which is straightforward between MR images and anatomic slices is less evident with ultrasound images. When comparing an ultrasound image and an anatomic slice, it is difficult to obtain a “layer by layer” comparison. Often it remains elusive to which interface, as seen on ultrasound images, a certain anatomic structure corresponds.
Although some correlations between ultrasound and anatomic images have been published [6], few articles have addressed the methodology of this type of correlation in detail, and authors have used similar methods to those described for MR imaging. Hence, we studied the methodology of ultrasound–anatomic correlation, and developed an optimised technique for obtaining correlations on slices or entire specimens.
Materials and methods
Two embalmed hand specimens and two non-embalmed hand specimens were obtained from the department of anatomy. Informed consent had been obtained prior to death to use tissues for research purposes. The required joint specimen was removed from the cadaver, ensuring that the relationship at the studied joint was kept as intact as possible. The non-embalmed specimens were frozen immediately after the individuals’ deaths (both were elderly subjects). Embalming of the other two specimens was performed using a solution of 10 l of water containing 250 g of chloral hydrate, 125 g of sodium sulphate, 125 g of magnesium sulphate, 250 g of potassium nitrate, 1 l of phenol, 1 l of a mixture of alcohol (96%) and 20 g of thymol, 1 l of formol, and 250 cc of glycerine. The solution was introduced in the cadaver via placement of a catheter in the femoral artery, and positioning of the solution 1 m above the level of the cadaver that lay on a table in supine position. In both embalmed and non-embalmed specimens, investigations were performed 4–8 months after death.
Prior to dissection, investigators experienced in musculoskeletal ultrasound examined the flexor and extensor side of the hand (second to fourth fingers) in the embalmed and non-embalmed specimens (MDM, KVD). The images obtained were saved in digital form (DICOM format) and then converted into JPEG format. The specimens or slices were photographed during the different stages of dissection. To illustrate our methodology, we focused on the extensor complex at the level of the proximal phalanx, on the dorsal hood area, and the flexor system at the metacarpophalangeal joint. An ATL HDI 5,000 system (Philips, Eindhoven, The Netherlands) was employed for all ultrasound studies (12 MHz transducer). The compound function of this system was used liberally by the investigators, both for fresh and embalmed specimens. The ultrasound image quality of embalmed and non-embalmed specimens was compared using a four-point ordinal scale (1, bad; 2, moderate; 3, good; 4, excellent). Limited MR imaging of the above-mentioned structures in the fresh specimens was performed for illustrative purposes. A proton density-weighted sequence (TR1800/TE17; FOV, 100; slice thickness, 3 mm; 3 signals acquired) and surface coil were used on an Intera clinical system (Philips, Best, The Netherlands).
The second phase of the investigation consisted of a progressive dissection of the specimens. During the different stages of dissection, ultrasound was repetitively performed by consensus of two investigators. The dissections were made by the principal investigator and by a senior anatomist. After ultrasound and dissection, the non-embalmed specimens were refrozen. In contradistinction, the embalmed specimens were placed in a 2% phenol solution. The specimens were handled carefully and the investigators wore appropriate protection (surgical mask, glasses, surgical gloves, and protective coat). Direct skin contact with the specimens (especially the fresh) was avoided and contact with medical equipment was limited when possible. The specimens were transported in a plastic box and the ultrasound transducer was coated with a surgical glove. If any contact occurred between the specimen and medical equipment, the latter was disinfected appropriately. After completion of a session of dissection, ultrasound, and photography of the embalmed specimens, the removed skin was placed over the dissected area and the specimen was preserved in a 2% phenol solution. This prevents the specimen from drying between sessions. For the next dissection session, the specimen was removed from the solution and put on a dry towel for 20 min to remove excessive fluid. The area of interest was also dabbed with a towel before the new dissection started.
After a new layer or structure had been removed, at dissection, the specimen was moved to the ultrasound department and ultrasound was performed.
With removal of the overlying structures, the ultrasound transducer can be precisely positioned on the anatomic structure of interest for a confident correlation between ultrasound observations and anatomic specimens or slices. In fact, specimens can also be sliced into 5-mm-thick sections and the described method can then be employed using the slice rather than the specimen.
Results
Image quality
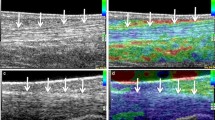
The initial results of ultrasound in fresh specimens prior to dissection showed a deterioration of image quality in both specimens used. Hence the subjective quality rating was judged moderate in the fresh specimens and excellent in the embalmed specimens. After denudation of the structures of interest, the quality in fresh specimens was judged good and in embalmed specimens excellent. These differences were related to multiple artefacts secondary to the presence of tissue gas (Fig. 1). The small gas bubbles had occurred in some areas of the specimen, other areas being spared. Nevertheless, a systematic analysis of the specimen by ultrasound was not possible. This phenomenon occurred despite the meticulous use of a method that was previously described for MR imaging for specimen conservation [1]. In contradistinction, the image quality in the embalmed specimens was excellent in all cases. There were no areas containing deposits of gas.
Photograph of the dissection of the extensor side of the hand in an embalmed specimen. Ultrasound sections were obtained at indicated levels (black lines). At the level of the proximal phalanx extensor tendon (e) and interosseous (I), tendon extensions are seen. Proximal to the level of the dorsal hood, the extensor tendon is made up of different portions (t). The connexus (C) is also shown, as is the dorsal hood area
Further progressive removal of fascia, connective tissue, retinaculum and tendons allowed a very precise correlation with ultrasound observations. Obviously, when a structure was no longer seen on ultrasound after it had been removed by dissection, it was considered consistent proof that this structure corresponded to the reflection, as seen on ultrasound images.
Anatomic results
In our experiment chosen to illustrate this (Fig. 2) technique, the extensor tendon complex of the fingers was studied at the level of the proximal phalanx and at the level of the dorsal hood complex, both in the transverse imaging plane [6]. The extensor complex at the level of the proximal phalanx is made up of fibres from the extensor digitorum tendon at the dorsal side of the phalanx. On both sides of the phalanx there are contributions from the interosseous tendons, and in addition on the radial side there are contributions from the lumbricalis tendon. All these structures intermingle, resulting in a cap-like configuration of the extensor complex at the level of the proximal phalanx (Fig. 3). The image can be compared to that of a “Chinese hat”. The extensor complex proper is quite thin, which became evident after removal of skin and subcutaneous fatty and connective tissue. For additional comparison we obtained MR images from fresh cadaveric specimens (Fig. 4). Our ultrasound investigations during dissection also showed that the extensor tendons appear flattened at the level of the midhand. The extensor tendon reaching the metacarpophalangeal head may originate from the fusion of different separate tendinous structures at midhand level. When reaching the dorsal hood, the tendon becomes more oval shaped (Fig. 5). The dorsal hood [7] is an area where the extensor system is kept centred over the metacarpophalangeal joint by extensive fibrous reinforcements on both sides of the tendon. The dorsal hood is also often referred to as the sagittal bands. Our dissections during ultrasound revealed that the dorsal hood region is easily identifiable with ultrasound. Whereas the tendon is seen as an oval-like structure, the medial and lateral area of the dorsal hood are seen as triangular structures on both sides of the tendon. The image is very reminiscent of a “sphinx-like” appearance. The sphinx-like appearance indicates that the ultrasound probe is exactly at the level of the dorsal hood. More distally, the sphinx-like appearance disappears and at the level of the proximal phalanx, the tendon becomes flattened again. At the metacarpophalangeal level of the palmar side, the flexor digitorum profundus tendon is located deep to the flexor digitorum superficialis tendon. The flexor tendons were seen as separate tendons on the palmar side at the level of the metacarpophalangeal joints (Fig. 6).
Ultrasound image in the transverse plane prior to a and after b dissection of the dorsal hood. After removal of skin and connective tissue, a “sphinx-like” appearance of the extensor tendon at the dorsal hood level is seen. Note the triangular shape and slightly more hypoechoic aspect of the dorsal hood structures (asterisks in a and b). Centrally located extensor tendon is slightly more hyperechoic (t in a, between white lines in b)
a Embalmed specimen: Transverse ultrasound of the flexor tendons at the palmar side of the metacarpophalangeal joints. Flexor superficialis and profundus tendons are seen (white arrows). Note the homogeneous appearance of the subcutaneous tissue (curved arrow). b Fresh specimen: Comparable image in fresh specimen. Detail of flexor tendons is less well depicted (white arrows). Also note the more heterogeneous subcutaneous tissue (curved arrow). Hyperechoic foci probably correspond to small gas deposits (short white arrows)
Discussion
The use of tissue-simulating test objects or cadaveric specimens is common in musculoskeletal radiology research [2]. Different methods have been described for imaging–anatomic correlation [1, 3]. A few studies made correlations between anatomic specimens and ultrasound findings [4, 5]. We describe a new method for ultrasound–anatomic correlation. The method was developed by systematically comparing ultrasound images before and during dissection in both embalmed and non-embalmed specimens. We have used it since its conception in several investigations. The method does not require expensive or sophisticated equipment and can be performed as long as the cadaveric specimens are available. The specimens are embalmed by introduction of a catheter in the femoral artery and the use of a typical mixture used for embalming specimens, for educational purposes.
Ethical and legal considerations play an important role in the use of human tissue for experiments and ethical standards should be strictly followed in this regard. Some specimens may present hazards to the investigator due to sharp bony margins or the possible presence of infectious agents.
In our investigation we observed that the fresh specimens contained gas bubbles (Figs. 2, 6) in the soft tissues, making evaluation of underlying structures difficult. It did not seem possible to displace the gas bubbles by changing the angle of incidence or increasing transducer pressure.
This limitation of ultrasound in fresh specimens has not been mentioned in previous investigations. Nevertheless, it is a finding we have been aware of when conducting anatomic–ultrasound correlations [4]. We acknowledge that neither in our investigations nor, to our knowledge, in the publications of other authors has this limitation been pointed out. Perhaps this effect is much more important in the study of smaller joints such as fingers or toes compared to larger joints. Although all precautions were taken to preserve the specimens as fresh as possible, it was obvious that with ultrasound very small deposits of gas in subcutaneous or muscular tissue led to artefacts, making a systematic assessment of anatomic structures difficult. It is highly likely that the subcutaneous gas is related to small foci of tissue necrosis and subsequent gas formation.
In addition there appears to be an embalming effect that results in a more homogeneous appearance of the specimen, compared to fresh specimens, since after removal of skin and subcutaneous tissue the quality of embalmed specimens was still better at ultrasound (Fig. 6). This is in contradistinction to the situation with MR–anatomic correlations, where MR imaging is not adequate in embalmed specimens (M. De Maeseneer, J. Van Roy, personal observations).
Because the number of specimens was limited, we did not calculate intra-observer and inter-observer variation, and we acknowledge that our observations are mainly based on subjective interpretations. In addition, a statistical approach was not possible with this number of data. In addition, the limited number of specimens used may not take into account all possible anatomic variations.
In summary, we report a new technique for performing ultrasound–anatomic correlations consisting of dissection during ultrasound. This is a simple method that does not require sophisticated material and that can be easily performed. The correlations with ultrasound should preferably be done with embalmed specimens rather than non-embalmed specimens, because of the presence of artefacts in the latter (gas deposits). The described methodology can yield excellent results regarding precise identification of different interfaces and structures as observed at ultrasound.
References
Hodler J, Trudell D, Kang HS, Kjellin I, Resnick D (1992) Inexpensive technique for performing magnetic resonance-pathologic correlation in cadavers. Invest Radiol 27:323–325
De Maeseneer M, De Wilde V, Gosselin R, Osteaux M (2003) The use of phantoms and tissue simulating test objects in the evaluation of imaging methods. JBR-BTR 86:3–5
Rauschning W (1983) Computed tomography and cryomicrotomy of lumbar spine specimens: a new technique for multiplanar anatomic correlation. Spine 8:170–180
De Maeseneer M, Lenchik L, Starok M, Pedowitz R, Trudell D, Resnick D (1998) Normal and abnormal medial meniscocapsular structures: MR imaging and sonography in cadavers. AJR Am J Roentgenol 171:969–976
Trappeniers L, De Maeseneer M, Van Roy P, Chaskis C, Osteaux M (2003) Peroneal nerve injury in three patients with knee trauma: MR imaging and correlation with anatomic findings in volunteers and anatomic specimens. Eur Radiol 13:1722–1727
Hauger O, Chung CB, Lektrakul N, Botte MJ, Trudell D, Boutin RD, Resnick D (2000) Pulley system in the fingers: normal anatomy and simulated lesions in cadavers at MR imaging, CT and US with and without contrast material distension of the tendon sheath. Radiology 217:201–212
Landsmeer JM (ed) (1976) Atlas of anatomy of the hand. Churchill Livingstone, Edinburgh, London, New York, pp 179–295
Acknowledgements
The work was made possible by financial support from the Prof. Dr A.L. Baert Prize 2002 (KUL, Leuven, Belgium).
We thank Eric Barbaix from the Department of Anatomy, Jan Pieter Clarijs from the Department of Experimental Anatomy, and Paul Beeckman from the Department of Radiology, Tielt, Belgium, for their intellectual contributions to our musculoskeletal research group.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Maeseneer, M., Jager, T., Vanderdood, K. et al. Ultrasound during dissection of cadaveric specimens: a new method for obtaining ultrasound–anatomic correlations in musculoskeletal radiology. Eur Radiol 14, 870–874 (2004). https://doi.org/10.1007/s00330-003-2216-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-003-2216-x