Abstract
Climate change has exacerbated the frequency and severity of heat waves, which on occasion lead to mass mortalities. Here, we report a massive mortality event in Imperial Cormorant Leucocarbo atriceps chicks that took place during December 2016 at Punta León, one of the two largest colonies (> 6000 pairs) and the northernmost colony for the species in coastal Patagonia, Argentina. During a 2-day period, we estimate that approximately 86.5% of the chicks died. Our results suggest that the mortality event was heat-related, as consequence of an intense heat wave during the brooding period. During two consecutive days, chicks between 12 and 19 days old were exposed to air temperatures above the historical mean of maxima for a total of 25 h. On one of these days, the air temperature reached a maximum of 38.1 °C with records above 35 °C sustained during four consecutive hours. Chicks were found dead throughout the colony, mostly in the nests with no evidence of external injuries other than occasional scavenging by seagulls. Acute mortality from disease was ruled out based on clinical presentation and negative results for avian influenza virus, saxitoxins, and domoic acid (two common marine toxins). Our work underscores the importance of long-term studies in understanding heat associated breeding failure of one of the largest Imperial Cormorant colonies along its breeding range in coastal Patagonia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A mean increase in air temperature of almost 2.0 °C by the 2040s and 3.0 °C by the 2080s is predicted because of contemporary global change (IPCC 2014, 2021). These upward shifts can be particularly challenging for the thermoregulatory abilities of endotherms (i.e., birds and mammals) at their upper thermal limits (Pörtner and Farrell 2008; Boyles et al. 2011). Rising maximum environmental temperatures might impose direct physiological constraints (e.g., dehydration and hyperthermia) upon endothermic species much more rapidly than lagged indirect climatic effects through biotic interactions (e.g., competition and resource availability).
The effects of global warming on animals have been a focus of research for over four decades (Parmesan 2006; Paleczny et al. 2015; Sydeman et al. 2015; Urban 2015). To date, most studies on endothermic species have analyzed the indirect effects of climate as the drivers of distribution, population, phenology, and behavioral-level changes (Crick 2004; Berteaux et al. 2006; Chen et al. 2011; Grémillet and Boulinier 2009; Ovaskainen et al. 2013; Keogan et al. 2018; Jenouvrier et al. 2018; Descamps et al. 2019; Somveille et al. 2020; Osborne et al. 2020) while the direct physiological impacts of climatic change appear underrepresented in the published literature. However, recent studies on land bird species clearly deal with how heat affects bird’s physiology and showed that high temperatures increase the demand for evaporative heat dissipation to maintain physiologically stable body temperatures and avoid lethal thermal limits (Albright et al. 2017; Conradie et al. 2019). It´s clear that heat-related massive bird mortality can arise from lethal hyperthermia if environmental temperature exceeds their heat tolerance and/or lethal dehydration if water demands for evaporative cooling exceed their dehydration tolerance limits (Albright et al. 2017). Moreover, body mass is one of the most important factors affecting bird–environment interactions, with smaller species showing higher mass-specific rates of evaporative water loss and hence greater relative water demands (McKechnie and Wolf 2010). Small birds likely exceed their thermoregulatory capacity to maintain physiologically stable water balance and/or body temperature and thus encounter potentially lethal conditions much more frequently, over shorter daily intervals (Albright et al. 2017).
Seabirds, in particular, may be expected to face significant thermoregulatory challenges during breeding. They often breed in exposed locations, such as cliffs, rocky promontories, or flat and open terrains (Furness and Monaghan 1989) with little protection from climatological factors such as sun, wind and rain, forcing them to rapidly exchange heat (both gain and loss) (see Cook et al. 2020). Cormorant adult breeders for example, usually spend a considerable proportion of their time gular fluttering as a thermoregulatory response to air temperature (Bartholomew et al. 1968). Complementary behavioral adjustments to heat while breeding include, among others, postural alterations such as crouching or standing, which increase the vulnerability of eggs and chicks to suboptimal air temperatures and predation risks (Cook et al. 2020). Thus, postural adjustments (i.e., crouching or standing) by adults may incur thermoregulatory costs to chicks given that small chicks are unable to adjust their upright posture to dissipate heat and may thus be exposed to warmer temperatures leading them to dehydration and hyperthermia (Lasiewski and Snyder 1969).
At the same time, diving seabirds like penguins and cormorants deal with an additional challenge: water has very high specific heat and thermal conductivity, so they need efficient insulation to limit heat loss while foraging in the ocean (Grémillet et al. 2005). Consequently, adaptations to limit heat loss during foraging, or rapidly gain heat following dives, may be in direct evolutionary conflict with adaptations to dissipate excess heat or avoid heat gain while attending the nest (Oswald and Arnold 2012). Cormorant species that breed at high latitudes have both morphological and physiological adaptations to low ambient temperatures, including heavy insulating dark plumage and high basal metabolic rates (Gabrielsen et al. 1988; Bryant and Furness 1995). Moreover, the fact that they have to avoid hyperthermia while breeding under warmer climate conditions, but at the same time minimize heat loss while diving in ≤ 10–15 ºC waters, generates a particular thermoregulatory challenge. While their predominantly black plumage may be beneficial for rapid heat gain following dives, it also generates great heat loads from solar radiation during nest attendance (Cook et al. 2012).
The Imperial Cormorant, Leucocarbo atriceps, is a colony-breeder that belongs to the southerly distributed blue-eyed shag group of the Phalacrocoracidae family (Nelson 2005). In the Patagonian coast of Argentina, more than 50,000 Imperial Cormorant pairs reproduce in 57 colonies ranging from Punta León (43° 05′ S, 64° 30′ W) in the north, to the Beagle Channel (55° 04′ S, 66° 33′ W) in the south (Yorio et al. 1999, 2020; Frere et al. 2005). Colonies range in size from dozens to more than 6,000 breeding pairs (Frere et al. 2005; Yorio et al. 2020). Punta León, located in Chubut Province, is one of the two largest colonies (> 6000 pairs) and the northernmost for the species (Frere et al. 2005; Quintana et al. in press). This colony extends over a flat de-vegetated terrain of sedimentary substrate (Yorio et al. 1998) highly exposed to environmental conditions. At Punta León, Imperial cormorants lay up to three eggs (mean = 2.7 ± 0.5) between early October/middle November (Yorio et al. 1994; Svagelj and Quintana 2011), both parents incubate the eggs for 28–29 days and feed the chicks for approximately 3 months. The brooding period occurs between late November and middle December (Svagelj and Quintana 2011).
The present study arose from the observation of a massive chick mortality event affecting Imperial cormorants at Punta León during the 2016 breeding season. This breeding population has been systematically monitored since 1980 (Malacalza 1984; Yorio et al. 1994; Quintana et al. in press) and has been also studied for more than three decades by Quintana and collaborators who have garnered a wealth of information on the species breeding biology, behavior and foraging patterns (see Quintana et al. in press for a review). Indeed, such long-term research effort provides an important baseline against which potential effects of environmental/climatological changes may be identified.
We hypothesized that the massive chick mortality event of 2016 could have been a consequence of an intense heat wave during the brooding period. To address this question, we analyzed the following: (a) a long-term data set of the breeding success (2004–2016), (b) long-term air temperature records for the area (2004–2016), (c) the amount of time 12 to 19 days old chicks were exposed to temperatures above the historical mean of maximum daily temperature during every season, and (d) chick carcasses for sign of disease or other acute mortality factors. The implications of extreme high environmental temperatures associated breeding failure in the face of increasing global change are discussed.
Materials and methods
Study site and nest monitoring
Fieldwork was conducted at Punta León colony (43° 05′ S, 64° 30′ W), Chubut, Argentina (Fig. 1) over 11 breeding seasons (2004–2006, 2008, 2010–2016) between early November and mid-December. The breeding phenology and reproductive success of Imperial cormorants from this colony was monitored annually since 2004 (except for 2007 and 2009). Each year, between 87 and 300 nests were visited every 3 to 5 days from shortly after the start of the laying period until chicks reached 30 days of age. On each visit the number of eggs or the number and age of chicks in each nest were recorded. Annual breeding success was estimated assuming that chicks had fledged if they reached 30 days of age due to the high probability of chick survival to independence at that age (Svagelj and Quintana 2011). Following the massive chick mortality event, during December 2016, the colony was still checked for 2 months to determine if adult cormorants initiated another breeding attempt.
Climate data
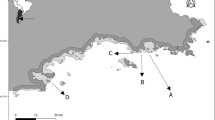
Atmospheric data were obtained by the Estación de Fotobiología de Playa Union (EFPU) (http://efpu.org.ar/) (43º 18′ S, 65º 02′ W) which is located on the coast of Chubut, 55 km south of Punta León (Fig. 1). Air temperature (°C), wind speed (m/s), and direction were measured continuously using a meteorological station (Tecmes, Pegasus), and a two-minute average was recorded automatically. From these data, the average, minimum, and maximum daily air temperature values were obtained.
During the massive mortality event of 2016, most chicks were between 12 and 19 days old. We thus calculated the maximum/minimum average air temperature for a given chick age (12 to 19 days old) to derive the historical mean of maximum/minimum for a period of 11 seasons (i.e., period 2004–2016 excepting 2007 and 2009) at each particular chick age. Additionally, we analyzed the number of consecutive hours that chicks between 12 and 19 days old were exposed to air temperatures below the historical mean of minima and above the historical mean of maxima.
Data analyses
To describe the variation in breeding success across years, we first calculated an overall mean using the weightedMean function from the R package matrixStats (Bengtsson 2017). The latter was decided because the number of nests checked differed between years and we wanted each year’s mean to contribute similarly to the overall value. After this, for each year, we calculated its deviation from the overall mean, by using a one-way analysis of variance (ANOVA) from the aov function of the stats R package (R Core Team 2019).
To explore a potential trend in mean and maximum daily air temperatures during December of the 11 studied years, we performed a Mann–Kendall Trend Test using the MannKendall function from the Kendall R package (McLeod 2011). Finally, differences between years in the daily maximum/minimum air temperature to which chicks between 12 and 19 days old were exposed were tested using an ANOVA test using the aov function from the stats R package. All statistical analyses were performed with a level of significance of p < 0.05.
Post-mortem examinations
We visited the colony the day following the discovery of the mortality event with the intention of performing post-mortem examinations and investigate the cause of death. Unfortunately, due to the elapsed time and extreme weather, the carcasses were in an advanced state of decomposition, preventing recovery of quality diagnostic samples. Notwithstanding, carcasses were examined for external injuries and signs of disease, and some of the freshest were collected for necropsy. Field observations including location and condition of carcasses, clusters, signs of stampede or predation, and so on were recorded for context. Five chick carcasses were recovered for necropsy on December 21, and four additional ones were sampled after the mortality event during follow-up visits on December 24 and January 14, 2017. The post 21 December dead chicks were fresh at the time of collection. They resembled expected chick mortality in that they were single animal deaths, in poor body condition, and the only new carcasses found during visits. We thus considered these deaths unrelated to the mass mortality event and will only focus on chicks sampled on December 21 in this study.
At necropsy, and despite decay, samples were collected from all identifiable tissues and stored both frozen and in 10% buffered formalin. In addition, oral and cloacal swabs were collected in viral transport media and stored frozen at − 80 °C. Finally, stomach content was preserved frozen. Given the condition of samples and local laboratory capacity, we limited diagnostics to two common causes of acute death in seabirds, avian influenza (Chen et al. 2006; Molini et al. 2020) and harmful algal blooms (Shumway et al. 2003). Cloacal swabs were screened for avian influenza virus by polymerase chain reaction at the Instituto de Virología of the Centro de Investigación en Ciencias Veterinarias y Agronómicas (CICVyA), Instituto Nacional de Tecnología Agropecuaria (INTA), Buenos Aires, Argentina. Samples from stomach content and tissue from intestines, stomach, liver, and kidney (only three chicks) were tested for saxitoxins and domoic acid by high-performance liquid chromatography at Instituto Nacional de Investigación y Desarrollo Pesquero (INIDEP), Mar del Plata, Argentina.
Results
Chick mortality event and breeding success across years
On our visit on December 13th, we monitored 293 nests and counted a total of 384 chicks. On the 17th, we received a report from a ranger that numerous chicks were dead at the colony. Chicks were found dead throughout the colony, mostly in the nests (Fig. 2a, b); however, there were several carcass clusters, mostly on the edge of the colony and the adjacent pebble beach (Fig. 2c, d). On our visit on the 20th, we found that only 52 (13.5%) of the 384 chicks from our monitored nests had survived. The few surviving chicks were monitored in successive visits. These chicks reached 30 days old (i.e., were assumed to have fledged) and did not present abnormalities nor clinical signs.
The mean breeding success for the whole study period was 0.93 ± 0.70 (range 0.16–1.33) chicks per nest and was extremely consistent across breeding seasons. A sharp decrease in breeding success was evident for 2016 (Fig. 3). The observed mortality event that took place between December 14th and 16th, 2016, was the only one, for chicks or adults, recorded throughout the study period.
Environmental conditions across breeding seasons
During December of 11 breeding seasons, the mean daily air temperature ranged between 10.0 and 29.6 °C (mean 19.4 °C) and no trend was detected across years (Mann Kendall test tau = − 0.01, p = 0.66). Likewise, there was no trend in mean daily maximum air temperature (Mann Kendall test tau = 0.03, p = 0.35), which ranged between 12.6 and 38.1 °C (mean 25.9 °C) over 11 breeding seasons. During the entire studied period, the warmest day was December 16th, 2016, coinciding with the time frame in which the massive chick mortality event took place (Fig. 4). On this particular day, the air temperature reached a maximum of 38.1 °C with records above 35 °C sustained during four consecutive hours. A similar extreme heat condition occurred during December 6th, 2008, with a maximum of 37.6 ºC (Fig. 4) that also persisted above 35 °C for 4 h.
During the 2016 massive mortality event, most chicks where between 12 and 19 days old. The mean daily maximum temperature to which chicks of this particular age-period were exposed was similar between seasons (F10,76 = 1.80, p = 0.08). However, the number of consecutive hours that chicks between 12 and 19 days old were exposed to temperatures above the daily historical mean of maxima for each particular age was particularly high during 2008 and 2016 (Fig. 5) Particularly, on December 6th, 2008 (the season with no massive chick mortality) most chicks were 16 days old when they were exposed to air temperatures above the historical mean of maxima for 13.5 h in a single day, with no more than 1.5 h of exposure above the historical maxima during the previous and following days (Fig. 5). In contrast, during 2016, chicks between 18 and 19 days old were exposed to air temperatures above the historical mean of maxima for a total of 25 h during two consecutive days (December 15th and 16th) (Fig. 5). Air temperatures exceeded the historical mean of maxima for 10.5 and 14.5 h on the 15th and 16th, 2016, respectively (Fig. 5). Moreover, during 2016, birds were also exposed to a contrasting climate scenario during the 2 days prior to the heat wave, with air temperature records below the historical mean of minima, for almost 30 consecutive hours (Fig. 5).
Temperature records while Imperial Cormorant, Leucocarbo atriceps, chicks were between 12 and 19 days old during 2008 and 2016. Air temperature records (orange), historical mean of maxima ± standard deviation (dark gray) and historical mean of minima ± standard deviation (light gray) for days in which Imperial Cormorant chicks were between 12 and 19 days old during a 2008 and b 2016. The number of hours that chicks of this age were exposed to temperatures below the historical mean of minima and above the historical mean of maxima, during c 2008 and d 2016 years are also shown. The light gray dotted line indicates 14 h
Post-mortem findings
All dead chicks were found with no evidence of external injuries other than occasional scavenging by seagulls. All were in an advanced state of decomposition as a result of the elapsed time and the prevailing high temperatures. No adult mortality was registered. Of the five study carcasses, two were males, one a female, and the others unconfirmed due to decomposition. Only the female was slightly less decomposed and allowed assessment of a good body condition, according to the lack of external injuries and signs of disease. All had digested remains in their stomachs. No significant macroscopic abnormalities were noted and were likely masked by severe decay. All samples were negative for avian influenza virus. Stomach and liver tissue from one of three chicks tested showed a slight trace of gonyautoxin that was too low for toxin identification by the methods used.
Discussion
Our results suggest that the Imperial Cormorant chick mortality event recorded at Punta León during 2016 season was heat-related, as consequence of an intense heat wave during the brooding period. During the 16th of December 2016, air temperature reached more than 38 °C exposing both adults and chicks Imperial cormorants to a risky thermal condition. In addition, such heat-related situation was preceded by two consecutive days with temperatures above the historical mean of maxima, with a total of 27 h over three consecutive days where adults and chicks were exposed to such extreme air temperatures. Such an extended time above upper critical temperatures can be critical for young chick survival. Small size birds may be particularly susceptible to heat waves given their typically high mass-specific rates of metabolism and water loss and will potentially have important impacts on their water balance (Albright et al. 2017). The lack of black-bulb temperature data in our study should not constitute a limitation for our results and conclusions. Cook et al. (2020) investigated the thermoregulatory responses of the Bank Cormorant, Phalacrocorax neglectus, and found that air temperature was strongly correlated with black-bulb temperature and that behavioral responses varied with air temperature in a similar manner to black-bulb.
The heat wave recorded at Punta León in the 2016 breeding season occurred during the early chick-rearing period (i.e., chicks less than three weeks old), a crucial and clearly determining moment driving the massive chick mortality event. Under such extreme environmental circumstances, evaporative cooling would not have been a viable option, forcing parents to exploit cooler environments such as the coastal waters nearby the colony at the cost of neglecting parental care. It is possible that adults moved to the coastal waters and left the small chicks unprotected from the extreme high air temperature. Imperial Cormorant chicks are altricial (Svagelj and Quintana 2011) and, in contrast to their parents, have no possibility of behavioral response (i.e., refreshing in the sea) to avoid overheating nor compensate for heat gain through behavioral adjustments and modulation of evaporative cooling by panting and gular fluttering. Moreover, at the age at which the young chicks were affected by the heat wave, their bodies were completely covered with black down which probably diminished even more their thermoregulation capacity (Hochscheid et al. 2002). The effect of a heat wave might have been much more critical at Punta León because of the features of the colony substrate: an open and flat ground-nesting area, virtually exposed to the sun with not shelter or shade availability. Moreover, the conical-shaped guano nests may have acted as a deadly trap for the young chicks who could have been further affected by the absorption of heat through long-wave radiation from their surroundings (i.e., a white guano-coated substrate). Our data highlight the growing evidence that hot weather events can directly impact animals by forcing trade-offs (mediated through both physiological and behavioral processes) between thermoregulatory demands and investment in other physiological and/or breeding functions (see Porter and Kearney 2009; Smit et al. 2016; Mitchell et al. 2018). The maximum air temperature reached on December 16th was barely below the critical upper temperature (40 °C) reported by Lasiewski and Snyder (1969) under experimental conditions for Double-crested Cormorant, Phalacrocorax auritus, 1-month-old chicks. Given that the thermoregulatory capacity of birds increases with age and body mass (Dunn 1976; Whittow and Tazawa 1991; Abraham and Evans 1990) and that chicks from Punta León were at least two weeks younger than those studied by Lasiewski and Snyder (1969), it is likely that the Imperial Cormorant chicks were exposed to air temperatures above their critical limit for thermoregulation.
Even when there is much literature considering sudden food shortage as a possible contributor to the mass mortality of chicks in seabird colonies (see Schreiber and Burger 2001), the long-term environmental stability reported for the marine areas surrounding the colony, preclude the idea of a sudden food shortage as responsible of the massive chick mortality event occurred during 2016 breeding season in Punta León. Recently, Quintana et al. (in press) reported the long-term variability of phenology, breeding traits, at-sea distribution, and foraging effort of breeding Imperial cormorants from Punta León. This study strongly suggests predictable and stable environmental conditions surrounding Punta León, enabling healthy and steady chick productivity during, at least, the last 16 years (Quintana et al., in press). Thus, even though heat events are quite rare in the Patagonian coast and die-offs due to overheating have not been reported in other northern cormorant colonies, the heat wave reported in the present work is an early warning for future constraints (under global warming scenarios).
In addition to catastrophic weather events, other factors for acute mass mortalities of seabirds include a few pathogens and toxins, such as Pasteurella multocida (de Lisle et al. 1990; Crawford et al. 1992; Waller and Underhill 2007; Bodenstein et al. 2015; Jaeger et al. 2018) and saxitoxins (Shumway et al. 2003; Van Hemert et al. 2020). However, neither of these agents would selectively only affect chicks, nor rage through a 6000 plus colony in just 24–48 h. Despite our inability to properly investigate the etiology, the hyper-acute, chick-only mortality scenario at Punta León is not indicative of an infectious origin. Notwithstanding, some cormorant species are known to be highly susceptible to pasteurellosis (Crawford et al. 1992; Waller and Underhill 2007) and paramyxovirus type 1 (Kuiken 1999) and thus warrant monitoring. The Punta León event also fails to resemble a toxic event, which usually extends over several days, affects mainly adults, and often involves several species (Work et al. 1993; Shumway et al. 2003; Van Hemert et al. 2020). The finding of hints of gonyautoxin in one cormorant chick is not unexpected, since toxin-producing algal blooms are relatively common in the study area (Uhart et al. 2008; Wilson et al. 2016; D’Agostino et al. 2019) and seabirds are often exposed to toxins with no deleterious effects (Van Hemert et al. 2020). Nevertheless, harmful algal blooms are predicted to increase in coastal areas due to global warming and human influence (Gilbert et al. 2014) and should thus remain a key differential diagnostic when marine wildlife mortality events occur.
Finally, considering that during 2016 breeding season, the number of active nests at Punta León was 5617 (Yorio et al. 2020), a simple extrapolation of the monitored number of chicks per nest right before and after the heat wave to the whole colony, allowed us to estimate a reduction in the number of chicks from 7358 to 997 (86.5%). We note that our monitored nests were localized near the edge of the colony where the absorption of heat through long-wave radiation from their surroundings (i.e., a white guano-coated substrate) was probably less that at the center of the colony. Thus, our simple extrapolation of chick mortality could be underestimated. With sufficient resources, Imperial cormorants may be capable of recovering from occasional catastrophic reproductive failures. However, it is unknown how resilient this species may be if events such as the one reported here are recurrent, more prolonged, and widespread, or if their food source is also affected. Recent seabird mass mortalities associated with food shortage from sustained heat waves such as the one in the northeast Pacific in 2014–2016 (Jones et al. 2018; Piatt et al. 2020) do not bode well for a future with increasing sea temperatures. This report provides early warning and valuable context to inform conservation efforts in the medium term in northern coastal Patagonia.
Availability of data and material
Data are available on request to the corresponding author.
Code availability
(software application or custom code) not applicable.
References
Abraham CL, Evans RM (1990) The development of endothermy in American white pelicans. Condor 101:832–841
Albright TP, Mutiibwa D, Gerson AR, Smith EK, Talbot WA, O’Neill JJ, McKechnie AE, Wolf BO (2017) Mapping evaporative water loss in desert passerines reveals an expanding threat of lethal dehydration. Proc Natl Acad Sci 114:2283–2288
Bartholomew GA, Lasiewski RC, Crawford EC (1968) Patterns of panting and gular flutter in cormorants, pelicans, owls, and doves. Condor 70:31–34
Bengtsson H (2017) matrixStats: functions that apply to rows and columns of matrices (and to vectors). R package version 0.52.2. https://github.com/HenrikBengtsson/matrixStats
Berteaux D, Humphries MM, Krebs CJ, Lima M, McAdam AG, Pettorelli N, Réale D, Saitoh T, Tkadlec E, Weladji RB, Chr SN (2006) Constraints to projecting the effects of climate change on mammals. Clim Res 32:151–158
Bodenstein B, Beckmen K, Sheffield G, Kuletz K, Van Hemert C, Berlowski B, Shearn-Bochsler V (2015) Avian cholera causes marine bird mortality in the Bering Sea of Alaska. J Wildlife Dis 51:934–937. https://doi.org/10.7589/2014-12-273
Boyles JG, Seebacher F, Smit B, McKechnie AE (2011) Adaptive thermoregulation in endotherms may alter responses to climate change. Integr Comp Biol 51:676–690
Bryant DM, Furness RW (1995) Basal metabolic rates of North Atlantic seabirds. Ibis 137:219–226
Chen H, Li Y, Li Z, Shi J, Shinya K, Deng G, Qi Q, Tian G, Fan S, Zhao H, Sun Y, Kawaoka Y (2006) Properties and dissemination of H5N1 viruses isolated during an influenza outbreak in migratory waterfowl in western China. J Virol 80:5976–5983. https://doi.org/10.1128/JVI.00110-06
Chen IC, Hill JK, Ohlemüller R, Roy DB, Thomas CD (2011) Rapid range shifts of species associated with high levels of climate warming. Science 333:1024–1026
Conradie SR, Woodborne SM, Cunningham SJ, McKechnie AE (2019) Chronic, sublethal effects of high temperatures will cause severe declines in southern African arid-zone birds during the 21st century. Proc Natl Acad Sci 116:14065–14070
Cook TR, Jewel OJD, Chivell W, Bester M (2012) An albino cape cormorant Phalacrocorax capensis. Mar Ornithol 40:72–77
Cook TR, Martin R, Roberts J, Häkkinen H, Botha P, Meyer C, Sparks E, Underhill LG, Ryan P, Sherley RB (2020) Parenting in a warming world: thermoregulatory responses to heat stress in an endangered seabird. Conserv Physiol 8:coz109
Crawford RJM, Allwright DM, He CW (1992) High mortality of Cape Cormorants (Phalacrocorax capensis) off western South Africa in 1991 caused by Pasteurella multocida. Colon Waterbirds 15:236–238. https://doi.org/10.2307/1521458
Crick HQ (2004) The impact of climate change on birds. Ibis 146:48–56
D’Agostino VC, Krock B, Degrati M, Sastre V, Santinelli N, Krohn T, Hoffmeyer MS (2019) Occurrence of toxigenic microalgal species and phycotoxins accumulation in mesozooplankton in Northern Patagonian gulfs, Argentina. Environ Toxicol Chem 38:2209–2223
De Lisle GW, Stanislawek WL, Moors PJ (1990) Pasteurella multocida infections in rockhopper penguins (Eudyptes chrysocome) from Campbell Island, New Zealand. J Wildlife Dis 26:283–285
Descamps S, Ramírez F, Benjaminsen S, Anker-Nilssen T, Barrett RT, Burr Z, Christensen-Dalsgaard S, Erikstad KE, Irons DB, Lorentsen SH, Mallory ML, Roberston GJ, Reierten TK, Strøm H, Varpe Ø, Lavergne S (2019) Diverging phenological responses of Arctic seabirds to an earlier spring. Glob Change Biol 25:4081–4409
Dunn EH (1976) Development of endothermy and existence energy expenditure of nestling Double-crested Cormorants. Condor 78:350–356
Frere E, Quintana F, Gandini P (2005) Cormoranes de la costa patagónica: estado poblacional, ecología y conservación. Hornero 20:35–52
Furness RW, Monaghan P (1989) Seabird feeding ecology. In: Seabird ecology. tertiary level biology. Springer, Boston, pp 23–34. https://doi.org/10.1007/978-1-4613-2093-7_3
Gabrielsen GW, Mehlum F, Karlsen HE (1988) Thermoregulation in four species of arctic seabirds. J Comp Physiol B 157:703–708
Glibert PM, Icarus Allen J, Artioli Y, Beusen A, Bouwman L, Harle J, Holmes R, Holt J (2014) Vulnerability of coastal ecosystems to changes in harmful algal bloom distribution in response to climate change: projections based on model analysis. Glob Change Biol 20:3845–3858. https://doi.org/10.1111/gcb.12662
Grémillet D, Boulinier T (2009) Spatial ecology and conservation of seabirds facing global climate change: a review. Mar Ecol Prog Ser 391:121–137
Grémillet D, Kuntz G, Woakes AJ, Gilbert C, Robin JP, Le Maho Y, Butler PJ (2005) Year-round recordings of behavioural and physiological parameters reveal the survival strategy of a poorly insulated diving endotherm during the Arctic winter. J Exp Biol 208:4231–4241
Hochscheid S, Grémillet D, Wanless S, du Plessis MA (2002) Black and white under the South African sun: are juvenile Cape gannets heat stressed? J Therm Biol 27:325–332
IPCC (2014) Climate change 2014: synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change [Core Writing Team, R.K. Pachauri and L.A. Meyer (eds.)]. IPCC, Geneva, Switzerland
IPCC (2021) Climate change 2021: the physical science basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change [Masson-Delmotte V, Zhai P, Pirani A, Connors SL, Péan C, Berger S, Caud N, Chen Y, Goldfarb L, Gomis MI, Huang M, Leitzell K, Lonnoy E, Matthews JBR, Maycock TK, Waterfield T, Yelekçi O, Yu R, Zhou B (eds)]. Cambridge University Press (In Press)
Jaeger A, Lebarbenchon C, Bourret V, Bastien M, Lagadec E, Thiebot J-B, Boulinier T, Delord K, Barbraud C, Marteau C, Dellagi K, Tortosa P, Weimerskirch H (2018) Avian cholera outbreaks threaten seabird species on Amsterdam Island. PLoS ONE 13:e0197291. https://doi.org/10.1371/journal.pone.0197291
Jenouvrier S, Desprez M, Fay R, Barbraud C, Weimerskirch H, Delord K, Caswell H (2018) Climate change and functional traits affect population dynamics of a long-lived seabird. J Anim Ecol 87:906–920
Jones T, Parrish JK, Peterson WT, Bjorkstedt EP, Bond NA, Ballance LT, Bowes V, Hippfner JM, Burgess HK, Dolliver JE, Lindquist K, Lindsey J, Nevins HM, Roberston RR, Roletto J, Wilson L, Joyce T, Harvey J (2018) Massive mortality of a planktivorous seabird in response to a marine heatwave. Geophys Res Lett 45:3193–3202. https://doi.org/10.1002/2017GL076164
Keogan K, Daunt F, Wanless S, Phillips RA, Walling CA, Agnew P, Ainley DG, Anker-Nilssen T, Ballard G, Barret RT, Barton KJ (2018) Global phenological insensitivity to shifting ocean temperatures among seabirds. Nat Clim Change 8:313–318
Kuiken T (1999) Review of Newcastle disease in cormorants. Waterbirds 22:333–347
Lasiewski RC, Snyder GK (1969) Responses to high temperature in nestling double-crested and pelagic cormorants. Auk 86:529–540
Malacalza VE (1984) Aves Guaneras. Relevamiento de especies en tres cormoraneras continentales de la Provincia del Chubut (Argentina) (Pelecaniformes-Phalacrocoracidae). Centro Nacional Patagónico 84:1–13
McKechnie AE, Wolf BO (2010) Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol Lett 6:253–256
McKechnie AE, Rushworth IA, Myburgh F, Cunningham SJ (2021) Mortality among birds and bats during a extreme heat event in easter South Africa. Austral Ecol 46:687–691
McLeod AI (2011) Kendall: Kendall rank correlation and Mann-Kendall trend test. R package version 2.2. https://CRAN.R-project.org/package=Kendall
Mitchell D, Snelling EP, Hetem RS, Maloney SK, Strauss WM, Fuller A (2018) Revisiting concepts of thermal physiology: predicting responses of mammals to climate change. J Anim Ecol 87:956–973
Molini M, Aikukutu G, Roux JP, Kemper J, Ntahonshikira C, Marruchella G, Khaiseb S, Cattoli G, Dundon WG (2020) Avian influenza H5N8 outbreak in African Penguins (Spheniscus demersus), Namibia, 2019. J Wildlife Dis 56:214–218. https://doi.org/10.7589/2019-03-067
Nelson B (2005) Pelicans, cormorants, and their relatives. Oxford University Press, Oxford
Osborne OE, Hara PD, Whelan S, Zandbergen P, Hatch SA, Elliott KH (2020) Breeding seabirds increase foraging range in response to an extreme marine heatwave. Mar Ecol Prog Ser 646:161–173
Oswald SA, Arnold JM (2012) Direct impacts of climatic warming on heat stress in endothermic species: seabirds as bioindicators of changing thermoregulatory constraints. Integr Zool 7:121–136
Ovaskainen O, Skorokhodova S, Yakovleva M, Sukhov A, Kutenkov A, Kutenkova N, Shcherbakov A, Meyke E, Delgado MdM (2013) Community-level phenological response to climate change. Proc Natl Acad Sci 110:13434–13439
Paleczny M, Hammill E, Karpouzi V, Pauly D (2015) Population trend of the world’s monitored seabirds, 1950–2010. PLoS ONE 10:e0129342
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Piatt JF, Parrish JK, Renner HM, Schoen SK, Jones TT, Arimitsu ML, Kuletz KJ, Bodenstein B, García-Reyes M, Duerr RS et al (2020) Extreme mortality and reproductive failure of common murres resulting from the northeast Pacific marine heatwave of 2014–2016. PLoS ONE 15:e0226087. https://doi.org/10.1371/journal.pone.0226087
Porter WP, Kearney M (2009) Size, shape, and the thermal niche of endotherms. Proc Natl Acad Sci 106:19666–19672
Pörtner HO, Farrell AP (2008) Physiology and climate change. Science 322:690–692
Quintana F, Wilson RP, Prandoni N, Svagelj WS, Gómez-Laich A (In press) Long-term ecology studies in Patagonian seabirds: a review with the Imperial Cormorant as a case study. In: Helbling W, Villafañe V, Narvarte M, Gonzalez R (eds) Global change in Atlantic coastal Patagonian ecosystems: a journey through time. Springer, Berlin
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing: 2019, Vienna, Austria. https://www.r-project.org
Schreiber EA, Burger J (2001) Seabirds in the marine environment. In: Schreiber EA, Burger J (eds) Biology of marine birds. CRC Press, Washington, pp 19–34
Shumway SE, Allen SM, Boersma PD (2003) Marine birds and harmful algal blooms: sporadic victims or under-reported events? Harmful Algae 2:1–17. https://doi.org/10.1016/S1568-9883(03)00002-7
Smit B, Zietsman G, Martin RO, Cunningham SJ, McKechnie AE, Hockey PAR (2016) Behavioural responses to heat in desert birds: implications for predicting vulnerability to climate warming. Clim Change Responses 3:1–14. https://doi.org/10.1186/s40665-016-0023-2
Somveille M, Dias MP, Weimerskirch H, Davies TE (2020) Projected migrations of southern Indian Ocean albatrosses as a response to climate change. Ecography 43:1683–1691
Svagelj W, Quintana F (2011) Breeding performance of the Imperial Shag (Phalacrocorax atriceps) in relation to year, laying date and nest location. Emu 111:162–165
Sydeman WJ, Poloczanska E, Reed TE, Thompson SA (2015) Climate change and marine vertebrates. Science 350:772–777
Uhart M, Karesh W, Cook R (2008) ¿Es el Mar Patagónico un ecosistema saludable? In: Estado de Conservación del Mar Patagónico y Áreas de Influencia. Foro para la Conservación del Mar Patagónico y Áreas de Influencia. Latingráfica, Buenos Aires, pp 303–324. http://www.marpatagonico.org
Urban MC (2015) Accelerating extinction risk from climate change. Science 348:571–573
Van Hemert C, Schoen SK, Wayne Litaker R, Smith MM, Arimitsu ML, Piatt JF, Holland WC, Ransom Hardison D, Pearce JM (2020) Algal toxins in Alaskan seabirds: evaluating the role of saxitoxin and domoic acid in a large-scale die-off of Common Murres. Harmful Algae 92:101730. https://doi.org/10.1016/j.hal.2019.101730
Waller LJ, Underhill LG (2007) Management of avian cholera Pasteurella multocida outbreaks on Dyer Island, South Africa, 2002–2005. Afr J Mar Sci 29:105–111. https://doi.org/10.2989/AJMS.2007.29.1.9.74
Whittow GC, Tazawa H (1991) The early development of thermoregulation in birds. Physiol Zool 64:1371–1390
Wilson C, Sastre AV, Hoffmeyer M, Rowntree VJ, Fire SE, Santinelli NH, Diaz Ovejero S, D´AgostinoMarónDoucette VCFGJ et al (2016) Southern right whale (Eubalaena australis) calf mortality at Península Valdés, Argentina: are harmful algal blooms to blame? Mar Mamm Sci 32:423–451
Work TM, Barr B, Beale AM, Fritz L, Quilliam MA, Wright JL (1993) Epidemiology of domoic acid poisoning in brown pelicans (Pelecanus occidentalis) and Brandt’s cormorants (Phalacrocorax penicillatus) in California. J Zoo Wildl Med 24:54–62
Yorio P, Quintana F, Campagna C, Harris G (1994) Diversidad, abundancia y dinámica espacio-temporal de la colonia mixta de aves marinas en Punta León, Patagonia. Ornitol Neotrop 5:69–77
Yorio P, Frere E, Gandini P, Harris G (1998) Atlas de la distribución reproductiva y abundancia de aves marinas del litoral patagónico Argentino. Fundación Patagonia Natural and Wildlife Conservation Society
Yorio P, Frere E, Gandini P, Conway W (1999) Status and conservation of seabirds breeding in Argentina. Bird Conserv Int 9:299–314
Yorio P, Pozzi L, Herrera G, Punta G, Svagelj WS, Quintana F (2020) Population trends of Imperial Cormorants (Leucocarbo atriceps) in northern coastal Argentine Patagonia over 26 years. Emu 120:114–122
Acknowledgements
We thank Dr. Pierre Anton Pistorius, Dr. Katrin Ludynia, one anonymous referee, and the editor Dr. Dieter for their suggestions that improved the manuscript. We express our gratitude to Tinio Resnik who reported the cormorant mortality on December 17th, 2016. We also thank La Chola, Miguel, Nicolás Prandoni, and Estancia El Pedral for assistance in various aspects of this research. We acknowledge Dr. Takashi Yamamoto for the pictures taken at the colony on our visit on December 20th, 2016. We specially thank W. Helbling for helpful comments on early versions of the manuscript and N. Montoya from Instituto Nacional de Investigación y Desarrollo Pesquero (INIDEP) for her help with the toxicological analysis. Logistical and institutional support was provided by the Instituto de Biología de Organismos Marinos (IBIOMAR-CONICET) and the One Health Institute, University of California, Davis. We thank the Ministerio de Desarrollo Territorial y Sectores Productivos and the Secretaría de Turismo de la Provincia de Chubut, Argentina, for the permits to work at Punta León protected area (permit 2016: 096-SsCyAP/16).
Funding
This study was funded by grants from the National Agency for Scientific and Technological Promotion of Argentina (PICT 2013 – 1229), the National Institute of Allergy and Infectious Diseases (NIAID) Center for Research on Influenza Pathogenesis (CRIP) (contract HHSN272201400008C), and the Instituto Nacional de Tecnología Agropecuaria (INTA) (PNSA 1115052 and PNSA 1115056).
Author information
Authors and Affiliations
Contributions
FQ, AGL, and MU conceptualized the initial research question. AGL, MU, and LG collected the data. AGL completed statistical analysis with the help of FQ. MBM performed the toxicological analysis, and AR performed the virological testing. FQ wrote the original manuscript. AGL, MU, and LG contributed to reviewing and editing. FQ and MU acquired funding. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest/competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Quintana, F., Uhart, M.M., Gallo, L. et al. Heat-related massive chick mortality in an Imperial Cormorant Leucocarbo atriceps colony from Patagonia, Argentina. Polar Biol 45, 275–284 (2022). https://doi.org/10.1007/s00300-021-02982-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-021-02982-6