Abstract
Information on incubation behaviour and influencing factors is important for species whose incubation is monoparental and of conservation concern. We assessed the incubation pattern of a noteworthy endangered southern South America marine duck species, the Chubut steamerducks (Tachyeres leucocephalus), in the Northern San Jorge Gulf, Patagonia, Argentina, in their core breeding area during 2015, 2016, and 2018. We described the laying and incubation period, characterized the nest incubation strategy, and determined the influence of environmental variables on its incubation strategy considering that weather conditions influence the incubation pattern. Our results showed that female Chubut steamerducks laid eggs every two days during sunrise; incubation starts once the female has full clutch size, then she increases nest attentiveness and takes nocturnal recesses ending in the morning. We suggested that nocturnal recesses during the incubation period contribute to minimizing avian predation. Even if weather conditions are one of the most important factors influencing the breeding behaviour of birds, we did not detect an environmental temperature and wind speed effect on the Chubut steamerducks’ breeding behaviour. This is the first description of Chubut steamerducks’ incubation rhythm, and we also present quantified measures of the incubation pattern. Our research provides valuable baseline information and contributes to increase knowledge on previously unknown life history features of this flightless, endemic, and vulnerable waterfowl species in the core of its narrow distribution range.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The reproduction of birds is divided into four well-distinguished phases: nest building, egg production, incubation, and care of nestlings (Nilsson et al. 2008; Mainwaring and Hartley 2013). The last phase is the most expensive in terms of energy expenditure (Williams 1996). However, the incubation period may be as expensive as rearing nestlings (Croston et al. 2020). This is because parents must maintain the proper physical environment for egg development while meeting their own metabolic needs (Reid et al. 2002; Tinbergen and Williams 2002) and limiting predation risk to themselves and their eggs (Afton and Paulus 1992). In species where incubation is monoparental, competing needs result in patterns of nest incubation characterized by periodic breaks in incubation during which adults leave the nest to self-maintenance activities (Croston et al. 2020).
Considering that adult incubation behaviour and the microclimate of developing eggs are closely linked, incubating adults adjust their nest attendance behaviour to mitigate a fluctuating environment and thus maintain an optimal incubation temperature (Martin et al. 2007; Coe et al. 2015). Some researchers have reported that incubation temperature affects a suite of post-hatching traits critical to the fitness of young birds (Ardia et al. 2010; Wada et al. 2015), as well as secondary sex ratios (Eiby et al. 2008; DuRant et al. 2016). These findings suggest that the incubation period is central to ecological processes and should be considered a critical stage in avian conservation efforts (Cooper et al. 2005; Martin 2008; DuRant et al. 2013, 2019). The information on nest attendance and factors that shape the incubation pattern are important as well because a real understanding of avian reproductive strategies will additionally contribute to avian conservation efforts.
Steamerducks (Tachyeres spp.) are large diving ducks limited in distribution to southern South America. Four species are currently recognized (Weller 1976), including three flightless species, the Chubut steamerduck (Tachyeres leucocephalus), the magellanic steamerduck (T. pteneres), and the Falkland steamerduck (T. brachypterus), and one flying species, the flying steamerduck (T. patachonicus). Flying steamerducks breed in both freshwater and marine habitats, whereas the three flightless species are strictly marine throughout their annual cycle.
Chubut steamerducks are endemic to a limited section of the coast of Chubut Province, Patagonia, Argentina, with an estimated total population of 3500 adults and a minimum of 2000 juveniles (Agüero et al. 2012). Owing to the combination of its restricted distribution, small population size, flightlessness, and the potential threats to which it is exposed, this species has been recently listed as “Vulnerable” on the IUCN Red List of Threatened Species (IUCN 2022). The Interjurisdictional Marine Park in San Jorge Gulf contains about 46% of the entire population of Chubut steamerducks (Agüero et al. 2012) and may provide some protection from human disturbance and habitat destruction within its jurisdiction. However, there are other potential threats to Chubut steamerducks that marine park designation does not protect against, such as oil spills and the introduction of invasive species.
During the last decade, there were some reports on the basic breeding ecology of Chubut steamerducks. For instance, Humphrey and Livezey (1985) made the first description of the nest, eggs, and downy young of Chubut steamerducks, and Agüero et al. (2010) described the general breeding habitat and determined, for the first time, the environmental features that this species selected for nesting within the main breeding area. Whereas Svagelj et al. (2012) quantified the variation in egg size at the species level, Agüero and García Borboroglu (2013) contributed information on aspects of behaviour and breeding phenology of this species (egg laying, incubation, hatching and fledging), highlighting that only females incubate, and males patrol the territory in shallow water in front of the nest.
In this study, we examined the breeding strategies of Chubut steamerducks nesting in northern San Jorge Gulf, Patagonia, Argentina. Two objectives were pursued. First, we described breeding behaviour in terms of incubation constancy, frequency, and length of incubation breaks. Secondly, we examined the influence of environmental variables on incubation strategy, specifically ambient temperature and wind speed as weather conditions influencing breeding behaviour.
Materials and methods
Study area
This study was conducted in the core of the Chubut steamerduck’s (T. leucocephalus) distribution range, particularly on nine islands within the Interjurisdictional Marine Park in Northern San Jorge Gulf, Patagonia, Argentina (132,124 ha, Fig. 1) (45° 2′ 6.41″ S–65° 51′ 50.55″ W). This marine protected area is characterized by the presence of multiple islands, sheltered bays, inlets with shallow water and a coastline with sandy and rocky/gravel bottom intertidal zones (Yorio 2001). The climate is temperate and semiarid, with an average annual temperature of 13 °C and an average annual precipitation of 200 mm. The predominant winds are from the southwest with wind speeds averaging 45 km/h but gusting up to 140 km/h (Camacho 1979).
Field nest monitoring
Data were collected during three breeding seasons from September to December (2015, 2016, and 2018). Each island was surveyed by walking transects that covered the whole area following the methods described in Agüero et al. (2010). Eggs are incubated only by females, whereas males were found invariably between the nest and the sea or swimming in shallow water in front of the nest (Agüero and García Borboroglu 2013). The nest-searching method exploited female behaviour during incubation. When humans approach to a nest, females tend to leave it very conspicuously, giving clues about the location of the nest.
To monitor the temperature inside each nest, we used temperature data loggers (HOBO Pendant UA-001-64), which record and store temperature data at intervals set by us. Temperature data loggers were placed in 34 nests (11 in 2015, 12 in 2016, and 11 in 2018) with a maximum of two eggs at the time of the finding.
Data loggers were placed inside a hollow “dummy” egg made with clay (hereafter “logger”) (Ketru Clay Lab). This material was chosen because it is a natural, cheap, and abundant heat-conductive material that turns hard, strong, and waterproof. A monofilament line was attached to each logger and sent through the bottom or side of the nest cup, taking care to avoid damaging the nest structure. The end of the monofilament was secured to the surrounding vegetation anchor points (branches), keeping the logger flush inside the nest (among the real eggs) to discourage the female from ejecting it. Loggers were set to record the temperature every five minutes; this interval represented the best trade-off between resolution and the memory capacity of the loggers with minimal disturbance to nesting ducks. In this way, the data were downloaded in the field every 10 days using a portable computer, minimizing human disturbance of the incubating female. On every visit, we recorded the presence of females in the nests, the number of eggs laid, date, and time. After each data download, the loggers were reset for a new recording period.
Weather data
As it was not possible to access the weather in the microhabitat via the sensor in the individual nests, data on weather conditions were obtained from the ERA5 atmospheric reanalysis models in the study region for the sampling period (Hersbach et al. 2020) and used for incRscan calibration (see “Measurements of the incubation pattern” section) and subsequently to score laying and incubation periods. Data on weather conditions (daily temperature and wind speed) used to build GLMMs were downloaded from http://www.smn.gob.ar.
Measurements of the incubation pattern
Before data analysis, the whole data set was split into two subsets: before the last egg laid (“laying period”), and after the last egg laid (“incubation period”). Through the HOBO software, we graphically visualized nest temperature against time, and using the fluctuations in temperature it was possible to determine when a female left or returned to the nest (Fig. 2).
An example of temperature (°C)–time data set for a single monitored Chubut steamerduck’ nest during 3 days of 2018 on Vernaci Sudoeste Island, Northern San Jorge Gulf. Off-bouts (black circles) and On-bouts (grey circles) were quantified using incRscore function. The continuous line corresponds to environmental temperature (°C). Time is expressed as decimal time
To handle the big data set of nest temperature, we used the R package incR, which takes advantage of nest temperature fluctuations to calculate the presence or absence of the female in the nest.
Following Capilla-Lasheras (2018), before running incR, we calibrated incRscan to find the optimal values of its main arguments (upper.time, lower.time, sensitivity, temp.diff, temp.diff.threshold and maxNightVariation) (see Capilla-Lasheras 2018 for more details). Therefore, we simultaneously deployed infrared camera traps (Bushnell Prime 24 Mpx) at five extra nests with loggers (see “Field nest monitoring” section). Cameras were set to trigger by motion. These data were collected during the breeding season of 2015 on Patria Island, northern San Jorge Gulf (Fig. 1).
We built a matrix containing a combination of values of the most important user-defined arguments of the incRscan function (see Capilla-Lasheras 2018 for more details) and ran it for every argument combination. Then, we compared incRscan-based incubation scores with video-based incubation scores and computed the percentage of agreement between them. In this way, the combination of parameters with the highest percentage of agreement was used to run incRscan on the big data set.
Finally, we extracted biologically relevant metrics for laying period [eggs laid by day, number of times spent continuously on the nest (“on-bouts” henceforth) and their duration, percentage of time spent by the female on the nest during a 24 h period (“attentiveness” henceforth)], and for incubation periods [daily number of periods spent by adults away from the nest (“off-bouts” henceforth), their duration and attentiveness]. These last variables were used to correlate with our set of predictors to determine the influence of environmental features.
Statistical analysis
We evaluated the relationship between incubation behaviours (off-bout number, off-bout duration, and nest attentiveness during the incubation period as response variables) and predictor variables (year, daily minimum temperature, daily maximum wind speed, incubation day, and clutch size) using Generalized Linear Mixed Models (GLMMs). These models account for a lack of independence between repeated observations of the same nest throughout the incubation period (Zuur et al. 2009). Therefore, we included “Nest ID” as a random effect in all models for each of the response variables. All models were validated through the residual analysis, and we calculated the proportion of total variance explained only by fixed effects and by the entire model (both fixed and random effects).
The distribution of the off-bout number is compatible with a negative binomial, while gamma and beta distributions were appropriate for off-bout duration and nest attentiveness, respectively. In all models, we used a log link function; the clutch size variable was log-transformed to be considered an offset variable, and interactions between year and daily minimum temperature, daily maximum wind speed, and incubation day were included.
We conducted all statistical analyses in R software, version 4.1.2 (incR, lme4, glmmTMB, MASS) (R Core Team 2021).
Results
We collected 9,463,744 temperature data points from 34 nests of Chubut steamerduck (T. leucocephalus) from September to December 2015, 2016, and 2018. To calibrate the main arguments of incRscan, we collected 33,656 temperature data points and 11,382 photos from five extra nests during the 2015 breeding season. We observed that the spring of 2015 and 2016 was slightly cooler and warmer, respectively, than in the other sampling years (Table 1).
Laying period
The average number of days between the beginning of nesting and the final clutch size was 7 days (3–11 days). Estimating that Chubut steamerducks lay 0.5 ± 0.3 eggs day−1 (ẋ ± SD, N = 28), the clutch size in our study averaged 5.7 ± 1.4 eggs (ẋ ± SD range = 3–9). The average attentiveness during this period was 39.0% ± 22.8 (ẋ ± SD range = 0.5–91.3%), with an on-bout frequency of 3.4 ± 2.1 times day−1 (ẋ ± SD range = 1–11). Additionally, more frequent on-bouts started between 00:00 and 01:00 AM, but longer on-bout durations (9:49 h) were between 04:00 and 06:00 AM, decreasing towards sunset (Fig. 3). Likewise, females kept an average nest temperature of 26.7 °C ± 6.9 (ẋ ± SD range = 11–36).
A Distribution of nest sessions and recesses exhibited by Chubut steamerducks across 24 h-interval, during laying (dark grey) and incubation period (light grey) on Northern San Jorge Gulf from 2015, 2016, and 2018 breeding season. B The mean duration of nest sessions and recesses across 24 h-interval during laying (dark grey) and incubation period (light grey)
Incubation period
After the final egg was laid, females increased nest attentiveness to 79% ± 19 (2–100%), with an average off-bout frequency of 3.4 ± 3 times day−1 (range = 0–18). Moreover, 29.5% of off-bouts occurred more frequently during the night (9:00 PM–02:00 AM), almost constantly after sunrise (Fig. 3).
Among off-bouts happening often, the longest (03:21 h) was between 00:00 and 02:00 AM decreasing to a minimum of 41 min at sunrise (05:00–09:00 AM) (Fig. 3). This pattern was coincident with the on-bout start time, showing the females came into the nest at sunrise (Fig. 3). The incubation period lasts ~ 34 days ± 6 days (range = 21–44), and incubating females warmed eggs to 32.9 °C ± 3.4 °C across daily on-bouts.
Environmental effects on the incubation strategy
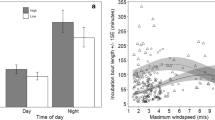
For the off-bout number model, explanatory variables only explain 8.76% of this response variable variation. After the nest random effect was included, the fit improved, explaining 72.38% of the response variable variation. Predicted off-bout frequency showed some tendencies of change through each explanatory variable. However, β coefficient estimates and their confidence intervals indicate that the apparent effects of these variables were without significant biological meaning (Fig. 4). Models for the off-bout duration and nest attentiveness showed poor fit (R2 < 50%).
Discussion
The Chubut steamerduck (T. leucocephalus) is not a colonial species and egg laying is asynchronous, occurring from September to February with the bulk of eggs laid in the middle of October and early November (Agüero and García Borboroglu 2013).
Females showed low nest attentiveness during the laying period but increased attentiveness after the last egg was laid. Eggs were holding above physiological zero for embryonic development during this period (25 °C and 27 °C) (Batt et al. 1992; Decuypere and Michels 1992). In this way, as in other duck species, partial incubation occurs before clutch completion, delaying the onset of full incubation when the final clutch size is reached (Batt et al. 1992; Watson et al. 1993; Wang and Beissenger 2011). In general, partial incubation is important to maintain the viability of earlier-laid eggs by initiating embryo development because waterfowl eggs lose viability under ambient conditions (Arnold et al. 1987). From our results, according to the time of the day when longer on-bouts occur, we discovered that female Chubut steamerducks laid eggs during sunrise. Once the female had a full clutch, she increased nest attentiveness and took nocturnal recesses that ended in the morning. Although some authors stated that dusk recess is uncommon for ducks (Batt et al. 1992), this pattern has also been reported for other wild species such as mallards (Anas platyrhynchos) (Afton and Paulus 1992; Legagneux et al. 2011), common pochards (Aythya ferina) (Legagneux et al. 2011) and northern shovelers (Anas clypeata) (Afton 1980). Chubut steamerduck’s nocturnal off-bouts may function to minimize predation by eyesight predators that need daylight to forage (Legagneux et al. 2011; Bueno-Encino et al. 2017). According to Agüero et al. (2010), Chubut steamerducks were found nesting on islands and islets in sympatry and syntropy with other species that were observed stealing eggs of this duck species as kelp gulls (Larus dominicanus) and southern caracara (Caracara plancus) (Yorio 2001; Formoso et al. 2019). In that sense, adding to the fact that Chubut steamerducks select nest sites with high vegetation cover to better protect from avian predators (Agüero et al. 2010), nocturnal recesses during the incubation period probably contribute to minimise avian predation.
Weather conditions can influence the breeding behaviour of birds (Amininasab et al. 2016; Carrol et al. 2018). Therefore, we examined the importance of ambient temperature and wind speed on breeding behaviour. However, our results showed that environmental measured variables do not affect recess frequency, duration, or nest attentiveness of incubating Chubut steamerducks.
It is known that environmental variables such as temperature and wind influence the cooling rate of eggs (Webb 1987; Turner 2002) and thus, the energy that a female has to expend to rewarm and maintain the optimal temperature of the eggs (Haftorn and Reinertsen 1985; Reid et al. 1999; Tinbergen and Williams 2002; Cresswell et al. 2004). However, notwithstanding that 2015 was a cooler spring than the other sampling years (Hersbach et al. 2020) incubating females of Chubut steamerducks did not show significant behavioural adjustments. As in many duck species (Drever and Clark 2007; McClintock et al. 2014; Grimaudo et al. 2020) Chubut steamerducks increase the amount of down as laying and incubation period progress, minimizing the cooling rate during night recesses. In this respect, the effects of environmental measured variables, particularly the cooler spring of 2015, on incubation strategy would be masked by the thermal effect of down material (McClintock et al. 2014).
Nonetheless, our results pointed out that further studies and access to larger sample sizes are needed to ascertain which and how environmental conditions (i.e. habitat features, wind direction, night temperature) in combination with intrinsic factors (i.e. body mass, female age, hormonal status) could potentially impact nest attendance strategies to better understand what drives incubation behaviour.
This study is the first to describe Chubut steamerducks’ incubation rhythm and present quantified measures of the incubation pattern. Our research provides valuable baseline information and contributes to increase knowledge on previously unknown life history features of this flightless, endemic, vulnerable waterfowl species at the core of its narrow distribution range, which could be used for future conservation purposes.
Data availability
The data supporting this study's findings are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Afton AD (1980) Factors affecting incubation rhythms of Northern Shovelers. Condor 82:132–137
Afton AD, Paulus SL (1992) Incubation and brood care. In: Batt BDJ, Afton AD, Anderson MG, Ankney CD, Johnson DH, Kadlec JA, Krapu GL (eds) Ecology and management of breeding waterfowl. University of Minnesota Press, Minneapolis, pp 62–108
Agüero ML, García Borboroglu P (2013) Breeding biology of the Chubut Steamerduck (Tachyeres leucocephalus). Ornitol Neotrop 24:85–93
Agüero ML, García Borboroglu P, Esler D (2010) Nesting habitat of Chubut Steamerducks in Patagonia, Argentina. Emu 110:302–306
Agüero ML, García Borboroglu P, Esler D (2012) Distribution and abundance of Chubut Steamerducks: an endemic species to Central Patagonia, Argentina. Bird Conserv Int 22:307–315
Amininasab SM, Kingma SA, Birker M et al (2016) The effect of ambient temperature, habitat quality and individual age on incubation behaviour and incubation feeding in a socially monogamous songbird. Behav Ecol Sociobiol 70:1591–1600
Ardia DR, Perez JH, Clotfelter ED (2010) Experimental cooling during incubation leads to reduced innate immunity and body condition in nestling tree swallows. Biol Sci 277:1881–1888. https://doi.org/10.1098/rspb.2009.2138
Arnold TW, Rohwer FC, Armstrong T (1987) Egg viability, nest predation, and the adaptive significance of clutch size in prairie ducks. Am Nat 130:643–653
Batt RJ, Afton BD, Anderson AD, Ankney MG, Johnson CD, Kadlec DJA, Krapu GL (eds) (1992) The ecology and management of breeding waterfowl. University of Minnesota Press, Minneapolis
Bueno-Enciso J, Barrientos R, Sanz JJ (2017) Incubation behaviour of blue Cyanistes caeruleus and great tits Parus major in a Mediterranean habitat. Acta Ornithol 52:21–34
Camacho H (1979) Descripción geológica de la hoja 47h48g, Bahía Camarones, Provincia de Chubut. Boletín N° 153. Servicio Geológico Nacional, Buenos Aires
Capilla-Lasheras P (2018) incR: a new R package to analyse incubation behaviour. J Avian Biol. https://doi.org/10.1111/jav.01710
Carroll RL, Craig AD, Fuhlendorf SD, Elmore RD, DuRant SE, Carroll JM (2018) Avian parental behavior and nest success influenced by temperature fluctuations. J Therm Biol 74:140–148
Coe BH, Beck ML, Chin SY, Jachowski C, Hopkins WA (2015) Local variation in weather conditions influences incubation behavior and temperature in a passerine bird. J Avian Biol 46:385–394
Cooper CB, Hochachka WM, Butcher G, Dhondt AA (2005) Seasonal and latitudinal trends in clutch size: thermal constraints during laying and incubation. Ecology 86:2018–2031
Cresswell W, Holt S, Reid JM, Whitfield DP, Mellanby RJ, Norton D, Waldron S (2004) The energetic costs of egg heating constrain incubation attendance but do not determine daily energy expenditure in the pectoral sandpiper. Behav Ecol 15:498–507
Croston R, Hartman CA, Herzog MP, Casazza ML, Feldheim CL, Ackerman JT (2020) Timing, frequency, and duration of incubation recesses in dabbling ducks. Ecol Evol 10:2513–2529
Decuypere E, Michels H (1992) Incubation temperature as a management tool: a review. Worlds Poult Sci J 48:28–38. https://doi.org/10.1079/WPS19920004
Drever MC, Clark RG (2007) Spring temperature, clutch initiation date and duck nest success: a test of the mismatch hypothesis. J Anim Ecol 76:139–148. https://doi.org/10.1111/j.1365-2656.2006.01183
DuRant SE, Hopkins WA, Hepp GR, Walters JR (2013) Ecological, evolutionary, and conservation implications of incubation temperature-dependent phenotypes in birds. Biol Rev Camb Philos Soc 88:499–509
DuRant SE, Hopkins WA, Carter AW, Kirkpatrick LT, Navara KJ, Hawley DM (2016) Incubation temperature causes skewed sex ratios in a presocial bird. J Exp Biol 219:1961–1964
DuRant SE, Willson JD, Carroll RB (2019) Parental effects and climate change: will avian incubation behavior shield embryos from increasing environmental temperatures? Integr Comp Biol 59:1068–1080
Eiby YA, Wilmer JW, Booth DT (2008) Temperature-dependent sex-based embryo mortality in a bird. Proc R Soc B 275:2703–2706
Formoso AE, Agüero ML, Udrizar Sauthier D (2019) Diet of the Southern Caracara in a near-shore insular system in southern Patagonia, Argentina. J King Saud Univ Sci 31:1339–1343
Grimaudo AT, Hope SF, DuRant SE, Kennamer RA, Hallagan JJ, Hopkins WA (2020) Ambient temperature and female body condition are related to night incubation behavior in wood ducks Aix sponsa. J Avian Biol 51:1–11
Haftorn S, Reinertsen RE (1985) The effect of temperature and clutch size on the energetic cost of incubation in a free-living blue tit Parus caeruleus. Auk 102:470–478
Hersbach H, Bell B, Berrisford P et al (2020) The ERA5 global reanalysis. Q J Rev Meteorol Soc 146:1999–2049
Humphrey PS, Livezey BC (1985) Nest, eggs, and downy young of the White-headed Flightless Steamerduck. Ornithol Monogr 36:944–953
IUCN (2022) The IUCN Red List of Threatened Species. Version 2022-2. https://www.iucnredlist.org
Legagneux P, Emeriau S, Giraudeau M, Duval C, Caizergues A (2011) Combining field and aviary approaches to monitor incubation in ducks: importance of clutch size, body mass, and weather. Bird Study 58:421–434
Mainwaring MC, Hartley IR (2013) The energetic costs of nest building in birds. Avian Biol Res 6:12–17
Martin TE (2008) Egg size variation among tropical and temperate songbirds: an embryonic temperature hypothesis. Proc Natl Acad Sci USA 105:9268–9271
Martin TE, Auer SK, Bassar RD, Niklison AM, Lloyd P (2007) Geographic variation in avian incubation periods and parental influences on embryonic temperature. Evolution 61:2558–2569
McClintock ME, Hepp GR, Kennamer RA (2014) Plasticity of incubation behaviors helps wood ducks (Aix sponsa) maintain an optimal thermal environment for developing embryos. Auk 131:672–680
Nilsson JF, Stjernman M, Nilsson JA (2008) Experimental reduction of incubation temperature affects both nestling and adult blue tits Cyanistes caeruleus. J Avian Biol 39:553–559
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/. Accessed 4 May 2022
Reid JM, Monaghan P, Ruxton GD (1999) The effect of clutch cooling rate on starling, Sturnus vulgaris, incubation strategy. Anim Behav 58:1161–1167
Reid JM, Monaghan P, Nager RG (2002) Incubation and the costs of reproduction. In: Deeming DC (ed) Avian incubation. Behavior, environment and evolution. Oxford University Press, New York, pp 314–325
Svagelj WS, Agüero ML, García Borboroglu P (2012) Variation in the size of eggs of Chubut Steamerducks (Tachyeres leucocephalus). Emu 112:67–172
Tinbergen JM, Williams JB (2002) Energetics of incubation. In: Deeming DC (ed) Avian incubation: behavior, environment, and evolution. Oxford University Press, Oxford, pp 299–313
Turner JS (2002) Maintenance of egg temperature. In: Deeming DC (ed) Avian incubation: behavior, environment, and evolution. Oxford University Press, Oxford, pp 118–142
Wada H, Kriengwatana B, Allen N, Schmidt KL, Soma KK, Macdougall-Shackleton SA (2015) Transient and permanent effects of suboptimal incubation temperatures on growth, metabolic rate, immune function and adrenocortical responses in zebra finches. J Exp Biol 218:2847–2855
Wang JM, Beissinger SR (2011) Partial Incubation in Birds: its occurrence, function, and quantification. Auk 128:454–466
Watson MD, Robertson GJ, Cooke F (1993) Egg-laying time and laying interval in the common eider. Condor 95:869–878. https://doi.org/10.2307/1369424
Webb DR (1987) Thermal tolerance of avian embryos: a review. Condor 89:874–898
Weller MW (1976) Ecology and behaviour of steamer ducks. Wildfowl 27:45–53
Williams JB (1996) Energetics of avian incubation. In: Carey C (ed) Avian energetics and nutritional ecology. Springer, Boston, pp 375–416
Yorio P (2001) Costa Argentina (Patagonia). In: Canevari P, Davidson I, Blanco D, Castro G, Bucher E (eds) Los humedales de América del Sur: una agenda para la conservación de la biodiversidad y las políticas de desarrollo. Wetlands International, Buenos Aires, pp 34–43
Zuur A, Ieno E, Walker N, Saveliev A, Graham M (2009) Mixed effects models and extensions in ecology with R. Springer, New York, pp 101–142
Acknowledgements
This research was funded by Rufford Small Grant Foundation (N° Ref. 17689-1) and National Council Research (PICT-2012-1399). We thank CESIMAR-CENPAT (CONICET) for institutional support; Soriano S.A. and Abril family. Permits for the work were issued by the Provincial Wildlife Bureau (Dirección de Fauna y Flora Silvestre and Secretaría de Turismo de la Provincia de Chubut) and the National Park authorities. We are grateful to the rangers of Interjurisdictional Marine Park, Camarones, Chubut for field assistance, Patricia Flischman for lab support, and Oscar Frumento for meteorological data analysis.
Author information
Authors and Affiliations
Contributions
MLA developed the idea and design. VD supervised all work. Field design, material preparation, and fieldwork were performed by MLA and AA. EB contributed to the data collection. Statistical analysis was done by EL and GM. The first draft of the manuscript was written by MLA and all authors commented on the versions of the manuscript. All authors read and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
Dra. María Laura Agüero and Dra. Verónica D’Amico are researchers at CESIMAR-National Council Research of Argentina (CONICET). Dra. Eliana Lorenti and Professor Graciela Minardi are professionals of CEPAVE–CONICET. Eloisa Berrier is a student at National University (Universidad Nacional de la Patagonia San Juan Bosco), Ariel Agu is a professional in electronics who has contributed to our studies for 10 years. Dra. María Laura Agüero has received financial support from the Rufford Small Grants Foundation and FONCyT- MINCyT.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Agüero, M.L., Lorenti, E., Minardi, G. et al. First description of the incubation pattern of an endemic and endangered marine duck species, the Chubut steamerduck (Tachyeres leucocephalus), and related environmental characteristics. Polar Biol 46, 1029–1037 (2023). https://doi.org/10.1007/s00300-023-03180-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-023-03180-2