Abstract
Aquatic insects are often consumed by terrestrial predators in Arctic tundra. However, this aquatic-terrestrial linkage may be disrupted by rapid warming that is causing a decrease in freshwater habitats across large areas of the Arctic. In this study, we investigated emerging mosquitoes (Diptera: Culicidae) as a resource subsidy for wolf spiders (Araneae: Lycosidae) in western Greenland, an area where significant pond drying has occurred in recent decades. We used pitfall trapping to compare the abundance, size, and fecundity of wolf spiders collected near (< 1 m) versus far (75–100 m) from the margins of three tundra ponds before, during, and after mosquito emergence. Nearly 90% of the wolf spiders collected in our study were Pardosa glacialis, the species that subsequently became the focus of our analyses. P. glacialis abundances, sizes, and the proportion of females with an egg sac were similar throughout the season both near and far from ponds. However, females near ponds produced about 20% more eggs per egg sac. Stable isotope analyses and a laboratory experiment confirmed mosquito consumption by P. glacialis and demonstrated that individuals collected near tundra ponds were significantly depleted in 13C relative to those in upland habitats, indicating differences in food resources among habitats. Our evidence indicates that mosquitoes do indeed serve as a subsidy to wolf spiders in western Greenland, but the demographic effects on spiders appear to be modest. Thus, P. glacialis abundance in the landscape may be relatively robust to pond drying and associated biotic and abiotic changes. Further studies will be needed to assess the broader effects for tundra ecosystems of disruptions to this and other aquatic-terrestrial linkages via the drying of ponds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Arctic is warming at over twice the rate of the global average and has already experienced major ecological changes (Post et al. 2019). Advances in the timing of snowmelt and spring are impacting the phenology of plants and invertebrates (Høye et al. 2007; Høye and Forchhammer 2008), and extended growing seasons are causing shrub cover to increase in tundra ecosystems (Sturm et al. 2005; Myers-Smith et al. 2011). Some species may benefit from these changes if they gain increased access to resources (Kerby and Post 2013) whereas others may experience population declines due to processes such as habitat change and phenological mismatch (McKinnon et al. 2012; Loboda et al. 2018). Linking these ecological processes with their direct and indirect effects on populations is critical for predicting community and ecosystem level impacts in rapidly changing Arctic systems.
One prominent effect of Arctic warming is changes to the distribution and abundance of freshwater habitats, which has the potential to disrupt aquatic-terrestrial linkages. Some areas of Arctic tundra are getting wetter (Walter Anthony et al. 2018) whereas others are becoming drier (e.g., Smith et al. 2005; Andresen and Lougheed 2015; Finger Higgens et al. 2019). Arctic freshwater ecosystems provide important water access to plants and animals during the growing season especially because summer precipitation can be low (Elberling et al. 2008). They also support the production of large amounts of insect biomass (Gratton et al. 2008). Aquatic insect prey provide important subsides to terrestrial predators (Nakano and Murakami 2001; Fausch et al. 2002; Paetzold et al. 2005; Schindler and Smits 2017), including in the Arctic where they are very abundant and can alter terrestrial food webs and ecosystem function (Gratton et al. 2008; Dreyer et al. 2012; Hoekman et al. 2012; Sanchez-Ruiz et al. 2018).
Arctic drying and an associated decrease in the abundance of emerging aquatic insects may have impacts on predator populations that rely on this aquatic subsidy. Wolf spiders (Araneae: Lycosidae) are dominant terrestrial arthropod predators in Arctic tundra (Bowden and Buddle 2010; Wyant et al. 2011; Bowden et al. 2018; Gillespie et al. 2020) that often reside in wet tundra habitats (Böcher et al. 2015; Bowden et al. 2018) and are known to consume aquatic Diptera (Wirta et al. 2015a, b; Eitzinger et al. 2019). Their growth, survival, and reproduction are being impacted by climate change in various ways. For example, longer growing seasons are permitting the production of multiple clutches per year (Høye et al. 2020) but may also expose wolf spiders to increasing rates of egg sac parasitism (Koltz et al. 2019). Climate-induced shifts in wolf spider body size (Høye et al. 2009) are also expected to affect rates of density-dependent cannibalism (Koltz and Wright 2020). Warming-induced decreases in freshwater habitat and aquatic insect prey could further impact survival, growth, and reproduction, but the effects of aquatic subsidies on Arctic wolf spider population dynamics have not been explored.
If aquatic insects are an important subsidy to wolf spiders, this may manifest in their abundances, sizes, and fecundity varying across the tundra landscape and with the timing of aquatic insect emergence. Wolf spiders could have an aggregated spatial distribution, with higher abundances near freshwater habitats. If an increase in spider abundance occurs near freshwater habitats in synchrony with the timing of aquatic insect emergence, this could reflect an ability of spiders to move across the landscape in response to a resource pulse (Morse 2002). Regardless of patterns in abundance and distribution, wolf spiders near freshwater habitats may have greater access to food resources (i.e., aquatic insects), and thus, females may reach a larger size at reproduction and produce larger clutches (Kreiter and Wise 2001; Puzin et al. 2011) compared to spiders without access to aquatic subsidies.
We tested these hypotheses using pitfall trapping to compare the abundance, size, and fecundity of wolf spiders collected near versus far from the margins of shallow tundra ponds in western Greenland. Mosquitoes emerge in large numbers from these shallow freshwater habitats once a year during a two- to four-week period, typically in early June (Culler et al. 2015). We have observed wolf spiders consuming mosquitoes at pond margins, and in general, spiders are known predators of mosquitoes throughout various stages of the mosquito lifecycle (Garcia and Schlinger 1972; Service 1973; Breene et al. 1988; Perevozkin et al. 2004; Futami et al. 2008; Becker et al. 2010, Jackson and Cross 2015). Our pitfall trapping covered the periods before, during, and after mosquito emergence, which allowed us to determine if and how wolf spiders responded to the presence of this ephemeral resource.
We paired the pitfall trapping with the use of stable isotopes and a lab experiment to confirm and quantify mosquito consumption by wolf spiders. The ratios of stable isotopes of carbon (13C:12C = δ13C) and nitrogen (15N:14N = δ15N) are an indicator of resources base and trophic position, respectively (Post 2002; Boecklen et al. 2011). Spiders that consume aquatic insect prey have δ13C values that more closely match those of aquatic versus terrestrial insects (Akamatsu et al. 2004) and predators tend to be enriched in 15N relative to their prey (Post 2002). Thus, if mosquitoes are being consumed by wolf spiders, we expected that the δ13C of wolf spiders collected near ponds, compared to spiders far from ponds, would more closely match the δ13C values of mosquitoes. In addition, we expected spiders to be enriched in 15N relative to mosquitoes. To test these predictions, we measured stable isotope ratios of field-collected wolf siders and ran a laboratory feeding experiment to determine how mosquito consumption impacted the δ13C and δ15N values of wolf spiders.
Methods
Study system
Our study was conducted in tundra near Kangerlussuaq, Greenland (Fig. 1). Freshwater lakes and ponds cover about 15% of the landscape (Heindel et al. 2015), and a recent analysis revealed that these lakes and ponds have been decreasing in size and number since the 1960s (Finger Higgens et al. 2019). Most of these habitats are fishless and instead support invertebrate assemblages consisting of crustaceans (Ostracoda, Copepoda, Branchiopoda), molluscs (Gastropoda), and insects (Coleoptera, Diptera, and Trichoptera; DeSiervo et al. 2020). Diptera, and in particular, mosquitoes (Aedes nigripes Zett; Culicidae), emerge in large numbers from a subset of the smaller and shallower freshwater habitats in Kangerlussuaq (Culler et al. 2015, 2018). Mosquito emergence occurs once a year during an approximate two- to four-week period, typically in early June (Culler et al. 2015). Wolf spiders (Lycosidae) are conspicuous in this tundra landscape, including at the margins of ponds and on the water surface, but their abundances have not been quantified.
Points represent the three study sites near Kangerlussuaq, Greenland (star on inset map of Greenland). Map data: Google Earth, Image 2018 DigitalGlobe. Modified with permission from Culler et al. (2018)
We selected three previously studied mosquito ponds, referred to as Pond 1, 1b, and 3 to match the names used in previous studies (Fig. 1; Culler et al. 2015, 2018; DeSiervo et al. 2020). These ponds represent potential areas of high aquatic insect resource subsidies for wolf spiders and were isolated from other ponds such that it was possible to compare (1) wolf spiders at the pond margin (within 1 – 2 m) that were likely to regularly encounter emerging mosquitoes (hereafter referred to as the near location) and (2) wolf spiders that were 70 – 100 m from the pond margin that would be less likely to encounter emerging mosquitoes from any pond (hereafter referred to as the far location, Figure S1, Online Resource 1). Although one study showed that wolf spiders can move more than 40 m in response to prey availability (Morse 2002), a mark-recapture study of wolf spider movement in Sweden indicated that the average distance covered by two Pardosa species during a growing season ranged from 7.9 to 45.2 m (Hallander 1967). We assumed that spiders would not be regularly moving 70–100 m among the near and far locations within the time of our sampling.
Pitfall trapping
To collect spiders for comparison of abundance, size, and fecundity near versus far from ponds, we installed pitfall traps that passively trapped spiders before, during, and after mosquito emergence. At each pond, we installed three pairs of pitfall traps within 1–2 m of the pond margin (near location) and an additional three pairs of pitfall traps approximately 70–100 m from the margin of the pond (far location, Figure S1, Online Resource 1). At the near locations, we placed three replicate pairs of two cups around the pond, maximizing the distance between pairs of cups to the best extent possible (13–50 m between pairs, depending on the pond; for spatial arrangement and coordinates see Figure S1 and Table S1 in Online Resource 1). At the far locations, we installed the three pairs of cups at least 70 m from the margin of any mosquito pond, with approximation of the spatial arrangement of the near cups, and in mixed shrub and grass vegetation that was as similar as possible to the near habitat (Figure S1, Table S1, Online Resource 1). The two cups in each pair were installed ~ 1 m apart.
Each pitfall trap was a plastic cup (5-cm radius, 10-cm depth) filled with 300 mL water and a few drops of dish soap to break the surface tension. The traps were buried so that the rim was flush with the ground such that we collected ground-dwelling organisms as they moved around the landscape. Traps were installed as soon as the ground thawed in 2018 (between 19 and 25 May), and contents were emptied every 4–7 days until 16 July (10 or 11 sampling dates from each site, Table S2, Online Resource 1). On each sampling date, the two cups within a pair were combined to produce three replicates for each near and far location. Pitfall traps are passive traps, so catches were both a function of spider abundance and spider activity, which could be related to temperature (Høye and Forchhammer 2008; Saska et al. 2013). Thus, we also deployed two temperature loggers (Hobo, Onset, Bourne, MA, USA) that recorded hourly air temperatures at ground level at each pond and location. To compare temperatures among sites, we took the average of the hourly values recorded by each of the two data loggers and then calculated an average of hourly air temperatures for the period 25 May–13 July. We also calculated thermal sums as an indicator of spider activity (Høye and Forchhammer 2008) for each site and location from 25 May to 13 July (see Table S2, Online Resource 1) and during each sampling period (see Table S3, Online Resource 1) by summing all hourly air temperatures above 0 °C and dividing the daily sums by 24. On a subset of the sampling dates, we measured soil moisture at each site and location (Delta-T HH2 moisture meter, Dynamax, Online Resource 2).
Pitfall samples were sorted in the laboratory, and each wolf spider was identified to species. Of the 2360 wolf spiders collected, nearly 90% were Pardosa glacialis (see "Results"), which became the species of focus for the rest of our study. Each individual P. glacialis was classified as either male, female, subadult (if clear male/female sexual organs were in the process of developing), or immature (if no evidence of sex organ development was present, Pickavance 2001). We measured carapace width as a metric of body size (Hagstrum 1971; Jakob et al. 1996; Bowden et al. 2013) using a dissecting microscope (Leica MZ12.5). Egg sacs within each sample (often disconnected from females) were dissected to count the number of developing eggs and spiderlings and to note parasitism by ichneumonid wasps (Gelis sp.; Koltz et al. 2019), which was indicated by the visual presence of wasp eggs, larvae, or pupae.
Data analysis
To test for patterns in abundances of P. glacialis, we fitted linear mixed models to our abundance data that included pond, location (near versus far), date (as a continuous variable), pond × location, pond × date, and location × date as fixed effects, with cup as a random effect (nested within pond × location), as possible explanatory variables (proc glimmix SAS v9.4). The model also included number of days per trapping period (4–7) and thermal sum per trapping period (Table S3, Online Resource 1; Høye and Forchhammer 2008) as covariates. We fitted models for males and females separately. Abundance data were square root transformed prior to analysis, which satisfied the assumptions of normality and homoscedasticity of residuals.
To test if spider size (carapace width) varied near versus far from ponds and in relation to mosquito emergence, we used general linear mixed models that evaluated fixed effects of pond, location, date (3 levels), and sex, with cup as a random effect (nested within pond × location; proc glm, SAS v9.4). We ran separate models for adults, subadults, and immatures. For all life stages, the model also included the two-way interaction of location × date because we were specifically interested in testing if sizes differed over time near versus far from ponds. For the adults and subadults, the model also included sex × date. The levels of date were before mosquito emergence (Sampling Periods 1–4), during mosquito emergence (Sampling Periods 5–7), and after mosquito emergence (Sampling Periods 8–11, Table S2, Online Resource 1).
We also used the trapping data to estimate the expected number of progeny produced per female at each pond and location, with consideration of the probability of producing an egg sac, the number of progeny if an egg sac was produced, and the incidence of egg sac parasitism. For each combination of pond × location, we estimated the proportion of females with eggs sacs (and its associated standard error; following Cochran 1977) as the slope of a regression (forced through the origin) of number of egg sacs versus number of female spiders across all trap samples. We restricted these analyses to the subset of later season dates at each location when most adult females were carrying egg sacs. We standardized across sites to the same phenological interval in terms of thermal sums (dates 7–10 for ponds 1 and 1b and dates 8–11 for pond 3, Table S2, Online Resource 1). We similarly estimated the proportion of egg sacs that were parasitized at each pond × location. We compared female clutch size across study locations by comparing the number of eggs plus spiderlings per egg sac with a general linear model that included pond and location (proc glm, SAS v9.4).
Stable isotopes
To determine differences in resource base and trophic position of wolf spiders, we hand collected 31 adult P. glacialis from among the near (n = 18) and far (n = 13) locations at each pond for stable isotope analysis. Each individual spider was frozen, dried, ground, and analyzed at the Stable Isotope Facility at the University of California Davis for ratios of stable isotopes of carbon (13C:12C = δ13C, per mil enrichment relative to VPDP standard, decarbonation was not performed) as an indication of resources base and nitrogen (15N:14N = δ15N, per mil 15N enrichment relative to atmospheric N2) as an indication of trophic position (Post 2002; Boecklen et al. 2011). We tested how values of δ13C and δ15N of field-collected P. glacialis varied among ponds, locations, and sex using multivariate analysis of variance (proc glm; SAS v9.4).
Functional response experiment
We conducted a functional response experiment in June 2018 to quantify consumption of emerging mosquitoes by P. glacialis and how mosquito consumption impacted δ13C and δ15N of wolf spiders. The experiment was conducted in two small refrigerators (Danby Maitre’D DWC350BLP) set to a temperature of 15 °C, the approximate local daytime air temperature during the time of the experiment. The refrigerators had glass doors that permitted natural lighting from the windows in the lab (24 h daylight) and humidity levels were not controlled. Eighteen P. glacialis adults (12 females, 4 males, 2 unknown) were collected from the near location at pond 1 (Fig. 1) and housed individually in 20-mL vials for 20 h without food to standardize hunger levels. For prey, we collected pupae of A. nigripes from pond 1. Pupae were used in feeding trials because, as ground-dwelling spiders, P. glacialis were observed to hunt and consume newly eclosed adult mosquitoes.
Spiders were randomly assigned to six different levels of prey density (4, 8, 14, 22, 30, or 40 mosquito pupae; #Pupaeinitial). Each spider and its prey were was placed in a round 2-L plastic container (18 cm diameter by 11 cm height). Approximately 400 mL of pond water was poured into the bottom of each container along with 50 g (dry mass) of vegetation (Carex spp.) to simulate a natural pond margin environment. Midway through the experiment, containers were shuffled within and among temperature chambers to minimize possible effects of chamber positioning. On the third day (74 h after the experiment started), spiders were removed from containers and we recorded how many mosquitoes they had eaten by counting the numbers of remaining live mosquitoes (adults and pupae), as well as dead pupae and exuviae.
For each spider, we calculated the number of prey available for consumption (Eq. 1) as follows:
where #Pupaeend was the number of pupae that had not emerged and thus remained unavailable as prey for the spider. We calculated the number of prey consumed (Eq. 2) as follows:
where #Adultsend was the number of adult mosquitoes that were alive and thus were not consumed by the spider (Online Resource 2). We plotted the number of prey consumed (Eq. 2, y-axis) versus the number of prey available (Eq. 1, x-axis) and determined the shape of the relationship by fitting both a linear model (estimating the slope with intercept set to 0) and type II saturating model (Holling 1959). We compared the goodness of fit of each model using Akaike’s information criterion (AICc; Anderson 2008).
We analyzed stable isotopes ratios of δ13C and δ15N of all spiders used in the experiment and four samples of mosquito pupae to test if and how the number of mosquito prey consumed impacted δ13C and δ15N of P. glacialis. We fitted general linear models (ANCOVAs) that included number of mosquitoes consumed, spider sex (male versus female), and the interaction of sex with the number of prey consumed as explanatory variables (proc glm; SAS v9.4).
Results
Wolf spiders in Kangerlussuaq
We collected a total of 2,360 wolf spiders across the six study areas (near and far locations at each of three ponds). Most wolf spiders (87.9%) were P. glacialis; 10.8% were Arctosa insignita; and 1.3% were P. groenlandica. P. glacialis was ubiquitous in the study area but locally rare at the far location at pond 1 (Table S4, Online Resource 1). Similarly, A. insignita was found everywhere except at the far location at pond 1. Instead, at the far location at pond 1, which was the driest and warmest site (Table 1), we collected only 28 wolf spiders throughout the entire season compared to an average of 488 at the other sampling locations. Most of those 28 spiders were P. groenlandica, a species that was absent or at relatively low numbers at all other sites (Table S4, Online Resource 1). Further analyses focused exclusively on P. glacialis because they represented nearly 90% of all wolf spiders collected. Most of the P. glacialis captures were adults (86%, n = 1,785) and males were caught at nearly a 3:1 ratio to females (Fig. 2). P. glacialis captures included 237 immature and 52 subadults (31 females and 21 males).
The trap abundances of male (left panels) and female (right panels) Pardosa glacialis varied over time and by pond and location. Points represent the average number of adult spiders collected in two cups on each date (shown as the average of the three sets of two cups). Error bars represent ± one standard error of the back-transformed means. Shaded areas indicate the approximate time period during which mosquitoes were emerging (Pond 1: 6/20–7/2; Pond 1b: 6/6–6/15; Pond 3: 6/10–6/19)
Abundances of Pardosa glacialis
We did not find evidence of higher abundances of P. glacialis wolf spiders at the margins of ponds compared to the far locations (Fig. 2), except at pond 1 where the far location contained a different Pardosa species and retrospectively appeared to be a different habitat type (Fig. 2, Table S4, S5, Online Resource 1). For the remaining two ponds, there was no pattern of more spiders near ponds, although there was some tendency for more males near pond 3 and fewer females near pond 1b (Fig. 2). There was evidence of phenological differences in that peak captures occurred earlier at pond 1b (Fig. 2, Table S5, Online Resource 1). As expected, some variation in trap captures was related to number of days per trapping period and thermal sum per trapping period (Table S5, Online Resource 1). In all models, less than 2% of the random variance in trap captures was attributable to cup nested within pond × location (Table S5, Online Resource 1).
Body size variation
The body sizes of adult, subadult, and immature P. glacialis did not vary near versus far from ponds. Across all ponds and locations, adult size increased through the season (date: F2,1762 = 15.48, p < 0.0001) but patterns varied by sex (date × sex: F 2,1762 = 6.64, p = 0.0013): males increased in size later in the season whereas females increased in size early in the season (Fig. 3). There were no effects of pond, location, or date × location on adult size. There was marginal variation in the size of subadults among ponds: mean ± standard error = 2.46 ± 0.08 (n = 21), 2.26 ± 0.06 (n = 15), and 2.16 ± 0.07 (n = 16) mm at ponds 1, 3, and 1b, respectively; F2,9 = 4.37, p = 0.05). Carapace width of immatures did not vary among ponds, locations, or dates (mean ± standard error: 1.65 ± 0.03 mm, n = 237). The random effect of cup explained only 3, 7, and 8% of total random variance in size of adults, subadults, and immatures, respectively.
The average sizes of Pardosa glacialis adults changed through time with variations between females (solid lines) and males (dashed lines), but size did not vary among ponds or the near versus far locations. Points represent the average carapace width of females and males (averaged across all Ponds and Locations) ± SE relative to timing of mosquito emergence (Before, During, or After; see Table S2, Online Resource 1). The letters indicate significant differences revealed by a post-hoc multiple means comparison test with a Tukey-Cramer adjustment
Egg sacs and fecundity
The proportion of females with an egg sac was not always higher near ponds but the number of immatures per egg sac, i.e., clutch size, was 14–20% higher near ponds (Table 2). Egg sacs collected at near locations had on average 73 immatures per egg sac versus 63 from egg sacs at far locations (F1,73 = 8.16, p = 0.0056, Table 2). There was no effect of pond on number of immature spiders per egg sac (F2,73 = 0.47, p = 0.63). Pond 1b had the highest proportion of females with an egg sac and the lowest proportion of parasitized egg sacs compared to ponds 1 and 3. At the near location at pond 1b, all females had an egg sac and none were parasitized whereas 79% had an egg sac at the far location and 10% were parasitized (Table 2). In contrast, at pond 3, the proportion of females with an egg sac was slightly higher at the far location and parasitism rates were similar near versus far (Table 2). The near location at pond 1 had the lowest proportion of females with an egg sac (Table 2). The expected number of spiderlings produced by a female P. glacialis varied from 27 to 72 (Table 2). In the absence of parasitism, being near a pond increased expected spiderlings by 44% at pond 1b but did not significantly change expected spiderlings at pond 3 (Table 2). Overall, parasitism reduced the expected number of spiderlings by up to 24%.
Stable isotopes
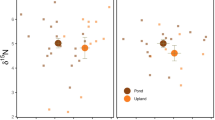
The multivariate analysis of variance indicated significant variation in the stable isotope values of P. glacialis and that the main drivers of this variation were effects of pond, location, and sex on δ13C (resource base) but not δ15N (trophic position). Individuals from the near locations were significantly depleted in 13C compared to far locations (mean δ13C ± standard error: − 26.7 ± 0.15 (n = 19) versus − 25.5 ± 0.22 (n = 12); F1,26 = 20.4, p = 0.0001). Spiders collected at pond 1 and 1b were significantly depleted in 13C compared to pond 3 (− 26.8 ± 0.30 (n = 6) and 26.2 ± 0.17 (n = 13) versus − 25.3 ± 0.18 (n = 12); F2,26 = 11.2, p = 0.0003), and females were significantly depleted in 13C compared to males (− 26.5 ± 0.14 (n = 21) versus − 25.7 ± 0.22 (n = 10); F2,26 = 10.3, p = 0.0035). δ15N values ranged from 2.2 to 6.7, but none of the variation was explained by pond, location, or sex (F4,26 = 1.2, p = 0.35).
Functional response experiment
Pardosa glacialis individuals consumed between 1 and 9 mosquito prey per day (Fig. 4) and those that consumed more mosquitoes tended to be depleted in 13C (F1,11 = 4.31; p = 0.06) and closer to the mean δ13C value (± standard error) for mosquitoes (− 33.2 ± 0.24, n = 4; Fig. 5). There were no effects of mosquito consumption rate on δ15N, but spiders were enriched in 15N relative to mosquitoes (mean ± standard error: 4.6 ± 0.16 (n = 15) versus 2.3 ± 0.07 (n = 4), respectively). During the experiment, two spiders died and a third produced an egg sac and so were excluded from analysis. For the fifteen that completed the experiment (4 males, 11 females), a linear model with the intercept set to 0 was a better fit to our data versus a type II model (ΔAICc = 2.65, and 10.21 for females and males, respectively). That is, there was no evidence of feeding saturation even at consumption of 9 mosquitoes per day. Females consumed 0.71 prey per prey offered per day compared to 0.31 for males (Fig. 4). Matching our field results, female spiders were significantly depleted in 13C compared to males (F1,11 = 8.00; p = 0.02; Fig. 5). The slope of δ13C versus the number of mosquitoes consumed did not vary for males versus females (F1,10 = 1.14; p = 0.31).
The numbers of emerging mosquitoes consumed by individual female (closed circles, n = 11) and male (open circles, n = 4) Pardosa glacialis per 24 h varied based on prey availability. The lines are linear models fit to data for females and males. We found no evidence of saturation indicating that female and male spiders could eat upwards of 9 and 4 emerging mosquitoes per day, respectively
As male (open circles) and female (closed circles) wolf spiders (Pardosa glacialis) consumed more mosquitoes, there was a trend of depletion in 13C relative to 12C (p = 0.06) but the slope of this trend line was not different for males (dashed line) versus females (solid line). In general, and consistent with our field sampling, males were enriched in 13C compared to females. The dotted line at -33.2 represents the average δ13C value for mosquito pupae
Discussion
Collectively, our results indicate limited effects on spider demographics from the resource subsidy of emerging mosquitoes. Contrary to the hypothesis of resource subsidies, we found similar abundances and sizes of P. glacialis among most of our sites, and there was no indication that P. glacialis moved towards ponds to forage for emerging mosquitoes. Most females at our sites produced an egg sac during the summer, with no consistent differences between locations near and far from ponds. One line of evidence supporting demographic effects from aquatic subsidies was that clutch sizes of P. glacialis females were up to 20% larger near ponds versus far from ponds. Furthermore, our feeding studies and stable isotope analyses demonstrated that P. glacialis consumed mosquitoes in the laboratory and field. The ephemeral pulse of food from emerging adult mosquitoes could boost fecundity in spiders near ponds, but possible confounding effects near versus far from ponds include differences in vegetation structure and prey community composition. A causal relationship between prey consumption and fecundity could be tested with measurements of clutch size following experimental augmentation of spider diets with aquatic insects. Fecundity data could be combined with estimates of spider survivorship, immigration, and emigration to quantify demographic effects of aquatic resource subsidies on landscape abundance of P. glacialis in tundra ecosystems.
Both P. glacialis and the second most common species, A. insignita, were ubiquitous in the most common habitat type of mixed grass and dwarf shrub tundra despite abiotic variation among these sites. The ecology of these species is, thus, relatively robust to at least some degree of variation in temperature and soil moisture. At the far location at pond 1, wolf spiders in general were less abundant and the dominant species was P. groenlandica. This location had the lowest percent soil moisture by volume (13%) and more closely resembled a dry erosion patch (Heindel et al. 2015), conditions that are known to favor P. groenlandica (Böcher et al. 2015). Although we matched vegetation types (mixed grass and dwarf shrub) at our near and far sites, the far location at pond 1 had taller but less dense shrubs compared to all other sites. Vegetation structure is known to impact the diversity and abundance of tundra arthropods (Rich et al. 2013), including spiders (Bowden and Buddle 2010; Hansen et al. 2016; Avila et al. 2017; Høye et al. 2018) and is a possible confounding factor in our study. Although P. glacialis was the most common species of wolf spider found in this study, more sampling is necessary to characterize wolf spider distributions across the broader tundra landscape in Kangerlussuaq.
There was no evidence of increased wolf spider abundances in areas with more mosquitoes. The trap abundances of P. glacialis increased consistently between near and far sites in early to mid-June, suggesting that activity of P. glacialis is not related to the timing of mosquito emergence. Furthermore, trap abundances of P. glacialis were also similar among ponds and did not track variation in abundances of emerging mosquitoes, which were 5X and 2X higher at pond 3 compared to pond 1 and 1b, respectively (DeSiervo et al. 2020). Instead, wolf spider activity and abundance are likely a function of snowmelt timing, ground thaw, air temperature (Saska et al. 2013), solar radiation (Høye and Forchammer 2008), general prey availability (aquatic and terrestrial), and the timing of mating (Høye and Forchammer 2008; Høye et al. 2020). Although pond 1 had the warmest air temperatures during our study, it had a later date of ground thaw and consequently had a later peak in spider activity. Our models also indicated, as expected that thermal sum per trapping period explained some of the variation in our spider abundance data. While this was not the focus of this study, further analyses (as in Saska et al. 2013) could reveal if temperature explains the variation in spider abundances that we found throughout the season, such as the second peak in abundances found at some of our sites.
Variation in aquatic subsidies among the ponds and locations was also not related to wolf spider size. Wolf spiders are generalists with determinate growth that depends on food availability (Miyashita 1968). In our study, the similarity in adult size among ponds and locations lends further support to the idea that prey were readily available throughout our study area. For P. glacialis in Greenland, growing season length may be more important in determining adult body size than habitat differences (Høye et al. 2009; Høye and Hammel 2010). Female spiders at low elevation sites in north Greenland (Disko Island) were larger than females at high elevation sites (Høye and Hammel 2010) and adult P. glacialis from east Greenland (Zackenberg) were larger in years with early snowmelt (Høye et al. 2009). However, Ameline et al. (2018) found no differences in adult body sizes of P. glacialis along an elevation gradient in south Greenland. Determining controls on wolf spider growth and adult size warrant continued study because larger females can produce bigger clutches (Ameline et al. 2018).
The proportion of females that produced an egg sac varied among sites (0.42–1.00), but there was no consistent relationship with mosquito availability. Females at pond 1b had the highest probability of producing an egg sac, and in fact, at the near location, every female we collected had produced an egg sac. In contrast, at the near location of pond 3, where mosquito availability was highest (DeSiervo et al. 2020), the proportion with an egg sac was only 0.57 (± 0.06) and was not significantly different from the proportion with an egg sac at the far location (0.70 ± 0.06). Even though we accounted for differences in thermal sums among our sites, we still expect that phenology and phenological variation among sites could explain the patterns we found in the proportion of females the produced an egg sac. First, female wolf spiders in Greenland produce egg sacs into the end of August (Ameline et al. 2018; Høye et al. 2020) and had we continued our sampling, we could have better estimated the reproductive success of females over the course of the growing season. Second, phenological differences in the beginning of the season may have an impact on the timing of egg sac production. Although the warmest site (pond 1) had the lowest proportion with an egg sac (0.42 ± 0.05), it also had a later date of ground and pond thaw and started off colder compared to other sites. These factors may have delayed spider foraging and mating activity, resulting in the lower proportion of gravid females seen during our sampling window. We have no reason to expect that variation in the proportion with an egg sac was related to the ability of females to find a mate. Males almost always outnumbered females (except at the far location at pond 1b) and can mate more than once (Vertainen et al. 2000).
Differences in potential fecundity near versus far from ponds were apparent when we looked at clutch size, i.e., the number of eggs produced per egg sac, suggesting that certain abiotic or biotic conditions near ponds may result in greater potential fecundity of an individual wolf spider. A similar pattern between reproductive output and habitat suitability was found in another species of lycosid spider, A. fulvolineata, in France (Pétillon et al. 2009). Egg sacs collected near mosquito ponds had 14–20% more eggs (an average of 73 compared to 63). A recent analysis of the clutch sizes of P. glacialis in northeast Greenland also found that females in wet areas produced larger clutches (Høye et al. 2020), estimating 97 and 75 eggs per egg sac in wet fen versus dry health locations, respectively (a 27% increase; Høye et al. 2020). We were unable to relate clutch size to female body size in our study because nearly all egg sacs became detached in the pitfall traps. We would expect female body size to impact clutch size because body size is typically associated with number of offspring produced (Honěk 1993; Brown et al. 2003; Hendrickx and Maelfait 2003; Hein et al. 2015, 2018), including in P. glacialis in Greenland (Ameline et al. 2018; Høye et al. 2020). In addition, there could be hidden effects on clutch size if female size impacts the probability of producing an egg sac. Female sizes were not different near versus far from ponds, so we are unable to attribute the variation in the number of eggs per egg sac to size differences near versus far from ponds.
Emerging mosquitoes may likely provide an influx of easy to capture nutrients during the critical period of egg sac production and allow females to produce more eggs per egg sac. Studies have shown that the nutrients that a female wolf spider consumes in the weeks before and during egg sac development go directly into the production of the egg sac (Kessler 1971; Rickers 2006). In our functional response experiment, the timing of which coincided with the period just before wolf spiders produced an egg sac in the field (in fact, one of the females produced an egg sac during the experiment), spiders consumed up to 9 mosquitoes per day. In their natural environment, spiders could consume even more mosquitoes than in our feeding experiment, which did not offer enough prey to reveal feeding saturation (Holling 1959). The degree to which wolf spiders preferentially consume emerging mosquitoes versus other prey types is likely a function of prey nutritional quality (Greenstone 1979; Mayntz et al. 2005; Schmidt et al. 2012), prey size (Henriques et al. 2021), encounter risk (Rendon et al. 2019), and the relative abundance of alternative prey (Kuusk et al. 2010; Wirta et al. 2015b; Eitzinger et al. 2019). It would be informative to compare the quality of mosquitoes as spider food compared to other prey, and though feeding trials, establish causal relationships between diet and fecundity (Toft 1999).
The significant differences we found in δ13C values of P. glacialis near versus far from ponds were consistent among the three sites and indicated that we were sampling populations with different resource bases. The lack of variation in δ15N values, both in our field-collected spiders and the spiders used in the experiment, suggests that male and female P. glacialis in our study area operate at a similar trophic position whether they consume many aquatic insects or few. At the far locations, P. glacialis had less access to mosquitoes. The enrichment of these individuals in 13C could reflect the consumption of prey more enriched in 13C compared to prey near ponds. The depletion in 13C of P. glacialis individuals collected at ponds could be due to consumption of emerging mosquitoes as was found in our lab experiment. Importantly, our functional response experiment showed just how rapidly δ13C values can change in response to consuming one type of prey. P. glacialis became depleted in 13C after just 74 h of consuming mosquitoes. One alternative explanation for the depletion in 13C of individuals near ponds is decreased cannibalism and intraguild predation (Koltz and Wright 2020); however, there was no evidence that P. glacialis near ponds held a lower trophic position. We did find that spider sex explained some variation in δ13C in both the field-collected individuals and the lab experiment. This suggests different resource bases for males versus females; however, we note that male spiders were less represented in the field and laboratory study and that this small sample size could be influencing our statistical results. Studies of feeding ecology using DNA barcoding (e.g., Wirta et al. 2015a; Eitzinger et al. 2019) are revealing that wolf spiders are generalists and eat terrestrial and aquatic prey. However, the proportions of their diet coming from different sources remains unknown. Further analysis of variation in prey community composition and the δ13C of different prey taxa near versus far from tundra ponds would help to confirm the relative importance of aquatic insects to the diets of P. glacialis and other Arctic wolf spiders in Kangerlussuaq.
The presence of P. groenlandica and absence of A. insignita and P. glacialis at the far site at pond 1, which has characteristics of future drier and warmer Arctic conditions, suggest that continued climate change in the Kangerlussuaq area may favor dry-tolerant species such as P. groenlandica. P. glacialis¸ the most common species in our study, may experience decreased fecundity in upland sites due to reduced aquatic subsidies or other factors, but drier conditions may also limit egg sac parasitism by wasps (Koltz et al. 2019). Although we did not find consistently lower rates of egg sac parasitism at our far sites in 2018, rates of egg sac parasitism in Kangerlussuaq in 2017 were lower in dry sites (Koltz et al. 2019). We found that parasitism can reduce the expected number of spiderlings produced per female by up to 24% and is, therefore, a potentially meaningful factor in spider population dynamics. We do not have comparable size, fecundity, and parasitism data for A. insignita or P. groenlandica, the other two wolf spiders found in Kangerlussuaq. There would be value in comparing the traits of multiple wolf spider species that co-occur in the same area (e.g., Ameline et al. 2018) and how they are shaped by environmental variation in space and time.
Freshwater and terrestrial habitats in Greenland are indeed linked through spider consumption of emerging aquatic insects (Wirta et al. 2015a; Eitzinger et al. 2019), but the consequences for mosquito and spider populations are not yet fully described. This is a previously unquantified source of mortality for Arctic mosquitoes, which are a notable pest of humans and wildlife (Joly et al. 2020) and whose abundances and population dynamics are shaped by abiotic change and species interactions (Culler et al. 2015, 2018; DeSiervo et al. 2020). For wolf spiders, we were only able to establish a correlative relationship between proximity to aquatic subsidies and the fecundity of P. glacialis. Confounding factors include differences in vegetation structure, soil moisture, and the soil invertebrate community that provides important food resources for Arctic wolf spiders (Koltz et al. 2017). Regardless of the mechanism, spiders are conspicuous and abundant arthropods in Arctic terrestrial ecosystems and any change to their population dynamics has the potential to affect birds (Wirta et al. 2015b) and other potential spider predators, arthropod community composition (Koltz et al. 2018a), and ecosystem functions such as decomposition (Koltz et al. 2018b). Understanding how cross-system linkages and related ecological processes are shaped by climate change is central to anticipating consequences for the broader tundra ecosystem.
Data availability
Data are available in Online Resource 2.
References
Akamatsu F, Toda H, Okino T (2004) Food source of riparian spiders analyzed by using stable isotope ratios. Ecol Res 19:655–662. https://doi.org/10.1111/j.1440-1703.2004.00680.x
Ameline C, Høye TT, Bowden JJ, Hansen RR, Hansen OLP, Puzin C, Vernon P, Pétillon J (2018) Elevational variation of body size and reproductive traits in high-latitude wolf spiders (Araneae: Lycosidae). Polar Biol 41:2561–2574. https://doi.org/10.1007/s00300-018-2391-5
Anderson D (2008) Model based inference in the life sciences: a primer on evidence. Springer, New York
Andresen CG, Lougheed VL (2015) Disappearing Arctic tundra ponds: Fine-scale analysis of surface hydrology in drained thaw lake basins over a 65-year period (1948–2013). J Geophys Res Biogeosci 120:466–479. https://doi.org/10.1002/2014JG002778
Avila AC, Stenert C, Rodrigues ENL, Maltchik L (2017) Habitat structure determines spider diversity in highland ponds. Ecol Res 32:359–367. https://doi.org/10.1007/s11284-017-1442-7
Becker N, Petric D, Zgomba M, Boase C, Madon M, Dahl C, Kaiser A (2010) Mosquitoes and their control. Springer, Berlin
Böcher J, Kristensen NP, Pape T, Vilhelmsen L (2015) The Greenland Entomofauna: an Identification Manual of Insects. Spiders and their Allies, Brill, Boston
Boecklen WJ, Yarnes CT, Cook BA, James AC (2011) On the use of stable isotopes in trophic ecology. Annu Rev Ecol Evol Syst 42:411–440. https://doi.org/10.1146/annurev-ecolsys-102209-144726
Bowden JJ, Buddle CM (2010) Determinants of ground-dwelling spider assemblages at a regional scale in the Yukon Territory, Canada. Ecoscience 17:287–297. https://doi.org/10.2980/17-3-3308
Bowden JJ, Høye TT, Buddle CM (2013) Fecundity and sexual size dimorphism of wolf spiders (Araneae: Lycosidae) along an elevational gradient in the Arctic. Polar Biol 36:831–836. https://doi.org/10.1007/s00300-013-1308-6
Bowden JJ, Hansen OLP, Olsen K, Schmidt NM, Høye TT (2018) Drivers of inter-annual variation and long-term change in High-Arctic spider species abundances. Polar Biol 41:1635–1649. https://doi.org/10.1007/s00300-018-2351-0
Breene RG, Sweet MH, Olson JK (1988) Spider predators of mosquito larvae. J Arachnol 16:275–277
Brown CA, Sanford BM, Swerdon RR (2003) Clutch size and offspring size in the wolf spider Pirata sedentarius (Araneae, Lycosidae). The Journal of Arachnology 31:285–296. https://doi.org/10.1636/m01-62
Cochran W (1977) Sampling Techniques. John Wiley & Sons, New York
Culler LE, Ayres MP, Virginia RA (2015) In a warmer Arctic, mosquitoes avoid increased mortality from predators by growing faster. Proc Roy Soc B 282:20151549. https://doi.org/10.1098/rspb.2015.1549
Culler LE, Ayres MP, Virginia RA (2018) Spatial heterogeneity in the abundance and fecundity of Arctic mosquitoes. Ecosphere 9:e02345. https://doi.org/10.1002/ecs2.2345
DeSiervo MH, Ayres MP, Virginia RA, Culler LE (2020) Consumer–resource dynamics in Arctic ponds. Ecology. https://doi.org/10.1002/ecy.3135
Dreyer J, Hoekman D, Gratton C (2012) Lake-derived midges increase abundance of shoreline terrestrial arthropods via multiple trophic pathways. Oikos 121:252–258. https://doi.org/10.1111/j.1600-0706.2011.19588.x
Eitzinger B, Abrego N, Gravel D, Huotari T, Vesterinen EJ, Roslin T (2019) Assessing changes in arthropod predator–prey interactions through DNA-based gut content analysis—variable environment, stable diet. Mol Ecol 28:266–280. https://doi.org/10.1111/mec.14872
Elberling B, Tamstorf MP, Michelsen A, Arndal MF, Sigsgaard C, Illeris L, Bay C, Hansen BU, Christensen TR, Hansen ES, Jakobsen BH, Beyens L (2008) Soil and plant community characteristics and dynamics at Zackenberg. Adv Ecol Res 40:223–248. https://doi.org/10.1016/S0065-2504(07)00010-4
Fausch KD, Power ME, Murakami M (2002) Linkages between stream and forest food webs: Shigeru Nakano’s legacy for ecology in Japan. Trends Ecol Evol 17:429–434. https://doi.org/10.1016/S0169-5347(02)02572-7
Finger Higgens RA, Chipman JW, Lutz DA, Culler LE, Virginia RA, Ogden LA (2019) Changing lake dynamics indicate a drier Arctic in western Greenland. J Geophys Res Biogeosci 124:870–883. https://doi.org/10.1029/2018JG004879
Futami K, Sonye G, Akweywa P, Kaneko S, Minakawa N (2008) Diving behavior in Anopheles gambiae (Diptera: Culicidae): avoidance of a predacious wolf spider (Araneae: Lycosidae) in relation to life stage and water depth. J Med Entomol 45:1050–1056. https://doi.org/10.1093/jmedent/45.6.1050
Garcia R, Schlinger EI (1972) Studies of spider predation on Aedes dorsalis (Meigen) in a salt marsh. Proceedings and Papers of the Annual Conference of the California Mosquito Control Association, Inc 40:117–118
Gillespie MAK, Alfredsson M, Barrio IC, Bowden JJ, Convey P, Coulson SJ, Culler LE, Dahl MT, Daly KM, Koponen S, Loboda S, Marusik S, Sandström JP, Sikes DS, Slowik J, Høye TT (2020) Circumpolar terrestrial arthropod monitoring: a review of ongoing activities, opportunities and challenges, with a focus on spiders. Ambio 49:704–717. https://doi.org/10.1007/s13280-019-01185-y
Gratton C, Donaldson J, Vander Zanden MJ (2008) Ecosystem linkages between lakes and the surrounding terrestrial landscape in Northeast Iceland. Ecosystems 11:764–774. https://doi.org/10.1007/s10021-008-9158-8
Greenstone MH (1979) Spider feeding behaviour optimizes dietary essential amino acid composition. Nature 282:501–503. https://doi.org/10.1038/282501a0
Hagstrum DW (1971) Carapace width as a tool for evaluating the rate of development of spiders in the laboratory and the field. Ann Entomol Soc Am 64:757–760
Hallander H (1967) Range and movements of the wolf spiders Pardosa chelata (O.F. Müller) and P. pullata (Clerck). Oikos 18:360–364. https://doi.org/10.2307/3565113
Hansen RR, Hansen OLP, Bowden JJ, Treier UA, Normand S, Høye TT (2016) Meter scale variation in shrub dominance and soil moisture structure Arctic arthropod communities. PeerJ 4:e2224. https://doi.org/10.7717/peerj.2224
Hein N, Feilhauer H, Löffler J, Finch OD (2015) Elevational variation of reproductive traits in five Pardosa (Lycosidae) species. Arct Antarct Alp Res 47:473–479. https://doi.org/10.1657/AAAR0013-111
Hein N, Brendel MR, Feilhauer H, Finch OD, Löffler J (2018) Egg size versus egg number trade-off in the alpine-tundra wolf spider, Pardosa palustris (Araneae: Lycosidae). Polar Biol 41:1607–1617. https://doi.org/10.1007/s00300-018-2301-x
Heindel RC, Chipman JW, Virginia RA (2015) The spatial distribution and ecological impacts of aeolian soil erosion in Kangerlussuaq, West Greenland. Ann Assoc Am Geogr 105:875–890. https://doi.org/10.1080/00045608.2015.1059176
Hendrickx F, Maelfait JP (2003) Life cycle, reproductive patterns and their year-to-year variation in a field population of the wolf spider Pirata piraticus (Araneae, Lycosidae). J Arachnol 31:331–339. https://doi.org/10.1636/m01-98
Henriques JF, Lacava M, Guzmán C, Gavín-Centol MP, Ruiz-Lupión D, De Mas E, Magalhães M-L (2021) The sources of variation for individual prey-to-predator size ratios. Heredity. https://doi.org/10.1038/s41437-020-00395-5
Hoekman D, Bartrons M, Gratton C (2012) Ecosystem linkages revealed by experimental lake-derived isotope signal in heathland food webs. Oecologia 170:735–743. https://doi.org/10.1007/s00442-012-2329-5
Holling CS (1959) Some characteristics of simple types of predation and parasitism. Can Entomol 91:385–398. https://doi.org/10.4039/Ent91385-7
Honěk A (1993) Intraspecific variation in body size and fecundity in insects: a general relationship. Oikos 66:483–492. https://doi.org/10.2307/3544943
Høye TT, Forchhammer MC (2008) Phenology of High-Arctic arthropods: effects of climate on spatial, seasonal and inter-annual variation. Adv Ecol Res 40:299–324. https://doi.org/10.1016/S0065-2504(07)00013-X
Høye TT, Hammel JU (2010) Climate change and altitudinal variation in sexual size dimorphism of arctic wolf spiders. Clim Res 41:259–265. https://doi.org/10.3354/cr00855
Høye TT, Post E, Meltofte H, Schmidt NM, Forchhammer MC (2007) Rapid advancement of spring in the High Arctic. Curr Biol 17:R449–R451. https://doi.org/10.1016/j.cub.2007.04.047
Høye TT, Hammel JU, Fuchs T, Toft S (2009) Climate change and sexual size dimorphism in an Arctic spider. Biol Let 5:542–544. https://doi.org/10.1098/rsbl.2009.0169
Høye TT, Bowden JJ, Hansen OLP, Hansen RR, Henriksen TN, Niebuhr A, Skytte MG (2018) Elevation modulates how Arctic arthropod communities are structured along local environmental gradients. Polar Biol 41:1555–1565. https://doi.org/10.1007/s00300-017-2204-2
Høye TT, Kresse J-C, Koltz AM, Bowden JJ (2020) Earlier springs enable high-Arctic wolf spiders to produce a second clutch. Proc Roy Soc B 287:20200982. https://doi.org/10.1098/rspb.2020.0982
Jackson RR, Cross FR (2015) Mosquito-terminator spiders and the meaning of predatory specialization. J Arachnol 43:123–142. https://doi.org/10.1636/V15-28
Jakob EM, Marshall SD, Uetz GW (1996) Estimating fitness: a comparison of body condition indices. Oikos 77:61–67. https://doi.org/10.2307/3545585
Joly K, Couriot O, Cameron MD, Gurarie E (2020) Behavioral, physiological, demographic and ecological impacts of hematophagous and endoparasitic insects on an Arctic ungulate. Toxins 12:334. https://doi.org/10.3390/toxins12050334
Kerby J, Post E (2013) Capital and income breeding traits differentiate trophic match-mismatch dynamics in large herbivores. Philos Trans R Soc B 386:1624. https://doi.org/10.1098/rstb.2012.0484
Kessler A (1971) Relation between egg production and food consumption in species of the genus Pardosa (Lycosidae, Araneae) under experimental conditions of food-abundance and food-shortage. Oecologia 8:93–109. https://doi.org/10.1007/BF00345629
Koltz AM, Wright JP (2020) Impacts of female body size on cannibalism and juvenile abundance in a dominant arctic spider. J Anim Ecol 89:1788–1798. https://doi.org/10.1111/1365-2656.13230
Koltz AM, Asmus A, Gough L, Pressler Y, Moore JC (2017) The detritus-based microbial-invertebrate food web contributes disproportionately to carbon and nitrogen cycling in the Arctic. Polar Biol 41:1531–1545. https://doi.org/10.1007/s00300-017-2201-5
Koltz AM, Schmidt NM, Høye TT (2018a) Differential arthropod responses to warming are altering the structure of Arctic communities. R Soc Open Sci 5:171503. https://doi.org/10.1098/rsos.171503
Koltz AM, Classen AT, Wright JP (2018b) Warming reverses top-down effects of predators on belowground ecosystem function in Arctic tundra. PNAS 115:E7541–E7549. https://doi.org/10.1073/pnas.1808754115
Koltz AM, Culler LE, Bowden JJ, Post E, Høye TT (2019) Dominant arctic predator is free of major parasitoid at northern edge of its range. Front Ecol Evol 7:250. https://doi.org/10.3389/fevo.2019.00250
Kreiter NA, Wise DH (2001) Prey availability limits fecundity and influences the movement pattern of female fishing spiders. Oecologia 127:417–424. https://doi.org/10.1007/s004420000607
Kuusk AK, Ekbom B (2010) Lycosid spiders and alternative food: feeding behavior and implications for biological control. Biol Control 55:20–26. https://doi.org/10.1016/j.biocontrol.2010.06.009
Loboda S, Savage J, Buddle CM, Schmidt NM, Høye TT (2018) Declining diversity and abundance of High Arctic fly assemblages over two decades of rapid climate warming. Ecography 41:265–277. https://doi.org/10.1111/ecog.02747
Mayntz D, Raubenheimer D, Salomon M, Toft S, Simpson SJ (2005) Nutrient-specific foraging in invertebrate predators. Science 307:111–113. https://doi.org/10.1126/science.1105493
McKinnon L, Picotin M, Bolduc E, Juillet C, Bêty J (2012) Timing of breeding, peak food availability, and effects of mismatch on chick growth in birds nesting in the High Arctic. Can J Zool 90:961–971. https://doi.org/10.1139/z2012-064
Miyashita K (1968) Growth and development of Lycosa T-insignita Boes. et Str. (Araneae: Lycosidae) under different feeding conditions. Appl Entomol Zool 3:81–88. https://doi.org/10.1303/aez.3.81
Morse DH (2002) Orientation and movement of wolf spiders Pardosa lapidicina (Araneae, Lycosidae) in the intertidal zone. J Arachnol 30:601–609. https://doi.org/10.1636/0161-8202(2002)030[0601:OAMOWS]2.0.CO;2
Myers-Smith IH, Forbes BC, Wilmking M, Hallinger M, Lantz T, Blok D et al (2011) Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environ Res Lett 6:045509. https://doi.org/10.1088/1748-9326/6/4/045509
Nakano S, Murakami M (2001) Reciprocal subsidies: Dynamic interdependence between terrestrial and aquatic food webs. PNAS 98:166–170. https://doi.org/10.1073/pnas.98.1.166
Paetzold A, Schubert CJ, Tockner K (2005) Aquatic terrestrial linkages along a braided-river: riparian arthropods feeding on aquatic insects. Ecosystems 8:748–759. https://doi.org/10.1007/s10021-005-0004-y
Perevozkin VP, Lukyantsev SV, Gordeev MI (2004) Comparative analysis of foraging behavior in aquatic and semiaquatic spiders of the genera Argyroneta, Dolomedes, Pirata, and Pardosa. Russ J Ecol 35:103–109. https://doi.org/10.1023/B:RUSE.0000018935.70179.85
Pétillon J, Puzin C, Acou A, Outreman Y (2009) Plant invasion phenomenon enhances reproduction performance in an endangered spider. Naturwissenschaften 96:1241–1246. https://doi.org/10.1007/s00114-009-0589-7
Pickavance JR (2001) Life-cycles of four species of Pardosa (Araneae, Lycosidae) from the island of Newfoundland, Canada. J Arachnol 29:367–378. https://doi.org/10.1636/0161-8202(2001)029[0367:LCOFSO]2.0.CO;2
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718. https://doi.org/10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2
Post E, Alley RB, Christensen TR, Macias-Fauria M, Forbes BC, Gooseff M, Iler A, Kerby JT, Laidre KL, Mann ME, Olofsson J, Stroeve JC, Ulmer F, Virginia RA, Wang M (2019) The polar regions in a 2 °C warmer world. Science Advances 5:eaaw9883. https://doi.org/10.1126/sciadv.aaw9883
Puzin C, Acou A, Bonte D, Pétillon J (2011) Comparison of reproductive traits between two salt-marsh wolf spiders (Araneae, Lycosidae) under different habitat suitability conditions. Anim Biol 61:127–138. https://doi.org/10.1163/157075511X566461
Rendon D, Taylor PW, Wildner SM, Whitehouse ME (2019) Does prey encounter and nutrient content affect prey selection in wolf spiders inhabiting Bt cotton fields? PLoS ONE 14:e0210296. https://doi.org/10.1371/journal.pone.0210296
Rich ME, Gough L, Boelman NT (2013) Arctic arthropod assemblages in habitats of differing shrub dominance. Ecography 36:994–1003. https://doi.org/10.1111/j.1600-0587.2012.00078.x
Rickers S, Langel R, Scheu S (2006) Dietary routing of nutrients from prey to offspring in a generalist predator: effects of prey quality. Funct Ecol 20:124–131. https://doi.org/10.1111/j.1365-2435.2006.01077.x
Sanchez-Ruiz JA, Phillips JS, Ives AR, Gratton C (2018) Responses of orb-weaving spider aggregations to spatiotemporal variation in lake-to-land subsidies at Lake Mývatn, Iceland. Polar Biol 41:1547–1554. https://doi.org/10.1007/s00300-017-2202-4
Saska P, van der Werf W, Hemerik L, Luff ML, Hatten TD, Honek A (2013) Temperature effects on pitfall catches of epigeal arthropods: a model and method for bias correction. J Appl Ecol 50:181–189. https://doi.org/10.1111/1365-2664.12023
Schindler DE, Smits AP (2017) Subsidies of aquatic resources in terrestrial ecosystems. Ecosystems 20:78–93. https://doi.org/10.1007/s10021-016-0050-7
Schmidt JM, Sebastian P, Wilder SM, Rypstra AL (2012) The nutritional content of prey affects the foraging of a generalist arthropod predator. PLoS ONE 7:e49223. https://doi.org/10.1371/journal.pone.0049223
Service MW (1973) Mortalities of the larvae of the Anopheles gambiae Giles complex and detection of predators by the precipitin test. Bull Entomol Res 62:359–369. https://doi.org/10.1017/S0007485300003862
Smith LC, Sheng Y, Macdonald GM, Hinzman LD (2005) Disappearing Arctic lakes. Science 308:1429. https://doi.org/10.1126/science.1108142
Sturm M, Schimel J, Michaelson G, Welker JM, Oberbauer SF, Liston GE, Fahnestock J, Romanovsky VE (2005) Winter biological processes could help convert arctic tundra to shrubland. Bioscience 55:17–26. https://doi.org/10.1641/0006-3568(2005)055[0017:WBPCHC]2.0.CO;2
Toft S (1999) Prey choice and spider fitness. The Journal of Arachnology 27:301–307. http://www.jstor.org/stable/3706001
Vertainen L, Alatalo RV, Mappes J, Parri S (2000) Sexual differences in growth strategies of the wolf spider Hygrolycosa rubrofasciata. Evol Ecol 14:595–610. https://doi.org/10.1023/A:1011080706931
Walter Anthony K, Schneider Von Deimling T, Nitze I, Frolking S, Emond A, Daanen R, Anthony P, Lindgren P, Jones B, Grosse G (2018) 21st-century modeled permafrost carbon emissions accelerated by abrupt thaw beneath lakes. Nat Commun 9:3262. https://doi.org/10.1038/s41467-018-05738-9
Wirta HK, Weingartner E, Hambäck PA, Roslin T (2015a) Extensive niche overlap among the dominant arthropod predators of the High Arctic. Basic Appl Ecol 16:86–92. https://doi.org/10.1016/j.baae.2014.11.003
Wirta HK, Vesterinen EJ, Hambäck PA, Weingartner E, Rasmussen C, Reneerkens J, Schmidt NM, Gilg O, Roslin T (2015b) Exposing the structure of an Arctic food web. Ecol Evol 5:3842–3856. https://doi.org/10.1002/ece3.1647
Wyant KA, Draney ML, Moore JC (2011) Epigeal spider (Araneae) communities in moist acidic and dry heath tundra at Toolik Lake, Alaska. Arct Antarct Alp Res 43:301–312. https://doi.org/10.1657/1938-4246-43.2.301
Acknowledgements
We thank Angela Spickard, Balt von Huene, and Reyn Hutten for help with lab and field work, the CH2MHill Polar Services team for excellent logistical support and Naalakkersuisut (the Government of Greenland) for permission to conduct research in Greenland. We also thank Julien Pétillon, Jesamine Bartlett, and one anonymous reviewer for providing valuable feedback on this manuscript during the review process.
Funding
This study was supported by a U.S. National Science Foundation award to LEC, MPA, and RAV (#1748137). Additional funding was provided to AMS through a Dartmouth College Undergraduate Advising and Research Program Honors Thesis Grant and to HMB through a Stefansson Fellowship from Dartmouth’s Institute of Arctic Studies. Funding for stable isotope analysis was provided to HMB from the Jerry Manne Fund in Environmental Studies.
Author information
Authors and Affiliations
Contributions
LEC, AMS, RAV, and MPA conceived and designed the study. AMS, HMB, MHD, and LEC conducted experiments and carried out field sampling. AMS processed spider pitfall samples and HMB processed stable isotope samples. AMS, MPA, and LEC analyzed field data and HMB, LEC, and RAV analyzed and interpreted stable isotope data. AMS and LEC wrote the manuscript and all authors read, edited, and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the special issue on the “Pathways and impacts of biotically-mediated marine and other stored nutrient transfer between polar ecosystems”, coordinated by Peter Convey, Katarzyna Zmudczyńska-Skarbek, and Stef Bokhorst.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Culler, L.E., Stendahl, A.M., DeSiervo, M.H. et al. Emerging mosquitoes (Aedes nigripes) as a resource subsidy for wolf spiders (Pardosa glacialis) in western Greenland. Polar Biol 47, 845–857 (2024). https://doi.org/10.1007/s00300-021-02875-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-021-02875-8