Abstract
Alien predator introduction is a global threat to amphibians. Yet, there is a lack of in situ studies of trophic interactions between alien predators and native amphibians, particularly concerning small predatory fish such as mosquitofish. Mosquitofish originate from the United States but have been introduced globally, including intentionally for mosquito control. They cause declines in many amphibian populations but the mechanisms involved have been seldom investigated. Trophic interaction studies (mainly ex situ) reveal negative effects on larval amphibian stages but do not consider interactions with adults. Some site-occupancy studies show no negative association with adult amphibians, suggesting potentially complex demographic impacts and calling for a better characterization of trophic interaction with adult amphibians. Here, we studied in situ trophic interactions between introduced Eastern mosquitofish (Gambusia holbrooki) and pond-breeding palmate newts (Lissotriton helveticus; larvae and adults) using gut content and stable isotope analyses. Mosquitofish had little trophic niche overlap with adult newts. Adult newts foraged mainly on burrowing benthic macroinvertebrates that were little exploited by mosquitofish, the latter focusing mainly on microcrustaceans. Both techniques suggested predation on newt eggs or larvae and cannibalism by mosquitofish. Since native newts were still abundant despite > 50 years of mosquitofish presence and reproductively active but without evidence of larval survival, we argue that ponds invaded by small predatory fish such as mosquitofish may pose a risk by acting as demographic sinks for newts due to their predatory impact on larvae and eggs, but potentially low impact on adults in terms of trophic niche overlap.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the most threatened vertebrates worldwide, amphibians are particularly affected by alien species introductions in their aquatic habitat (Denoël et al. 2019; Knapp 2005; Stuart et al. 2004). The negative impacts of alien species on amphibians are especially worrying as they play important roles in small streams, pond and lake ecosystems and their disappearance can have complex ecological consequences that propagate within and across ecosystems (Whiles et al. 2006). Because many amphibians have evolved in absence of functionally similar predators, they often lack the evolutionary history to produce behaviour or traits necessary to adequately respond to pressures from alien predators introduced in ecological time (Bucciarelli et al. 2014), making them appropriate models to study the impact of alien introductions on naïve native predators.
Introduced alien predators can impact amphibian populations by direct interactions such as predation on adults, eggs or larvae (Gamradt and Kats 1996; Knapp 2005; Orizaola and Braña, 2006; Pope 2008; Stenson and Aronsson 1995), competition for resources and modification of prey community composition or size structure (Cabrera-Guzmán et al. 2017; Knapp et al. 2001; Tiberti et al. 2014, 2019). Alien predators may also affect native amphibian species through various forms of non-consumptive negative interactions inducing detrimental behavioural changes (Winandy et al. 2015, 2016; Winandy and Denoël, 2015). Indirect detrimental effects such as alteration of habitat structure or ecosystem functioning (Hartel et al. 2007; Richardson et al. 1995; Rolla et al. 2018), depletion of terrestrial food sources due to the consumption of emerging insects (Tiberti et al. 2016) or pathogen transmission (Kiesecker et al. 2001) have also been documented. Predicting the outcome of species introduction is complex due to possible synergies between all these effects and other biotic or abiotic stressors (Bucciarelli et al. 2014), especially in a context of global changes (Jackson et al. 2016). But critically, there is a lack of in situ studies of trophic interaction between introduced aquatic predators such as fish and native amphibians. Most studies focus on predatory fish that are often considerably larger than native amphibians (e.g. salmonids), while comparatively little information is available on trophic impacts of fish that are within the size range or smaller than native amphibians and may therefore be unable to predate on adult amphibians (Cabrera-Guzmán et al. 2017; Komak and Crossland 2000; Mahony et al. 2013; Remon et al. 2016; Webb and Joss 1997). Furthermore, impact studies tend to focus on trophic interactions with amphibian larvae without assessing potential interactions with adults. Yet differential impact of introduced predators on larval and adult stages of native species can lead to complex impacts on their demography. Considering invasive predator’s trophic interactions with all life stages of native species may allow identification of phenomena such as demographic sinks (Howe et al. 1991). Demographic sinks may arise when the larvae of native species are consumed by alien predators but adults are still able to exploit invaded habitats because they do not suffer from competition or predation themselves and/or do not recognize the alien species as a threat (Woodford and Mcintosh 2010).
The eastern mosquitofish (Gambusia holbrooki) is a small freshwater fish (< 60 mm total length) of the family Poecillidae (Rauchenberger 1989). It is native to eastern North America but has been introduced and has established in multiple freshwater systems on every continent, except Antarctica (Pyke 2005, 2008). This is also the case for its sister species, G. affinis, from which G. holbrooki used to be considered a subspecies (Smith et al. 1989; Wooten et al. 1988). There is a large body of evidence on the negative impact of mosquitofish introductions on native fish populations in the scientific literature, ultimately leading to local population extinction (Pyke 2008). Direct predation by mosquitofish or constant harassment of native juvenile fish resulting in death have been demonstrated in many different study systems (Rowe et al. 2007; Schumann et al. 2015; Sutton et al. 2013). Similarly, several studies have highlighted predatory behaviour towards anuran eggs, hatchlings and tadpoles (Komak and Crossland 2000; Mahony et al. 2013; Remon et al. 2016; Vannini et al. 2018), as well as newt larvae (Cabrera-Guzmán et al. 2017; Gamradt and Kats 1996; Preston et al. 2012; Vannini et al. 2018). Mosquitofish were demonstrated to alter feeding and egg-laying behaviour of the adult pygmy newt (Cabrera-Guzmán et al. 2019) and paedomorphic Greek smooth newts (Toli et al. 2020) in experimental studies. Previous studies looking for site occupancy of amphibians (both anurans and newts) in the presence of invasive mosquitofish provided contrasting results; finding negative associations for newts but random associations for anurans (Bounas et al. 2020), or no apparent avoidance of invaded sites by both adult newts and anurans but strong negative associations with larvae, as well as larval predation in mesocosms experiments (Klop-Toker et al. 2018; Preston et al. 2012). These results call for more in situ studies to assess the trophic niche use and interaction between introduced mosquitofish and the different life stages of newts in natural systems. Studying the specificities of mosquitofish interactions with adult and larval newts could provide important insights to identify mechanisms potentially inducing demographic ‘sinks’ which may constitute a pernicious threat for natives, even when invasion appears limited at the landscape scale (Woodford and Mcintosh 2010).
Here, we coupled stable isotope and stomach content analyses to study direct trophic interactions between introduced mosquitofish (G. holbrookii) and native palmate newts L. helveticus in a pond where both predators have been known to coexist for more than 50 years (Gabrion et al. 1977). We hypothesized that because of their carnivorous diet but small size, mosquitofish would primarily represent a threat for newt eggs and larvae, while adult newts may still be able to coexist through resource partitioning by feeding on prey likely less accessible to mosquitofish such as burrowing macroinvertebrates. Following this hypothesis, adult newts might be able to coexist with introduced mosquitofish thanks to resource partitioning, but unable to renew their population due to predation on eggs or larvae, with invaded populations therefore potentially constituting demographic sinks.
Materials and methods
Study site and sampling
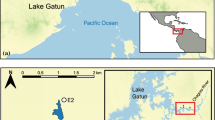
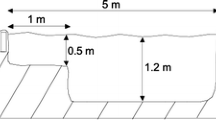
The selected pond is located on the Larzac plateau (Department of Hérault, France; 43° 79′ 89″ N, 3° 49′ 78″ E; 617 m elevation) (Fig. S1), hosted a population of native palmate newts Lissotriton helveticus and had introduced mosquitofish for at least 50 years (Gabrion et al. 1977) (Fig. 1). The Larzac region is a limestone plateau with traditionally managed habitats that is especially suited for this study as important palmate newt population declines have been correlated to fish introductions (Denoël et al. 2005; Denoël and Winandy 2015). Sampling were done from 7 to 10 June 2015 and on 28 May 2018, i.e. during the reproductive period of newts (Gabrion et al. 1977). In Larzac, newts typically stay several months in water after overwintering (Gabrion et al. 1977; Denoël and Ficetola 2014). Previous research from two other regions of France showed that palmate newts mature at 3–5 or 4–6 years and have an adult life expectancy of four to seven years, at around 270 and 880 m, respectively (Guyetant et al. 1991; Miaud 1991). The pond is artificial, built of concrete. In 2015, its diameter under water was of 14 m, for a maximum water depth of 1 m and with no canopy cover. It presented a low area of aquatic vegetation cover (Groenlandia densa) (~ 10% of water surface) and high water turbidity. Benthic habitat presented an accumulation of terrestrial leaf litter. The pond was invaded by a single alien fish species identified as the eastern mosquitofish Gambusia holbrooki based on morphological traits (Rauchenberger 1989) (Fig. S2).
Palmate newt and mosquitofish densities were estimated for comparison using a standardized dip-netting session (mesh-size = 1 mm) following (Denoël and Winandy 2015). Data is expressed in number of individuals per square meter for one hour of sampling. During censuses, newts and fish were kept in separated tanks filled with water from the pond and then counted and identified according to species and sex. Both species are morphologically sexually dimorphic (Denoël, 2007; Rauchenberger 1989). Newts and fish were measured to the nearest 0.5 mm (snout-vent-length for newts: from the tip of the snout to the end of the cloaca; and standard length for fish: from the tip of the mouth to the end of the fleshy part of the body) and weighed to the nearest 0.01 g using a mass balance. Stomach contents of newts and fish were obtained by stomach flushing of anaesthetized individuals (bath of phenoxyethanol 0.5 ml/l) (Lejeune et al. 2021), respectively using the techniques described by Joly (1987) and Brosse and Lepage (2000) immediately after sampling. Stomach contents of each individual were stored individually in vials filled with ethanol to reach a final concentration of 70% ethanol for preservation until back in the lab. Non-lethal samples of caudal skin for newts (Lejeune et al. 2018), and caudal fin membrane for fish were taken for stable isotope analysis. Following recommendations of Hayden et al. (2015), fin samples of fish were taken from the tip of the caudal fin membrane (2 mm) using surgical scissors and avoiding rays. In total 43 newts and 49 mosquitofish were sampled for body size, stomach content and stable isotopes in 2015. All newts and fish were kept in tanks filled with water from the pond until they were completely awake before being released. Because prey types such as amphibian eggs or hatchlings may be difficult to identify in gut contents of mosquitofish due to quick digestion (Pyke 2005), we decided to collect a larger sample of mosquitofish in 2018, looking specifically for vertebrate prey occurrence in the guts. In 2018, 140 mosquitofish were sampled in the same pond. These individuals were euthanized and preserved in 70% alcohol until back in the lab for dissection of the whole digestive tract. Detailed sample sizes for each test and year are available in Table S1.
All main potential food sources were collected to be implemented in stable isotope mixing models of predator’s assimilated diet. Macroinvertebrates, mosquitofish larvae and terrestrial prey found at the water surface were collected by dip netting (mesh size: 600 µm). Mesozooplankton were collected using towed nets (mesh size: 250 µm). Newt eggs were collected in the aquatic vegetation by hand. Macrophytes were collected by hand. Periphyton was scraped from a device holding six vertically oriented glass windows (8 × 12 cm) that were placed underwater for two weeks during sampling. Terrestrial leaf litter was collected by hand. All stable isotope samples were taken with n = 6 per taxa, rinsed with clear water, stored individually in glass vials and put on ice until back in the lab.
All sampling material was carefully disinfected before and after sampling with a solution of Virkon to avoid the spread of diseases to amphibians.
Stomach content analysis
Ingested prey were identified to the lowest taxonomic level given their state of digestion, counted and measured to the nearest 0.01 mm under a stereoscopic microscope (Zeiss Stemi 2000-C; Carl Zeiss, Jena, Germany). They were then grouped at higher taxonomic ranks to equalize the degree of precision of taxonomic identification across prey types. We calculated trophic niche widths based on prey abundance in the stomach contents for each individual using Shannon index (Shannon 1948), following the equation: H’ = − Σpi × ln pi, where pi is the proportion of prey i relative to the total number of prey in a given stomach. Differences in H’ and body length (Ln-transformed) were tested according to species and sexes using a two-way univariate permutational analysis of variance (PERMANOVA) that employed a similarity matrix based on Euclidean distances. We chose this test over ANOVA as it allows for more flexibility in terms of statistical assumptions (Anderson 2001). Diet composition (DC) was described and analysed in terms of prey abundance (DC(N)) and prey dry mass (DC(DM)). Prey DM were estimated from body length using taxon specific length–weight regressions for Acaria (Baumgärtner and Rothhaupt 2003), Cladocera and Cyclopoida (Rosen 1981), aquatic insects (Benke et al. 1999), Collembola (Ganihar 1997) and other terrestrial invertebrates (Gowing and Recher 1984). For Ostracoda, we used a mean dry mass value obtained by weighting samples from the studied pond (individual ostracod DM = 0.0065 ± 0.0012 mg after 48 h at 50 °C, n = 4). Differences in diet composition (DC) according to species and sexes were assessed using a two-way multivariate PERMANOVA that employed a similarity matrix based on Bray–Curtis distances calculated from dry mass proportion of prey per stomach for DC(DM) and from square root transformed proportion of prey abundance per stomach for DC(N) (Anderson 2001). Proportions were used to account for differences in stomach capacity and square root transformation in the case of DC(N) was carried out to balance the contribution from smaller but more abundant prey (e.g. microcrustaceans) relative to larger but less abundant ones (e.g. macroinvertebrates) (Anderson et al. 2008). We used the average Bray–Curtis similarity between group pairs as a measure of diet similarity (Anderson et al. 2008). We subsequently used a similarity percentage (SIMPER) analysis to assess the average percent contribution of each prey type to the dissimilarities between diets that corresponded to significant differences according to PERMANOVA (Clarke 1993), and conducted a two way univariate PERMANOVA (Euclidean distances) on each prey type to assess significant differences in individual prey type consumption according to species, sex and their interaction. Significant interaction terms in PERMANOVA were examined by pairwise comparisons using permutational t-tests. All permutation based tests were computed with 9,999 permutations to assess significance of the results. In cases where too few unique permutations would be allowed by the model, p-values were approximated using Monte-Carlo simulation (Clarke and Gorley 2006). Homogeneity of multivariate dispersion was tested prior to all PERMANOVA using permutational analysis of multivariate dispersion (PERMDISP). PERMDISP, PERMANOVA, permutational t-tests and SIMPER were performed using PRIMER version 7 software (Clarke and Gorley 2006) and the PERMANOVA + add-in (Anderson et al. 2008).
Stable isotope analysis
All samples were oven-dried at 60 °C for 72 h (BINDER B28) and ground into a homogeneous powder with mortar and pestle. Stable isotope ratios of carbon and nitrogen were measured using an isotope ratio mass spectrometer (Isoprime 100, Isoprime, UK) coupled in continuous flow to an elemental analyser (VarioMicro, Elementar, Germany). Stable isotope ratios were conventionally expressed as δ values in ‰ (Coplen 2011). Certified reference materials from the International Atomic Energy Agency (IAEA, Vienna, Austria) used were ammonium sulphate (IAEA-N2; δ15N = 20.3 ± 0.2‰) and sucrose (IAEA C-6; δ13C = − 10.8 ± 0.5‰). Both these reference materials are calibrated against the international references Vienna Pee Dee Belemnite for carbon samples and atmospheric air for nitrogen. Internal standards (glycine) were inserted into all runs at regular intervals to assess potential drift over time. Repetitive measurements of glycine (δ15N = 2.3 ± 0.3‰; δ13C = − 47.5 ± 0.3‰) were also used as an elemental standard. One of the samples was randomly selected and analysed multiple times (once every 15 analyses). Analytical precision (± SD) on replicated samples equalled 0.2‰ for δ13C and 0.3‰ for δ15N.
We used Stable Isotope Bayesian Ellipses in R (SIBER; Jackson et al. 2011) to generate bivariate standard ellipses representing core isotopic niches of each predator, using the package SIBER version 2.1.0 in R version 3.3.1. To account for sample size differences, areas of the ellipses associated with each group (Standard Ellipse Area B; SEAB) were computed using Bayesian inference (MCMC parameters: 2 chains, 200,000 iterations, 10,000 burn-ins, thins = 50, and using an inverted wishart prior). SEAB were calculated and compared according to species and sex using direct pairwise comparison of their posterior distributions. We calculated the percentage of overlap (PO) between each group based on single estimates of standard ellipses area corrected for small sample sizes (SEAc). Percentage of overlap between group a and b was calculated following the equation: PO = 100 × Overlap(ab)/(SEAc(a) + SEAc(b) − Overlap(ab)). Differences in location of the isotopic niches according to species, sex and their interaction were tested using two-way bivariate PERMANOVA (Euclidean distance) on carbon and nitrogen stable isotope ratios of the consumers. Significance of the interaction term was examined by pairwise comparisons using post hoc permutational t-tests.
We used Bayesian mixing models with ‘uninformative’/generalist priors to model the contribution of different food sources to the assimilated diet of predators using ‘Mixsiar’ ver. 3.1.7 (Stock et al. 2018). Sources implemented into the models were selected on the basis of stomach content data, as reflecting different microhabitats or feeding strategies. Multiple models were run, gradually pooling sources into ecologically relevant categories and according to their isotopic similarity while making sure that consumers remain within the source polygon. List of sources implemented in the final models are presented in Table S2 while isotope values of all collected samples are represented in Fig. S3. For newts, we applied trophic discrimination factors (TDFs) of amphibian skin: 2.3 ± 0.5‰ for δ15N and 0.1 ± 0.4‰ for δ13C (Cloyed et al. 2015), which were successfully used in previous studies on newts trophic ecology (Lejeune et al. 2018, 2021). For mosquitofish, we applied general TDFs from McCutchan et al. (2003) for invertebrate sources (1.4 ± 0.21‰ for δ15N and 1.3 ± 0.3‰ for δ13C), and vertebrate sources (3.3 ± 0.26‰ for δ15N and 1.3 ± 0.3‰ for δ13C), which were previously used to study mosquitofish trophic ecology (Remon et al. 2016). Models were set to account for process and residual errors. MCMC parameters were, for newts: 3 chains, 100,000 iterations, 50,000 burn-in, 50 thins, and for fish: 3 chains, 300,000 iterations, 200,000 burn-in, 100 thins. Markov Chain convergence was assessed by visual analysis of trace plots, complemented with Gelman-Rubin, Geweke, and Heidelberger and Welch diagnostics (Stock et al. 2018). We used Deviance Information Criterion (DIC) to compare model performances and select those that were most supported by the data (Spiegelhalter et al. 2002). While all models gave similar results and different ways of pooling sources had no consequences on their critical interpretation, only the most performant model was presented.
Results
Population densities and sexual size dimorphism
On average (mean ± SD), 6.5 ± 2.6 adult eastern mosquitofish and 0.7 ± 0.1 adult metamorphic palmate newts were captured per square meter over one hour of sampling, so that mosquitofish population could be estimated to be about 9 times larger than that of newts. During our two survey periods, palmate newts (Lissotriton helveticus) were the main caudate amphibian found in the pond (we found one marbled newt Triturus marmoratus). Marsh frogs Pelophylax ridibundus (kurtmueleri lineage) were also present but not abundant with only a few adults spotted at the pond, and no tadpoles were found during sampling. No newt larvae were captured or observed despite the observation of newt reproductive activity in the pond (courtship and egg-laying) and the occurrence of eggs in the aquatic vegetation.
Both palmate newt and mosquitofish depicted sexual size dimorphism (Pseudo-F1,85 = 94.6, p-value < 0.001), with males being smaller than females (mean ± SD = 41.7 ± 1.8 mm vs. 46.3 ± 4.5 mm, for palmate newts males and females, and 27.4 ± 1.6 mm vs. 40 ± 6.2 mm, for mosquitofish males and females) (Table S3 and S4). The sexual size dimorphism was stronger within mosquitofish than within palmate newts, with female mosquitofish having body size similar to that of male palmate newts (Table S5).
Stomach content analysis
Analyses of diet composition based on prey abundance (DC(N)) and dry mass (DC(DM)) provided congruent results (Table 1). Stomach contents of newts were largely dominated by Chironomidae larvae in terms of abundance (N = 2.7 ± 0.5 and 4.1 ± 0.7 items per individual for females and males, respectively), dry mass proportions (%DM = 82.5 ± 7.3% and 87.6 ± 8.0%) and percent frequency of occurrence (%FOO = 79 and 78%) (Table 1). The contribution of Chironomidae larvae was significantly less in mosquitofish (Pseudo-F1,74 = 49.36 and 88.58, p < 0.001 for both DC(N) and DC(DM), respectively; Table S6), with N = 1.1 ± 0.4 and 0.5 ± 0.2 items per individual, %DM = 18.2 ± 6.2% and 9.7 ± 7.7%, and %FOO = 38 and 26% in females and males, respectively. By contrast, mosquitofish relied significantly more on terrestrial invertebrates (Pseudo-F1,74 = 5.87 and 12.79, p < 0.05 and < 0.001 for DC(N) and DC(DM), respectively), with N = 0.4 ± 0.1 and 0.5 ± 0.1, %DM = 16.7 ± 6.1% and 41.6 ± 13.6%, and %FOO = 34 and 32% for females and males respectively. Diet of female mosquitofish was dominated by Ostracoda (N = 23.3 ± 10.2 items per individual, %DM = 30.7 ± 7.7% and %FOO = 72%), with a contribution significantly higher than in female newts, but similar contributions in males of both species. Male mosquitofish were significantly more reliant on Cladocera than the three other groups (N = 0.7 ± 0.3 items per individual, %DM = 23.3 ± 12.1% and %FOO = 21%) (see PERMANOVA interaction term and pairwise permutational t-tests in Table S6 and S7). No clear evidence of vertebrate prey ingestion could be found in gut contents of mosquitofish sampled in 2015. However, specific search for vertebrate prey ingestions based on dissection of whole digestive tracts of mosquitofish sampled in 2018 provided the following results: 10 out of 70 female mosquitofish had newt body parts in their guts (%FOO = 14.3%) with sizes corresponding to hatchlings or embryos in final stages of development. %FOO of newt body parts in male mosquitofish was 2.9% (2 out of 70 individuals). Cannibalism towards alevins was evident in two female mosquitofish (%FOO = 2.9%).
Analysis of DC(N) and DC(DM) using PERMANOVA confirmed global significant differences between newts and mosquitofish diet in 2015 (Pseudo-F1,74 = 16.42 and 24.34, respectively, p < 0.001 for both), without global sex effect, but with a significant species × sex interaction (Pseudo-F1,74 = 7.30 and 2.72, p < 0.001 and < 0.05, respectively) (Table S8). All pairwise differences were significant except between female and male palmate newts in both DC(N) and DC(DM) analyses (Table S9). Accordingly diet similarities were higher between female and male palmate newts (56.9% and 72.5% for DC(N) and DC(DM), respectively) than between any other consumer groups. Diet similarities between palmate newts and mosquitofish were low (22–31.1% and 10–19.9 for DC(N) and DC(DM), respectively). The lowest similarity was found between females and males mosquitofish (20.3%) for DC(N) and between males of both species (10%) for DC(DM). There were no significant differences in Shannon index (used as a measure of dietary niche width) between species (Pseudo-F1,74 = 0.01, p = 0.923), sex (Pseudo-F1,74 = 0.049, p = 0.83) or interaction (Pseudo-F1,74 = 4.61, p = 0.058) (Table S3 and S8).
Similarity percentage (SIMPER) analysis based on prey abundance and dry mass data yielded very similar results, identifying Chironomidae larvae as the main contributor to the observed dissimilarities between palmate newts and mosquitofish diet (33.3% and 44.4% for DC(N) and DC(DM), respectively, and contributing more to the diet of newts than to fish), followed by Ostracoda, Chironomidae pupae, terrestrial invertebrates and Cladocera, all contributing more to the diet of fish than to newts for a cumulative contribution of these five items > 90% to diet dissimilarities (Table S10).
Stable isotope analysis
There was no overlap between the core isotopic niches of the two species (Fig. 2), and the percentage of proportional overlap remained relatively low between sexes within each species (13 and 18% for newts and mosquitofish, respectively) (Table S11). Mosquitofish displayed higher δ15N values (mean ± SD = 5.6 ± 0.8 and 6.5 ± 0.8‰) than palmate newts (mean ± SD = 4.2 ± 0.5 and 4.2 ± 0.3‰ for females and males, respectively) and higher δ13C values (mean ± SD = − 24 ± 0.6 and − 23.5 ± 0.7‰) than palmate newts (mean ± SD = − 24.3 ± 0.2 and − 24.5 ± 0.2‰ for females and males, respectively) (Table S12). PERMANOVA and subsequent pairwise permutational t-tests confirmed differences in dietary niche location among all groups (p < 0.05), except between palmate newt females and males (significant interaction term in PERMANOVA: Pseudo-F1,85 = 10.11, p < 0.001; pairwise permutational t-test: t38 = 1.345, p = 0.177) (Table S13 and S14). The core isotopic niches of mosquitofish were on average 3 to 5 times wider than that of newts (SEAB = 0.83‰2 (0.58–1.21) and 1.22‰2 (0.79–2.02) for female and male mosquitofish vs. 0.26‰2 (0.18–0.41) and 0.12‰2 (0.07–0.20) for female and male newts, respectively) (Fig. 3; Table S12). Pairwise comparisons of niche area posterior distributions indicated that the differences were particularly robust between species (posterior probability = 100%) and between female and male newts (posterior probability = 99%), but not between female and male mosquitofish (posterior probability < 95%) (Table S15).
Stable isotope biplot depicting consumers in the isospace of carbon and nitrogen stable isotopes. Standard ellipses area represent the core isotopic niche of each group. Blue colour, triangles = native palmate newts (Lissotriton helveticus). Grey colour, circles = introduced eastern mosquitofish (Gambusia holbrooki). Full lines, plain symbols = Males. Dashed lines, hollow symbols = Females. Male newt and female fish illustrations to scale
Bayesian estimates of the Standard Ellipse Areas (SEAB) of palmate newts and mosquitofish. Blue = female (♀) and male (♂) palmate newts, Grey = female (♀) and male (♂) mosquitofish. Black dots indicate SEAB modes, rectangles encompass 50%, 75% and 95% credible intervals, from the darkest to the lightest, respectively. Male newt and female fish illustrations to scale
According to stable isotope mixing models, newts relied mainly on Chironomidae (modes: 43% (CI95 = 4–75) and 58% (CI95 = 15–80) for females and males, respectively) (Fig. 4). Chironomidae were on average less important in the diet of mosquitofish than in that of newts (modes: 18% (CI95 = 1–52) and 9% (CI95 = 1–33) for females and males, respectively). Conversely, mosquitofish relied much more on vertebrates (i.e. mosquitofish larvae and newt eggs, and potentially newt larvae or other amphibian eggs) which represented their main assimilated food source during the past months (modes: 41% (CI95 = 24–51) and 56% (CI95 = 40–65), for females and males, respectively) than for newts (modes: 15% (CI95 = 1–34) and 10% (CI95 = 2–20), for females and males, respectively). Reliance on terrestrial invertebrates was similar in the diets of newts and female mosquitofish (modes from 22 to 30% (CI95 = 9–49), while it was lower for male mosquitofish (mode: 8%, CI95 = 0–32). Reliance on mesozooplankton and benthic microcrustaceans (Ostracoda) was on average lower for newts and female mosquitofish, representing 10 to 15% (CI95 = 1–25) of their diet compared to male mosquitofish (mode: 24%, CI95 = 5–36).
Contribution of different food sources implemented in stable isotope mixing models to the assimilated diet of newts (blue) and mosquitofish (grey). Chironomidae = Chironomidae larvae. Terrestrial invert. = terrestrial invertebrates found drowning at the water surface. Vertebrates = palmate newt eggs and mosquitofish larvae. Microcrust = microcrustaceans (mesozooplankton and Ostracoda). ♀ = females, ♂ = males. Note that ‘Vertebrates’ contribution may also be influenced by the consumption of anuran eggs which often have stable isotope composition close to that of palmate newt eggs but were not sampled in this pond. Male newt and female fish illustrations to scale
Discussion
Both stomach contents and stable isotope analysis provided evidence of resource partitioning between introduced eastern mosquitofish Gambusia holbrooki and native palmate newts Lissotriton helveticus. Newt and potentially other amphibian eggs and larvae together with conspecific larvae were the main contributors to mosquitofish assimilated diet. Mosquitofish and newts partitioned available invertebrate resources in accordance with their body size and trophic morphology, with mosquitofish mainly consuming microcrustaceans (benthic Ostracoda and mesozooplankton) and newts focusing on burrowing benthic macroinvertebrates; both prey types found to be abundant in the studied pond. Together, these results suggest that overlap in resource use may not be the primary negative impact of mosquitofish on newts. Since adult newts were still able to exploit resources, court and lay eggs in this invaded habitat, but apparently without larval survival during the two years studied, this study raises concerns that mosquitofish likely exert important predation on eggs and larvae and that invaded habitats may act as demographic sinks for amphibians.
Mosquitofish partition resources with adult newts but predate on newt eggs and larvae
Both gut content and stable isotope niche modelling revealed little overlap between the dietary niche and the core isotopic niches of two species (22–31% or 10–20% for dietary niches based on prey abundance or dry mass proportions, respectively, and 0% for core isotopic niches). These corresponded to significant differences in all cases, suggesting that competition for resources could be low between adult palmate newts and mosquitofish at the time of sampling. Congruently, the estimated palmate newt population density (0.7 ± 0.1 individuals*m−2) fell within the range of what was found in other non-invaded ponds during the reproductive season (0.3–4.3 individuals*m−2) (Lejeune et al. 2021) despite the high abundance of mosquitofish in the pond. According to gut content analysis, palmate newts were relying mainly on burrowing macroinvertebrates such as Chironomidae, while mosquitofish were relying mainly on microcrustaceans (ostracods for females, mesozooplankton for males) and terrestrial invertebrates. According to stable isotopes, partitioning globally followed the same pattern, but with a lower contribution of ostracods and mesozooplankton to the diet of mosquitofish in favour of vertebrate prey. Newts relied mainly on Chironomidae according to mixing models (mean contribution = 43–58%), but the models identified ‘vertebrate’ source of food as the main contributor to the diet of mosquitofish (mean contribution = 41–56%). Terrestrial invertebrates also represented an important food source for both newts and female mosquitofish (mean contribution between 22 and 30%). These diet differences are consistent with body size differences of the two predators as body size is an important factor of niche differentiation and smaller aquatic predators are generally limited to smaller prey due to gape-size limitation (Cohen et al. 1993; Lejeune et al. 2021). It is also concordant with the trophic morphology of mosquitofish (e.g. superior mouth) which is more typical of surface feeders (Hugueny and Pouilly 1999) and may prevent them from foraging efficiently on burrowing prey.
Discrepancies between gut content and stable isotope analyses
A discrepancy existed between the gut content and stable isotope results regarding the importance of vertebrate sources in the diet of mosquitofish. ‘Vertebrate’ sources (i.e. amphibian eggs or larvae and/or mosquitofish larvae) were identified as the main food source for mosquitofish according to stable isotope mixing models, but no evidence of ingestion could be found in gut contents sampled in 2015. Yet, in samples collected from 2018, multiple instances of newt hatchlings and one instance of mosquitofish larvae ingestions were found. Newt hatchling ingestions were frequent in females but rare in males (frequency of occurrence = 14.3% and 2.9% respectively). Differences in sample size and sampling method (flushing vs. dissection) between 2015 and 2018 might partly explain the differences observed between the two years. But, a similar discrepancy between stable isotopes and stomach contents results was also noted in a study involving G. holbrooki foraging on anuran eggs (Remon et al. 2016). A mismatch between sampling time and the life-history of newts or inadequate dipnets mesh-size cannot explain the absence of detection of hatchlings or newt larvae from the pond or in mosquitofish gut contents in 2015. Indeed, the timing of sampling aligned with the life-history of palmate newts, which are long-term breeders, starting to court and lay eggs in late winter or early spring and continuing for several months in the study area; with larvae hatching around one to four weeks later depending on temperature (Gabrion et al. 1977; Galloy and Denoël, 2010). This is also confirmed by the observation and capture of both adults and larvae in similar but uninvaded ponds from the same region and altitude, and following the same protocol from April to June (2014–2015) (Lejeune et al. 2021). Multiple other factors may explain this discrepancy. Stable isotopes integrate diet information over a longer time period than stomach content, the latter being only a snapshot. High digestion rate of carnivorous fish such as mosquitofish may lower chances of identification of eggs or hatchlings in gut contents (Pyke 2005) and while gut contents provide direct information on food uptake, not all ingested prey are equally assimilated, or assimilated at all (Prestidge 1979; Rudnick and Resh 2005). By contrast, stable isotopes provide indirect information on the assimilated diet, and higher assimilation rate of vertebrate food sources compared to invertebrate food sources may contribute to explaining this result (McCutchan et al. 2003). In this study, it was not possible to distinguish between the contribution of mosquitofish larvae and newt eggs to ‘vertebrate’ source as both had undiscernible signal in the mixing models and therefore had to be grouped in the final model. It is therefore also possible that part of the “vertebrate” isotopic signal relates to cannibalism which may be more frequent in early spring when other prey may be less available in the pond (Remon et al. 2016). Multiple studies have demonstrated the importance of cannibalism in mosquitofish, especially when population density is high (Dionne 1985; Pyke 2005), which appears plausible in our situation. Finally, anuran eggs sometimes have an isotopic composition close to that of newt eggs (Lejeune et al. 2021). “Vertebrate” signal might incorporate anuran eggs and tadpoles which were absent at the time of sampling but present earlier in the season (M. Denoël, personal observation of bufonid tadpoles in the studied pond). Besides these considerations, it appears clear from the absence of newt larvae in the pond despite reproductive activity spotted during sampling (courtship and egg-laying), the presence of eggs in the aquatic vegetation and their high frequency in the gut contents of dissected mosquitofish from 2018, that this isotope signal includes signs of predation on newt eggs or hatchlings. Several studies have demonstrated that mosquitofish effectively predate on amphibian larvae, including newts, even when alternative types of prey are abundant (Cabrera-Guzmán et al. 2017; Gamradt and Kats 1996; Goodsell and Kats 1999; Remon et al. 2016). Conversely, Reynolds (2009) found that albeit not consuming amphibian eggs directly, G. holbrooki were consuming amphibian hatchlings but showed a preference for invertebrate prey (i.e. mosquito larvae and Daphniidae) whenever available. The increased microcrustacean consumption in stomach contents compared to stable isotope information may reflect their seasonal increased availability in the pond ecosystem. Similarly, and although speculative, higher dietary contribution of vertebrate food sources according to stable isotopes compared to gut content analysis (amphibian eggs or larvae, or mosquitofish larvae) might reflect potential predation earlier in the season. Switching from a diet strongly influenced by vertebrate consumption in early spring towards increased consumption of ostracods for female and Cladocera for male mosquitofish might contribute to explaining the lower overlap between their dietary niches compared to isotopic niches.
Demographic implications of coexistence without larval survival
Overall, our results suggest that introduced mosquitofish may not constitute a direct threat for adult palmate newts in the studied pond, as they still manage to forage on abundant burrowing prey that appeared to be less consumed by mosquitofish. However, because mosquitofish consume newt eggs, hatchlings or larvae (Cabrera-Guzmán et al. 2017; Gamradt and Kats 1996; Preston et al. 2012; Vannini et al. 2018), the population dynamic may be strongly affected. If the potential for resource partitioning between adult newts and introduced mosquitofish persists throughout the reproductive period, newt recruitment from other ponds within migration distance could continue successfully over time, therefore constituting a demographic sink with a global detrimental effect on palmate newts at the metapopulation level (Woodford and Mcintosh 2010). This hypothesis is consistent with the fact that newts could still be found in abundance in this pond more than 50 years after mosquitofish introduction (Gabrion et al. 1977), and successfully produce eggs but without apparent larval survival. The studied pond was not isolated. Previous research showed that ponds can be quickly colonised by palmate newts in the study area (Denoël and Winandy 2015) and the nearest fishless pond inhabited by palmate newts was located 340 m away. However, it cannot be excluded that newts were more abundant than usual or that mosquitofish pressure was higher in the studied year; therefore calling for in-depth analyses of changes in newts and mosquitofish populations over time for a complete understanding of mosquitofish impacts on newts. Despite this, our results are congruent with research conducted on other newt species, such as evidenced by Preston et al. (2012) showing no significant influence of introduced mosquitofish presence on California newts (Taricha torosa) occupancy in wetlands despite evidence of predation on larvae in mesocosms experiments. This suggests that in some cases, adult newts may be unable to identify mosquitofish as a threat or at least not be driven to exclusion from the habitat by competition. Amphibians are generally recognized as more naïve towards introduced predators due to the heterogeneity of predation regimes in freshwater systems compared to terrestrial and marine systems where functionally equivalent predators are often widely and homogeneously distributed (Cox and Lima 2006). Yet, studies involving palmate newt exposure to other introduced fish species such as the goldfish revealed significant patterns of avoidance and negative effects on newt activity (Winandy et al. 2016; Winandy and Denoël, 2015) or even escape from the aquatic environment (Winandy et al. 2015). This was also demonstrated regarding mosquitofish impact on paedomorphic newts (adults which retained larval features following an alternative developmental pathway) (Toli et al. 2020). More studies on the behavioural response of metamorphic newts to mosquitofish may shed light on whether they perceive this species as a threat or not to help understand such coexistence cases. Captured newts did not show particular signs of attacks by mosquitofish (e.g. notched caudal fins, missing limbs) as can sometimes be observed in ponds inhabited by large populations of mesopredators such as Aeshnidae or Dytiscidae (B. Lejeune and M. Denoël, pers. obs.). Yet the possibility of non-consumptive negative interactions between mosquitofish and palmate newt still exists.
Perspectives
With respect to species conservation, one may argue that ‘sink’ habitats are more detrimental to newts than a simple disappearance of the habitat patch, because native newts would keep wasting their reproductive potential in an unsuitable habitat, year after year, while they could have instead contributed to population turnover in other suitable habitats within their reach. But on the other hand, in such situations, newts could potentially survive for years in the presence of the introduced fish, which may provide time to implement conservation measures and hopefully restore the population by eliminating the fish. Indeed, in newts, even if larval survival is reduced or suppressed, survival of the adults may be more important in maintaining stable populations (Biek et al. 2002). In situations where ponds are more isolated and source-sink dynamics cannot occur due to migration constraints, mosquitofish introductions may directly provoke local population extinctions. External factors or differences among species biology might also influence population survival for instance via boom years of newt or mosquitofish reproduction. Long-term studies incorporating information on potential newt recruitment from nearby ponds in more complex settings (e.g. comparing demographic parameters and trophic ecology across areas subject to different pressures of mosquitofish invasion) and different newt species would ultimately help forecast consequences for native populations and help identify if ponds invaded by mosquitofish effectively act as demographic sinks for newts or other native species to inform potential conservation measures.
Data availability
The datasets generated and analyzed during the current study are available on request to the corresponding author.
References
Anderson MJ (2001) Permutation tests for univariate or multivariate analysis of variance and regression. Can J Fish Aquat Sci 58:626–639. https://doi.org/10.1139/cjfas-58-3-626
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: a guide to software and statistical methods. PRIMER-E: Plymouth, UK
Baumgärtner D, Rothhaupt KO (2003) Predictive length–dry mass regressions for freshwater invertebrates in a pre-alpine lake littoral. Int Rev Hydrobiol 88:453–463. https://doi.org/10.1002/iroh.200310632
Benke AC, Huryn AD, Smock LA, Wallace JB (1999) Length-mass relationships for freshwater macroinvertebrates in North America with particular reference to the southeastern United States. J N Am Benthol Soc 18:308–343. https://doi.org/10.2307/1468447
Biek R, Funk WC, Maxell BA, Mills LS (2002) What is missing in amphibian decline research: insights from ecological sensitivity analysis. Cons Biol 16:728–734. https://doi.org/10.1046/j.1523-1739.2002.00433.x
Bounas A, Keroglidou M, Toli E-A, Chousidis I, Tsaparis D, Leonardos I, Sotiropoulos K (2020) Constrained by aliens, shifting landscape, or poor water quality? Factors affecting the persistence of amphibians in an urban pond network. Aquat Conserv Mar Freshw Ecosyst 30:1037–1049. https://doi.org/10.1002/aqc.3309
Brosse L, Lepage M (2000) First results on the diet of the young Atlantic sturgeon Acipenser sturio L., 1758 in the Gironde estuary. Bol Inst Español Oceanogr 16:75–80
Bucciarelli GM, Blaustein AR, Garcia TS, Kats LB (2014) Invasion complexities: the diverse impacts of nonnative species on amphibians. Copeia 2014:611–632. https://doi.org/10.1643/OT-14-014
Cabrera-Guzmán E, Díaz-Paniagua C, Gomez-Mestre I (2017) Competitive and predatory interactions between invasive mosquitofish and native larval newts. Biol Invasions 19:1449–1460. https://doi.org/10.1007/s10530-017-1369-5
Cabrera-Guzmán E, Díaz-Paniagua C, Gomez-Mestre I (2019) Invasive mosquitofish (Gambusia holbrooki) affect egg-laying and behaviour of Spanish pygmy newts (Triturus pygmaeus). Amphib Reptil 40:103–112. https://doi.org/10.1163/15685381-20181019
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
Clarke KR, Gorley RN (2006) PRIMER v6: User Manual/Tutorial (Plymouth Routines in Multivariate Ecological Research). PRIMER-E, Plymouth, UK.
Cloyed CS, Newsome SD, Eason PK (2015) Trophic discrimination factors and incorporation rates of carbon- and nitrogen-stable isotopes in adult green frogs, Lithobates clamitans. Physiol Biochem Zool 88:576–585. https://doi.org/10.1086/682576
Cohen JE, Pimm SL, Yodzis P, Saldaña J (1993) Body sizes of animal predators and animal prey in food webs. J Anim Ecol 62:67–78. https://doi.org/10.2307/5483
Coplen TB (2011) Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun Mass Sp 25:2538–2560. https://doi.org/10.1002/rcm.5129
Cox JG, Lima SL (2006) Naiveté and an aquatic-terrestrial dichotomy in the effects of introduced predators. Trends Ecol Evol 21:674–680. https://doi.org/10.1016/j.tree.2006.07.011
Denoël M (2007) Le Triton palmé, Triturus helveticus (Razoumowski, 1789). In: Jacob JP, Percsy C, De Wavrin H et al (eds) Amphibiens et Reptiles de Wallonie. Aves-Raînne & Région wallonne, Namur, pp 86–95
Denoël M, Ficetola GF (2014) Heterochrony in a complex world: disentangling environmental processes of facultative paedomorphosis in an amphibian. J Anim Ecol 83:606–615
Denoël M, Winandy L (2015) The importance of phenotypic diversity in conservation: resilience of palmate newt morphotypes after fish removal in Larzac ponds (France). Biol Conserv 192:402–408. https://doi.org/10.1016/j.biocon.2015.10.018
Denoël M, Džukić G, Kalezić ML (2005) Effects of widespread fish introductions on paedomorphic newts in Europe. Conserv Biol 19:162–170. https://doi.org/10.1111/j.1523-1739.2005.00001.x
Denoël M, Ficetola GF, Sillero N, Džukić G, Kalezić ML, Vukov T, Muhovic I, Ikovic V, Lejeune B (2019) Traditionally managed landscapes do not prevent amphibian decline and the extinction of paedomorphosis. Ecol Monogr 89:e01347. https://doi.org/10.1002/ecm.1347
Dionne M (1985) Cannibalism, food availability, and reproduction in the mosquito fish (Gambusia affinis): a laboratory experiment. Am Nat 126:16–23. https://doi.org/10.1086/284392
Gabrion J, Sentein P, Gabrion C (1977) Les populations néoténiques de Triturus helveticus des Causses et du Bas-Languedoc I. Répartition et caractéristiques. La Terre La Vie 31:489–506
Galloy V, Denoël M (2010) Detrimental effect of temperature increase on the fitness of an amphibian (Lissotriton helveticus). Acta Oecol 36:179–183. https://doi.org/10.1016/j.actao.2009.12.002
Gamradt SC, Kats LB (1996) Effect of introduced crayfish and mosquitofish on California newts. Conserv Biol 10:1155–1162. https://doi.org/10.1046/j.1523-1739.1996.10041155.x
Ganihar SR (1997) Biomass estimates of terrestrial arthropods based on body length. J Biosci 22:219–224. https://doi.org/10.1007/BF02704734
Goodsell JA, Kats LB (1999) Effect of introduced mosquitofish on pacific treefrogs and the role of alternative prey. Conserv Biol 13:921–924. https://doi.org/10.1046/j.1523-1739.1999.98237.x
Gowing G, Recher HF (1984) Length-weight relationships for invertebrates from forests in south-eastern New South Wales. Aust J Ecol 9:5–8. https://doi.org/10.1111/j.1442-9993.1984.tb01612.x
Guyetant R, Pinston H, Herold JP, Rougeot JC (1991) Étude de populations de tritons: Triturus alpestris et T. helveticus dans une mare temporaire d’altitude (est de la France–Massif du Jura). In: Bagnilières JL, Castanet J, Conand F, Meunier FJ (eds) Tissus durs et âge individuel des vertébrés. ORSTIOM-INRA, pp 355–362
Hartel T, Nemes S, Cogǎlniceanu D, Öllerer K, Schweiger O, Moga CI, Demeter L (2007) The effect of fish and aquatic habitat complexity on amphibians. Hydrobiologia 583:173–182. https://doi.org/10.1007/s10750-006-0490-8
Hayden B, Soto DX, Jardine TD, Graham BS, Cunjak RA, Romakkaniemi A, Linnansaari T (2015) Small tails tell tall tales - intra-individual variation in the stable isotope values of fish fin. PLoS ONE 10:1–18. https://doi.org/10.1371/journal.pone.0145154
Howe RW, Davis GJ, Mosca V (1991) The demographic significance of ‘sink’ populations. Biol Conserv 57:239–255. https://doi.org/10.1016/0006-3207(91)90071-G
Hugueny B, Pouilly M (1999) Morphological correlates of diet in an assemblage of West African freshwater fishes. J Fish Biol 54:1310–1325. https://doi.org/10.1111/j.1095-8649.1999.tb02057.x
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER - stable isotope Bayesian ellipses in R. J Anim Ecol 80:595–602. https://doi.org/10.1111/j.1365-2656.2011.01806.x
Jackson MC, Loewen CJG, Vinebrooke RD, Chimimba CT (2016) Net effects of multiple stressors in freshwater ecosystems: a meta-analysis. Glob Change Biol 22:180–189. https://doi.org/10.1111/gcb.13028
Joly P (1987) Le régime alimentaire des amphibiens méthodes d’étude. Alytes 6:11–17
Kiesecker JM, Blaustein AR, Miller CL (2001) Transfer of a pathogen from fish to amphibians. Conserv Biol 15:1064–1070. https://doi.org/10.1046/j.1523-1739.2001.0150041064.x
Klop-Toker K, Valdez J, Stockwell M, Clulow S, Clulow J, Mahony M (2018) Community level impacts of invasive mosquitofish may exacerbate the impact to a threatened amphibian. Austral Ecol 43:213–224. https://doi.org/10.1111/aec.12558
Knapp RA (2005) Effects of nonnative fish and habitat characteristics on lentic herpetofauna in Yosemite National Park, USA. Biol Conserv 121:265–279. https://doi.org/10.1016/j.biocon.2004.05.003
Knapp RA, Matthews KR, Sarnelle O (2001) Resistance and resilience of alpine lake fauna to fish introductions. Ecol Monogr 71:401–421. https://doi.org/10.1890/0012-9615(2001)071[0401:raroal]2.0.co;2
Komak S, Crossland MR (2000) An assessment of the introduced mosquitofish (Gambusia affinis holbrooki) as a predator of eggs, hatchlings and tadpoles of native and non-native anurans. Wildl Res 27:185–189. https://doi.org/10.1071/WR99028
Lejeune B, Sturaro N, Lepoint G, Denoël M (2018) Facultative paedomorphosis as a mechanism promoting intraspecific niche differentiation. Oikos 127:427–439. https://doi.org/10.1111/oik.04714
Lejeune B, Bissey L, Didaskalou EA, Sturaro N, Lepoint G, Denoël M (2021) Progenesis as an intrinsic factor of ecological opportunity in a polyphenic amphibian. Funct Ecol 35:546–560. https://doi.org/10.1111/1365-2435.13708
Mahony MJ, Hamer AJ, Pickett EJ, McKenzie DJ, Stockwell MP, Garnham JI, Keely CC, Deboo ML, O’Meara J, Pollard CJ, Clulow S, Lemckert FL, Bower DS, Clulow J (2013) Identifying conservation and research priorities in the face of uncertainty: a review of the threatened bell frog complex in eastern Australia. Herpetol Conserv Biol 8:519–538
McCutchan JHJ, Lewis WM, Kendall C, McGrath CC (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102:378–390. https://doi.org/10.1034/j.1600-0706.2003.12098.x
Miaud C (1991) La squelettochronologie chez les Triturus (amphibiens, urodèles) à partir d'une étude de T. alpestris, T. helveticus et T. cristatus du sud-est de la France. In: Bagnilières JL, Castanet J, Conand F, Meunier FJ (eds) Tissus durs et âge individuel des vertébrés. ORSTIOM-INRA, pp 363–384
Orizaola G, Braña F (2006) Effect of salmonid introduction and other environmental characteristics on amphibian distribution and abundance in mountain lakes of northern Spain. Anim Conserv 9:171–178. https://doi.org/10.1111/j.1469-1795.2006.00023.x
Pope KL (2008) Assessing changes in amphibian population dynamics following experimental manipulations of introduced fish. Conserv Biol 22:1572–1581. https://doi.org/10.1111/j.1523-1739.2008.00998.x
Prestidge RA (1979) Ingestion and assimilation efficiency of Aeshna brevistyla and Hemicordulia australiae larvae (Odonata). New Zeal J Mar Freshw Res 13:193–199. https://doi.org/10.1080/00288330.1979.9515794
Preston DL, Henderson JS, Johnson PTJ (2012) Community ecology of invasions: direct and indirect effects of multiple invasive species on aquatic communities. Ecology 93:1254–1261. https://doi.org/10.1890/11-1821.1
Pyke GH (2005) A review of the biology of Gambusia affinis and G. holbrooki. Rev Fish Biol Fish 15:339–365. https://doi.org/10.1007/s11160-006-6394-x
Pyke GH (2008) Plague minnow or mosquito fish? A review of the biology and impacts of introduced Gambusia species. Annu Rev Ecol Evol Syst 39:171–191. https://doi.org/10.1146/annurev.ecolsys.39.110707.173451
Rauchenberger M (1989) Systematics and biogeography of the genus Gambusia (Cyprinodontiformes: Poecilidae). Am Museum Novit 1–74.
Remon J, Bower DS, Gaston TF, Clulow J, Mahony MJ (2016) Stable isotope analyses reveal predation on amphibians by a globally invasive fish (Gambusia holbrooki). Aquat Conserv Mar Freshw Ecosyst 26:724–735. https://doi.org/10.1002/aqc.2631
Reynolds SJ (2009) Impact of the introduced Poeciliid Gambusia holbrooki on amphibians in southwestern Australia. Copeia 2009:296–302. https://doi.org/10.1643/CH-08-101
Richardson MJ, Whoriskey FG, Roy LH (1995) Turbidity generation and biological impacts of an exotic fish Carassius auratus, introduced into shallow seasonally anoxic ponds. J Fish Biol 47:576–585
Rolla M, Biffoni G, Brighenti S, Iacobuzio R, Liautaud K, Pasquaretta C, Tiberti R (2018) Predation by introduced fish can magnify the terrestrial arthropod subsidies in mountain lakes. Can J Fish Aquat Sci 75:1453–1464. https://doi.org/10.1139/cjfas-2017-0121
Rosen RA (1981) Length-dry weight relationships of some freshwater zooplankton. J Freshw Ecol 1:225–229. https://doi.org/10.1080/02705060.1981.9664034
Rowe DK, Smith JP, Baker C (2007) Agonistic interactions between Gambusia affinis and Galaxias maculatus: Implications for whitebait fisheries in New Zealand rivers. J Appl Ichthyol 23:668–674. https://doi.org/10.1111/j.1439-0426.2007.00912.x
Rudnick D, Resh V (2005) Stable isotopes, mesocosms and gut content analysis demonstrate trophic differences in two invasive decapod crustacea. Freshw Biol 50:1323–1336. https://doi.org/10.1111/j.1365-2427.2005.01398.x
Schumann DA, Hoback WW, Koupal KD (2015) Complex interactions between native and invasive species: Investigating the differential displacement of two topminnows native to Nebraska. Aquat Invasions 10:339–346. https://doi.org/10.3391/ai.2015.10.3.0
Shannon CE (1948) A mathematical theory of communication. Bell Syst Tech J 27:379–423. https://doi.org/10.1145/584091.584093
Smith MH, Scribner KT, Hernandez JD, Wooten MC (1989) Demographic, spatial, and temporal genetic variation in Gambusia. In: Meffe GK, Snelson FF (eds) Ecology & evolution of livebearing fishes (Poeciliidae). Prentice Hall, Englewood Cliffs, NJ, pp 235–257
Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A (2002) Bayesian measures of model complexity and fit. J R Stat Soc Ser B Stat Methodol 64:583–616. https://doi.org/10.1111/1467-9868.00353
Stenson JAE, Aronsson S (1995) Newt-fish interactions in a small forest lake. Amphibia-Reptilia 16:177–184. https://doi.org/10.1163/156853895X00352
Stock BC, Jackson AL, Ward EJ, Parnell AC, Phillips DL, Semmens BX (2018) Analyzing mixing systems using a new generation of Bayesian tracer mixing models. PeerJ 6:e5096. https://doi.org/10.7717/peerj.5096
Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306:1783–1786. https://doi.org/10.1126/science.1103538
Sutton TM, Zeiber RA, Fisher BE (2013) Agonistic behavioral interactions between introduced western mosquitofish and native topminnows. J Freshw Ecol 28:1–16. https://doi.org/10.1080/02705060.2012.688492
Tiberti R, Brighenti S, Iacobuzio R, Pasquini G, Rolla M (2014) Behind the impact of introduced trout in high altitude lakes: Adult, not juvenile fish are responsible of the selective predation on crustacean zooplankton. J Limnol 73:593–597. https://doi.org/10.4081/jlimnol.2014.969
Tiberti R, Rolla M, Brighenti S, Iacobuzio R (2016) Changes in the insect emergence at the water–air interface in response to fish density manipulation in high altitude lakes. Hydrobiologia 779:93–104. https://doi.org/10.1007/s10750-016-2801-z
Tiberti R, Bogliani G, Brighenti S, Iacobuzio R, Liautaud K, Rolla M, von Hardenberg A, Bassano B (2019) Recovery of high mountain Alpine lakes after the eradication of introduced brook trout Salvelinus fontinalis using non-chemical methods. Biol Invasions 21:875–894. https://doi.org/10.1007/s10530-018-1867-0
Toli EA, Chavas C, Denoël M, Bounas A, Sotiropoulos K (2020) A subtle threat: behavioral and phenotypic consequences of invasive mosquitofish on a native paedomorphic newt. Biol Invasions 22:1299–1308. https://doi.org/10.1007/s10530-019-02181-9
Vannini A, Bruni G, Ricciardi G, Platania L, Mori E, Tricarico E (2018) Gambusia holbrooki, the ‘tadpolefish’: the impact of its predatory behaviour on four protected species of European amphibians. Aquat Conserv Mar Freshw Ecosyst 28:476–484. https://doi.org/10.1002/aqc.2880
Webb C, Joss J (1997) Does predation by the fish Gambusia holbrooki (Atheriniformes: Poeciliidae) contribute to declining frog populations? Aust Zool 30:316–324. https://doi.org/10.7882/AZ.1997.007
Whiles MR, Lips KR, Pringle CM, Kilham SS, Bixby RJ, Brenes R, Connelly S, Jose CG, Hunte-Brown M, Huryn AD, Montgomery C, Peterson S (2006) The effects of amphibian population declines on the structure and function of neotropical stream ecosystems. Front Ecol Environ 4:27–34. https://doi.org/10.1890/1540-9295(2006)004[0027:TEOAPD]2.0.CO;2
Winandy L, Denoël M (2015) The aggressive personality of an introduced fish affects foraging behavior in a polymorphic newt. Behav Ecol 26:1528–1536. https://doi.org/10.1093/beheco/arv101
Winandy L, Darnet E, Denoël M (2015) Amphibians forgo aquatic life in response to alien fish introduction. Anim Behav 109:209–216. https://doi.org/10.1016/j.anbehav.2015.08.018
Winandy L, Colin M, Denoël M (2016) Temporal habitat shift of a polymorphic newt species under predation risk. Behav Ecol 27:1025–1032. https://doi.org/10.1093/beheco/arw008
Woodford DJ, Mcintosh AR (2010) Evidence of source-sink metapopulations in a vulnerable native galaxiid fish driven by introduced trout. Ecol Appl 20:967–977. https://doi.org/10.1890/08-1909.1
Wooten MC, Scribner KT, Smith MH (1988) Genetic variability and systematics of Gambusia in the southeastern United States. Copeia 2:283–289. https://doi.org/10.2307/1445867
Acknowledgements
We are grateful to V. Cagnati, L. Fieschi-Meric and L. Prats for field help and to the pond owner for providing access to his pond. We thank the two anonymous reviewers for their constructive comments. M. Denoël and G. Lepoint are, respectively, a Research Director and a Senior Research Associate of the F.R.S.—FNRS. B. Lejeune was a PhD student funded by Fonds pour la Formation à la Recherche dans l’Industrie et dans l’Agriculture (FRIA). The capture permit of newts was issued by Direction Générale de l’Environnement, de l’Aménagement et du Logement Languedoc Roussillon. All manipulations followed ethical standards. The ethical aspects of the protocol were approved by the Conseil National de la Conservation de la Nature (France).
Funding
This research benefited from F.R.S.-FNRS (Fonds de la Recherche scientifique) grants J.0008.13, J.0112.16, and T.0070.19 and a Fonds Spéciaux pour la Recherche grant C15/63 (Univ. of Liège) to M. Denoël.
Author information
Authors and Affiliations
Contributions
MD, GL and BL contributed to the study conception and design, and supervised lab and field work. BL performed data collection, sample preparation, processing and analysis of stable isotopes and gut contents. VC and TN performed gut content analyses. The first draft of the manuscript was written by BL. MD and GL revised subsequent drafts. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lejeune, B., Clément, V., Nothomb, T. et al. Trophic interactions between native newts and introduced mosquitofish suggest invaded ponds may act as demographic sinks. Biol Invasions 25, 2993–3007 (2023). https://doi.org/10.1007/s10530-023-03089-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-023-03089-1