Abstract
Microorganisms dominate most of Antarctic ecosystems and play a crucial role in their functioning. They are called extremophilic microorganisms with unique and versatile metabolic properties with possible biotechnological applications in several areas. The aim of the present study was to identify psychrotolerant microorganisms from Antarctic continent samples and to screen them for antimicrobial effects. Phylogenetic analyses revealed that most isolates were closely related to recognized species, including those recovered previously from Antarctica, which belonged to the major phyla Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria (classes Alpha, Beta, and Gammaproteobacteria). A total of 326 bacterial isolates, distributed in 39 different genera, were recovered and identified based on sequencing of the 16S rRNA gene. The main representative genera were Arthrobacter, Psychrobacter, Pseudoalteromonas, and Rhodococcus. Antimicrobial screening revealed fifteen isolates capable of inhibiting growth of at least one of the indicator strains: Escherichia coli, Micrococcus luteus, Staphylococcus aureus, Bacillus subtilis, and Candida albicans. One psychrotolerant bacterium, Pseudomonas sp. isolate 99, showed a broad antimicrobial range, in addition to antiproliferative and antiparasitic activity. Overall, the small number of antibiotic-producing isolates obtained and the weakness of their inhibition halos corroborated previous findings suggesting that cold-loving bacteria from Antarctica are not as good as their relatives from mesophilic environments for antimicrobial prospecting. Nonetheless, antiproliferative and antiparasitic results observed are promising and suggest that there is an untapped wealth in Antarctic environments for bioprospecting compounds with pharmaceutical potential application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antarctica is located south of the Antarctic Polar circle and is called the continent of extremes. It has the most extreme weather conditions, including low temperatures which may reach up to − 89 °C, high incidence of UV radiation, and water and nutrient deficit (Margesin et al. 2008; Margesin and Miteva 2011). It has the highest average altitude and is the most isolated of all continents. This geographic isolation probably resulted in the selection/speciation of unusual microorganisms, and also prevented their spread to other continents. That unique biome means that much of its biological heritage cannot be found anywhere else on the planet (Nichols et al. 1999).
Without human colonization, the continent still preserves the records of historical processes by which the earth passed in the last millennia. Thus, there is a possibility of confrontation and comparison, past and present, between environmental conditions, both biological and climatic (Wilmotte et al. 2012). So, an effort to provide a “database” on microbial composition is necessary to forecast possible future changes in diversity and taxonomic composition, as a result of variations in the ecosystem and/or human introductions.
Microorganisms dominate most Antarctic ecosystems and play a crucial role in their functioning and primary productivity. Several studies have shown that there is great biodiversity in the polar regions and different types of biological activities (Graumann et al. 1996; Morita et al. 1999; Deming 2002b; Bowman 2004). Cultivable bacteria from Antarctica have been studied by several authors (Bowman et al. 1997; Van Trappen et al. 2002; Webster and Bourne 2007; Chong et al. 2012; Huang et al. 2013; Shivaji et al. 2013). The main representative bacterial groups isolated belonged to four major phyla: Actinobacteria, Bacteroidetes, Proteobacteria, and Firmicutes. However, compared to the temperate and tropical regions, and despite their ecological importance, little is known about diversity of microbes and their geographical distribution in Antarctic systems (Taton et al. 2003; Vyverman et al. 2010).
Physiological adaptations allowed microorganisms to thrive in Polar Regions. Cold-shock proteins empower specific activities as the temperature drops. Cold active enzyme tends to reduce activation energy leading to high catalytic efficiency (Gerday et al. 1997). Expression of antifreeze-nucleating proteins and exopolysaccharide promotes survival at subzero temperature (Nichols et al. 1999; Muryoi et al. 2004; Nichols et al. 2005). In addition, increase in the proportion of unsaturated fatty acids in the cellular membranes helps to maintain a semifluid state at low temperatures (Deming 2002a; Häggblom and Margesin 2005; Newman and Hill 2006; Margesin et al. 2007, 2008; Shivaji and Prakash 2010).
These particular traits and unique adaptations already revealed suggest that the Antarctic environment represents a huge pool of microbial biodiversity and exploitable biotechnology. This untapped diversity has resulted in increasing interest in the study of cold-adapted microorganisms exploiting their ability to produce novel metabolites/compounds with potential biotechnological applications (Kennedy et al. 2008); for example, antimicrobial compounds have already been identified (Bruntner et al. 2005; Shekh et al. 2011; Liu et al. 2013) as well as antiproliferative molecules (Mojib et al. 2010, 2011), which could be used as anticancer drugs.
The evolution of antibiotic resistance by major human pathogens has made the first antibiotics, and most of their successors, mostly ineffective (Spellberg et al. 2004; 2013). Despite the significant efforts in the search for new antibiotics, bacteria are getting resistant faster than the appearance of new drugs to the market.
The search for new drugs is therefore of great importance to modern society and a promising alternative is to explore biodiversity in environments with unique characteristics, which contain microorganisms with particular physiological and metabolic properties that allow them to adapt and survive in inhospitable conditions, such as the Antarctic continent.
This work aimed to identify a broad collection of psychrotolerant bacteria isolated from diverse Antarctic samples and evaluate their potential for producing antimicrobial, antiproliferative, and antiparasitic compounds.
Materials and methods
Sampling
Samples used in this study were collected during an expedition to Antarctica in the austral summer (2013 and 2015) by the MycoAntar—Brazilian Antarctic Program team. The sources and places from which samples were obtained are detailed in Table 1 and Fig. 1. Samples were collected aseptically and placed in sterile plastic bags. Terrestrial samples as soil, penguin soil, sediment, and biofilm were frozen right after sampling and lichen, sponges, sea star, and other bryozoans were kept refrigerated at 4 °C and transported to the Division of Microbial Resources (DRM), Campinas, Brazil, for further processing. A flowchart depicting the methodological strategy adopted in this work for screening the bioactive compounds is shown in Fig. 2.
Bacterial isolation and identification
Isolation of bacteria was carried out on R2A agar (Yeast extract 0.5 g, Proteose Peptone 0.5 g, Casamino acids 0.5 g, Glucose 0.5 g, Soluble starch 0.5 g, Na-pyruvate 0.3 g, K2HPO4 0.3 g, MgSO4 × 7 H2O 0.05 g, Agar 15 g; Reasoner and Geldreich 1985), NA (Nutrient agar: Beef Extract 3 g, Peptone 5 g, Agar 15 g), and TSA (Trypticase soy agar: tryptone 15 g, soya peptone 5 g, sodium chloride 5 g, Agar 15 g). Artificial seawater (ASW) was used for isolation of bacteria from marine samples and distillated water for terrestrial samples, both supplemented with cycloheximide (300 µg.mL−1). R2A medium was used aiming to recover slow-growing bacterial species that would quickly be suppressed by fast-growing species on a richer culture medium (Margesin et al. 2012). Samples were pre-washed with sterile seawater to avoid the isolation of surface microorganisms and cut into small fragments, which were placed onto the culture media cited above. The plates were kept at 5 and 15 °C for 10 days. Bacterial growth was monitored every 24 h and colonies were transferred to the same medium used for isolation. All purified bacteria were preserved at − 80 °C in 20% glycerol in the research holding of the Brazilian Collection of Environmental and Industrial Microorganisms (CBMAI) at Chemical, Biological and Agricultural Pluridisciplinary Research Center (CPQBA) at the Campinas State University (UNICAMP). For bacterial identification, genomic DNA from the pure cultures was obtained according to the protocol described by (Pitcher et al. 1989). PCR amplification with 10f and 1100r primers (Lane 1991), sequencing and phylogenetic analyses of partial 16S rRNA gene fragments were carried out as described previously by Belgini et al. (2014). The Ribosomal Database Project (RDP) was used to select the most related species for further phylogenetic reconstruction.
Antimicrobial activity assay
Bacterial isolates obtained from Antarctica were tested as antimicrobial substance (AMS) producers using the agar diffusion method described by (Anthony et al. 1972). Bacteria were spot on NA-ASW agar and incubated at 15 °C for 7 days. After that, bacterial cells were killed by exposure to chloroform vapor during 15 min. After evaporation of residual chloroform, the plates were covered with a layer of semi-solid medium (0.5% agar) inoculated with one of the indicator strains: E. coli ATCC 11775, Micrococcus luteus ATCC 4698, Staphylococcus aureus ATCC 6538, Bacillus subtilis ATCC 6051, and Candida albicans ATCC 10231. Inhibition halo around the colony indicated the production of AMS.
Cultivation of AMS-producing bacteria
The selected AMS-producing bacterial isolate was grown in 5 L of TSB-ASW (Tryptic Soy Broth) for 72 h at 15 °C to produce biomass for subsequent chemical analyses. Cells were collected by successive centrifugations at 8.000×g (Eppendorf 5410) for 10 min at 4 °C and stored at 5 °C. Considering a possible accumulation of antimicrobial substance in the culture medium, the supernatant fraction was also separated for chemical analyses.
Soxhlet extraction
Soxhlet is a continuous solid/liquid extractor that allows an unmanaged and unmonitored operation while efficiently recycling a small amount of solvent (Jensen 2007; Luque de Castro and Priego-Capote 2010; Schmidt et al. 2014). Cell biomass from each pellet underwent a successive soxhlet extraction using an increasing polar gradient. First, around 25 g of cells were extracted with 200 mL of hexane until exhaustion (24 h). The crude hexane extract was removed from the soxhlet system and reserved in an Erlenmeyer flask. Cells were then subjected to another extraction with 200 mL of methanol for 24 h until completion. Crude extracts were concentrated under vacuum in a rotary evaporator at 40 °C until complete dryness and weighed to calculate the yield of each extraction.
Liquid–liquid partition extraction
Partition is a method that separates compounds based on their relative solubility in two different immiscible liquids, the culture broth (polar) and ethyl acetate (non-polar) (Rezaee et al. 2006; Sant’Anna et al. 2016). Each portion of the microbial culture broth (1000 mL) was transferred to a 2000 mL separating funnel to be partitioned with Ethyl Acetate (500 mL, 3 times). After shaking, the organic phase was collected and treated with anhydrous sodium sulfate, followed by filtration. The extracts were then concentrated under vacuum and weighed for yield calculations (Harder et al. 2002).
Pharmacological assays for determining in vitro antiproliferative activity
This assay aimed to detect anticancer activities by evaluating antiproliferative action in human tumor cells (Monks et al. 1991). In vitro tests were performed with the crude extract of Pseudomonas sp. 99 on human tumor cell lines of different origins and characteristics, as follows: breast cancer (MCF-7), lung cancer (NCI-H460), and glioblastoma (U251) provided by the National Cancer Institute at Frederick MA-USA. Stock cultures were grown in complete medium [RPMI 1640 (GIBCO) supplemented with 5% fetal bovine serum (FBS, GIBCO) and 1% (v/v) penicillin:streptomycin (Nutricell, 1000 U/mL:1000 g/mL)] in a humidified atmosphere with 5% CO2, at 37 °C. For the experiments, cell lines were used between passages 4–12. Cells in 96-well plates (100 µL cells/well) were exposed to different bacterial extract concentrations in dimethyl sulfoxide (DMSO)/RPMI (0.25, 2.5, 25, and 250 μg/mL) and incubated at 37 °C with 5% of CO2 for 48 h. Final DMSO concentration did not affect cell viability. Doxorubicin chloride (Europharma, 0.025, 0.25, 2.5, and 25 μg/mL) was used as a positive control. Before (T0 plate) and after sample addition (T1 plates), cells were fixed with 50% trichloroacetic acid and cell growth determined by spectrophotometric quantification (540 nm) of cellular protein content using sulforhodamine B (SRB) assay. Three measurements were obtained: 1) time zero (T0, at the beginning of incubation); 2) 48 h post-incubation for compound free (C), and 3) tested (T) cells. Cell proliferation was determined according to the equation \(100 \times \left[ {{{\left( {{\text{T}} - {\text{T}}0} \right)} \mathord{\left/ {\vphantom {{\left( {{\text{T}} - {\text{T}}0} \right)} {{\text{C}} - {\text{T}}0}}} \right. \kern-0pt} {{\text{C}} - {\text{T}}0}}} \right]\), for T0 < T ≤ C, and \(100 \times \left[ {{{\left( {{\text{T}} - {\text{T}}0} \right)} \mathord{\left/ {\vphantom {{\left( {{\text{T}} - {\text{T}}0} \right)} {{\text{T}}0}}} \right. \kern-0pt} {{\text{T}}0}}} \right]\), for T ≤ T0. A concentration–response curve for each cell line was plotted using the software Origin 8.0 (OriginLab Corporation). From the concentration–response curve for each cell line, TGI (total growth inhibition or cytostatic effect) value was determined by non-linear regression analysis using the software Origin 8.0® (OriginLab Corporation) (Shoemaker 2006).
Antimicrobial activity by disk diffusion test and minimum inhibitory concentration (MIC)
The hexane extract of Pseudomonas sp. 99 (the selected AMS-producing bacterial isolate) was solubilized in 5% DMSO solution. Petri dishes containing Mueller Hinton medium were seeded with 100 μL of the indicator strain culture at 108 cells/mL, by the spread plate technique. Then 3 μL (1 mg) of the extract sample was spot shaped on the disk, followed by incubation at 35 °C. The absence of the indicator strain growth indicated the antimicrobial activity. MIC tests were carried out using a tissue culture test plate (96 wells) against the following strains: Escherichia coli ATCC 11775, Micrococcus luteus ATCC 4698, Staphylococcus aureus ATCC 6538, Bacillus subtilis ATCC 6051, Candida albicans ATCC 10231. The stock solution of the extract was diluted and transferred into the first well, and serial dilutions were performed in order to obtain the concentration range of 1.6–0.012 mg/mL. The microbial inoculum was added to all wells and the plates were incubated at 37 °C during 24 h (bacteria) or at 30 °C for 48 h (yeast). Antimicrobial activity was detected by adding 20 µL of 0.5% TTC (triphenyl tetrazolium chloride, Merck) aqueous solution. MIC was defined as the lowest concentration that inhibited visible growth, as indicated by the TTC staining (Eloff 1998).
Antiparasitic assay
Trypanosoma cruzi assay was performed as described elsewhere (Moraes et al. 2014), using the Y strain. For the single-concentration primary screening, samples were tested at 10 µg/mL; 100 µg/mL was the highest concentration in the confirmatory dose–response assay, with ten 2-fold dilution points, and 104 µg/mL was the highest concentration for reference compound benznidazole. The Operetta high-content automated imaging system (Perkin Elmer) was used to acquire and analyze images. The analysis output was based on several parameters: host cell number, ratio of infected cells, and number of parasites per infected cell. The ratio of infected to total number of cells was then calculated, and defined as the Infection Ratio (IR). The raw data for IR values were normalized to negative—DMSO (mock)-treated infected cells—and positive (non-infected cells) controls to determine the normalized antiparasitic activity, expressed as a percentage of activity in comparison to wells. Sample cytotoxicity was determined by the cell ratio (number of cells in the test well divided by the average number of cells in negative control wells).
Results
Bacterial identification
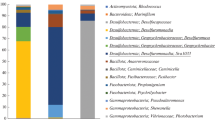
From 600 bacterial isolates recovered from the Antarctic samples, 326 were identified based on the sequencing and phylogenetic analysis of the 16S rRNA gene. Marine sediments yielded a higher number of strains (n = 109), followed by biofilms (n = 68), bryozoans (n = 48), sponge (n = 47), soil (n = 43), penguin soil (n = 7), and lichen (n = 4) (Table 2). They were distributed in four phyla Actinobacteria (115 isolates, 35%), Proteobacteria (153 isolates, 47%), Bacteroidetes (31 isolates, 10%), and Firmicutes (28 isolates, 8%) (Table 2).
Phylogenetic analysis, using the RDP tool and Bayesian classifier to select the closest related strains, permitted the identification of many bacterial isolates at the species level as shown in the phylogenetic trees (Electronic Supplementary Material—ESM). Species of the Alphaproteobacteria class (Online Resource 1) were identified as Sulfitobacter litoralis (n = 8), Sulfitobacter donghicola (n = 4), and Loktanella salsilacus (n = 2). The Betaproteobacteria class accounted with only one species, Polaromonas hydrogenivorans (n = 1). The Gammaproteobacteria group (Online Resource 2) was the most represented among the bacteria cultivated from the Antarctic samples, and thus isolates were identified based on three different phylogenetic trees: one for Pseudoalteromonas genus (Online Resource 3), one for Psychrobacter genus (Online Resource 4), and one for the other genera. Members of Psychrobacter genus were identified as follows: P. urativorans (n = 1), P. cibarius (n = 4), P. cryohalolentis (n = 4), and P. maritimus (n = 2). Pseudoalteromonas members could not be identified at the species level, probably because the 16S rRNA gene is not an appropriate phylogenetic marker for this group. The other Gammaproteobacteria species recovered were Pseudomonas frederikbergensis (n = 1), Marinomonas primoryensis (n = 10), Marinobacterium rhizophilum (n = 1), Marinobacter psychrophilus (n = 1), and Psychromonas arctica (n = 11).
Actinobacteria members were identified based on two phylogenetic trees: one for Arthrobacter genus (Online Resource 5) and one for the remaining genera (Online Resource 6). The Actinobacteria species recovered (bootstrap > 97%) were Leifsonia Antarctica (n = 3), Microbacterium testaceum (n = 5), Williamsia maris (n = 1), and Tomitella biformata (n = 1). Arthrobacter species recovered were Arthrobacter cryotolerans (n = 4) and Arthrobacter agilis (n = 2).
Species belonging to Firmicutes (Online Resource 7) (bootstrap > 72%) were Sporosarcina newyorkensis (n = 2), Sporosarcina aquimarina (n = 1), Planococcus kocurii (n = 1), Brevibacillus agri (n = 1), and Paenibacillus lautus (n = 1). Bacteroidetes species recovered (Online Resource 8) (bootstrap > 88%) were Maribacter arcticus (n = 3), Cellulophaga fucicola (n = 4), Cellulophaga algicola (n = 1), Bizionia argentinensis (n = 2), Psychroserpens mesophilus (n = 1), Polaribacter sejongensis (n = 2), Winogradskyella damuponensis (n = 2), Winogradskyella eximia (n = 1), and Cyclobacterium qasimii (n = 2).
Screening for antimicrobial activities
Bacterial isolates were tested in an antagonism assay against Gram-negative, Gram-positive, and yeast indicator strains: Escherichia coli ATCC 11775, Micrococcus luteus ATCC 4698, Staphylococcus aureus ATCC 6538, Bacillus subtilis ATCC 6051, and Candida albicans ATCC 10231. Among the 600 bacterial strains recovered from the Antarctic samples, 15 were positive against, at least, one indicator strain. The antimicrobial activity, taxonomic affiliation, and inhibition halo size of isolates are provided in Table 3. Bacillus safensis SG-32 and Streptomyces B-131, previously known as antimicrobial-producing strains, were used as positive controls (C +). Seven strains (Bacillus safensis H12, Sulfitobacter litoralis 103, Pseudomonas sp. 99, Bacillus sp. 98, Tsukamurella sp. 216, Cyclobacterium qasimii 172, and Pseudomonas frederiksbergensis 631) showed broad antibacterial spectrum activity against Gram-negative and Gram-positive target bacteria. Only three bacterial strains (Cellulophaga fucicola 418, Arthrobacter sp. 423, and Pseudoalteromonas sp. 485) showed inhibitory activity against Candida albicans.
Bacteria which presented the largest inhibition halos (++ or +++) had the inhibitory activity confirmed in another antagonism assay. Five of them (Bacillus sp. 98, Bacillus safensis H12, Pseudomonas sp. 99, Cyclobacterium qasimii 172, and Pseudomonas frederiksbergensis 631) presented a significant inhibitory halo and were selected for further MIC experiment (Table 4).
Three strains (H12, 99, and 631) presented satisfactory MIC activity. B. safensis H12 showed MIC of 1.6 mg/mL against E. coli and M. luteus, 0.4 mg/mL against S. aureus, and 0.8 mg/mL against B. subtilis. Pseudomonas sp. 99 showed the lowest values of MIC, 0.1 mg/mL against E. coli, and 0.2 mg/mL against S. aureus. Finally, P. frederiksbergensis 631 presented MIC of 0.25 mg/mL against S. aureus. No extract exhibited inhibition against C. albicans. Due to the lowest values observed in the MIC experiment, Pseudomonas sp. 99 was selected for extract preparation and further biological assays.
Pseudomonas sp. 99 extract preparation and biological activity evaluation
After proliferation, Pseudomonas sp. 99 biomass was separated by centrifugation from culture broth for further extraction. Biomass (25 g) extraction provided hexane (99 Hex, 99.8 mg) and methanol (99 Met, 386 mg) extracts while an ethyl acetate (99 Acet, 484 mg) extract was obtained from the culture broth (5 L). Results from the disk diffusion antimicrobial assay showed that all extracts inhibited S. aureus growth while 99 Acet and 99 Hex also inhibited M. luteus (Table 5).
The antiproliferative activity of the three extracts against three human tumor cell lines was expressed as TGI (Total growth inhibition—concentration that inhibited cell growth by 100%) (Table 6). According to Fouche et al. (2008), TGI values higher than 50 µg/mL represent inactive samples. This way, all extracts should be considered inactive however Pseudomonas sp. 99 hexane extract (99 Hex) seemed to contain antiproliferative substances in lower concentration as 99 Hex was able to slightly inhibit U251 (TGI = 81.24 µg/mL) and NCI-H460 (TGI = 98.29 µg/mL).
The three Pseudomonas sp. 99 extracts, at the single concentration of 10 µg/mL, were evaluated against T. cruzi Y strain, while the reference drug (positive control) benznidazole was tested at 104 µg/mL (400 µM) (Fig. 3). Again, Pseudomonas sp. 99 Hex was considered sufficiently active for further testing in dose–response as it presented normalized antiparasitic activity superior to 50% and host cell toxicity lower than 50%, while the other extracts 99 Met and 99 Acet were inactive. When tested in dose–response, sample 99-Hex presented only moderate anti-T. cruzi activity, with a maximum activity of 23.4%.
Discussion
Cultivated bacterial diversity from Antarctic samples
The psychrophilic bacteria isolated from different sites from the South Shetland Islands in Antarctica were taxonomically characterized based on 16S rRNA gene sequencing and phylogenetic analysis. Results showed that isolates were assigned to the major phylogenetic groups: Gammaproteobacteria (40%), Actinobacteria (35%), Bacteroidetes (10%), Firmicutes (8%), and Alphaproteobacteria and Betaproteobacteria (6%). The results obtained are corroborated by previous studies on microbial communities from various Antarctic habitats regardless of whether molecular or cultivation-based approaches were applied (Bowman et al. 1997; Van Trappen et al. 2002; Wilmotte et al. 2012; Carr et al. 2013).
Thirty-nine bacterial genera were identified out of 326 isolates. Most of the strains were closely related to other known cultured bacteria.
Phylogenetic analyses showed that 45 strains remained unaffiliated (Online Resource), possibly representing new species, as follows: isolates 128 (closely related with Antarctobacter heliothermus), 629 (closely related with Pseudomonas lutea), 163 and 436 (Pseudomonas sp.), 445, 108, 111, 276, 148, 188, and 219 (closely related with Psychrobacter luti), 409, 424-2, 245, 79, 212, 269, 281, 514, 567, 270, 561, 283, 514, r2a27, 60, and 258 (closely related with Psychrobacter cryohalolentis), 594 (closely related with Leifsonia rubeus), 595, 583, 493, 491, 228, 226, and 264 (Arthrobacter sp.), 171, 509, and 397 (Arenibacter sp.), 465, 118, 120, 168, 224, 395, and 457 (Zobellia sp). Four genera were shown to be predominant among the bacteria recovered from the Antarctic samples: Arthrobacter (n = 82), Psychrobacter (n = 62), Pseudoalteromonas (n = 38), and Rhodococcus (n = 13).
The genus Arthrobacter belongs to the Actinobacteria phylum and includes bacteria that are mostly mesophilic, with optimum growth below 30 °C, but some strains isolated from cold environments (Arctic, Antarctica, glaciers) are psychrotolerant or even psychrophilic (White et al. 2000; Pindi et al. 2010; Ganzert et al. 2011; Dsouza et al. 2015). Numerous Arthrobacter strains have been studied for the ability to degrade toxic compounds, such as 4-chlorophenol, 4-fluorophenol, 4-nitrophenol, or phenanthrene (Busse and Wieser 2014). Some strains were identified as a source of cold-adapted enzymes, such as chitinases and β-galactosidases (Lonhienne et al. 2001; Białkowska et al. 2009), and emulsifying agents (Rosenberg et al. 1979; Rosenberg et al. 2014). In this work 22 Arthrobacter sp. strains, more closely related to A. psychrochitiniphilus/A. alpinus, were accessed. A. psychrochitiniphilus strains are supposed to degrade chitin (Wang et al. 2009). Two strains were related to Arthrobacter agilis, a species associated with red carotenoid (bacterioruberin) production (Fong et al. 2001). A. psychrophenolicus has been described as degrading high phenol concentrations at low temperatures (Margesin et al. 2003).
Psychrobacter is an aerobic, osmotolerant, oxidase-positive, psychrophilic, or psychrotolerant bacterium. They occur in a wide range of wet, saline, and cold habitats (Dworkin 2006; Kim et al. 2012) but also in warm and slightly salty habitats. They have been studied for the production of cold-adapted enzymes such as β-lactamases (Feller et al. 1997). Isolation procedures used herein allowed the recovery of 62 isolates belonging to the Psychrobacter genus from Antarctic samples. However, identification of these isolates at the species level was not possible. According to Bakermans et al. (2006), gyrB gene represents a more reliable phylogenetic marker for the taxonomy of Psychrobacter species.
Pseudoalteromonas are marine bacteria, separated from the Alteromonas group in 1995 (Gauthier et al., 1995). They can be found in association with marine eukaryotes and may present antibacterial, bacteriolytic, agarolytic, and algicidal activities (Holmström et al. 1999). Taxonomic studies of Pseudoalteromonas group have revealed that 16S rRNA gene is highly conserved among the members of this taxon, not offering resolution for discriminating them at the species level. Romanenko et al. (2003) showed that Pseudoalteromonas agarivorans shared 99.9% 16S rRNA sequence similarity with Pseudoalteromonas distincta, Pseudoalteromonas elyakovii, Pseudoalteromonas atlantica, and Pseudoalteromonas espejiana. These reports are in accordance with the results observed in the phylogenetic reconstruction of the 38 isolates from Antarctic samples identified as Pseudoalteromonas sp.
Members of Rhodococcus genus are Gram-positive, aerobic, do not produce spore, and are closely related to Mycobacterium and Corynebacterium. Although some species are pathogenic, most are benign. They are found in many environments, including soil, water, and in association with eukaryotic cells (Finnerty 1992). The Rhodococcus members obtained in this study were mainly isolated from Antarctic biofilm and soil samples. Two species identified among the isolates, R. kyotonensis and R. yunnanensis, are associated to naphthalene-degrading activity (Anan`ina et al. 2011).
Antimicrobial-producing bacterial isolates
Biodiversity screenings, seeking for therapeutic and anti-tumor drugs from natural products, concentrates on metabolites with unusual properties. Consequently, extremophiles, which developed biomolecules to thrive in particular living conditions, have been viewed as valuable sources of novel bioproducts, including antimicrobials (Cavicchioli et al. 2002; Sánchez et al. 2009). The antibiotic production of cold-loving organisms has not been investigated as extensively as those of the mesophiles (O’Brien et al. 2004). One reason may be the difficulty to detect psychrophilic microorganisms that produce antibiotics in cold environments. For example, Sánchez et al. (2009) recovered only 0.16% from 8,000 bacteria screened for antimicrobial activities, and O’Brien et al. (2004) detected 0.29% on their screening. However, studies focused on obtaining bacteria with antimicrobial activities from mesophilic environments identified much higher rates. For example, Romanenko et al. (2013) isolated 177 bacteria from deep-sea sediment in Japan and found that 13% of them were antimicrobial-producing bacteria when cultivated at 28 °C. Kennedy et al. (2009) isolated over 52 bacteria from a sponge at 28 °C and found that 50% had antimicrobial activity. In this study, 15 out of 600 (2.5%) bacterial isolates tested at 15 °C had the ability to inhibit the growth of the indicator strains used.
Some of the AMS-producing strains isolated in this work have been previously described in Antarctica, such as the genera Arthrobacter, Pseudomonas (Lo Giudice et al. 2007), Flavobacterium, Marinobacter, Cyclobacterium, and Bacillus (Rojas et al. 2009). Members of the genus Tsukamurella have been isolated from hydrothermal vents and reported as AMS producer (Eythorsdottir et al. 2016). Romanenko et al. (2013) described two Sulfitobacter strains that displayed antimicrobial activity, but no reports were found specifically for Sulfitobacter litoralis, that exhibited herein an antimicrobial inhibition against the Gram-positive bacteria M. luteus and S. aureus. Similarly, no reports were found about Cellulophaga fucicola as AMS producer.
Five strains (Bacillus sp. 98, B. safensis H12, Pseudomonas sp. 99, Cyclobacterium qasimii 172, and P. frederiksbergensis 631) stood out for the size and/or sharpness of the inhibition halo. It is worth noting that two other assays were previously performed, according to the methods described by (Engelhardt et al. 2010) and (Ichikawa et al. 1971) for the screening of antibiotic-producing bacteria. None of the two methodologies, however, resulted in clearly visible inhibition halos. The method that best suited was the one described by Anthony et al. (1972), in which colonies were first exposed to chloroform vapor prior to testing the growth of indicator strains.
The capacity of Bacillus species to produce substances with antimicrobial activity against a wide variety of microorganisms is well documented (Walker and Abraham 1970; Nakano and Zuber 1990; Leifert et al. 1995). Similarly, antimicrobial production by Pseudomonas sp. has been reported, including P. frederiksbergensis (Prasad et al. 2011; Melo et al. 2016). Pseudomonas sp. has already been described as cold active enzyme producer (Im et al. 2013), larvicidal activity (Mageswari et al. 2015), and poly-β-hydroxybutyrate producer (Li et al. 2013).
Pseudomonas sp. 99 was selected for further evaluation of its antiproliferative and antiparasitic potential due to a broad spectrum activity in MIC tests. Its extract (99 Hex) promoted growth inhibition of two tumor cell lines in concentration lower than 100 μg/mL. A pure extract compound of Microbispora aerate, isolated from penguin excrements in Antarctic Livingston Island, presented a low antiproliferative and cytotoxic effect in L-929 mouse fibroblast cells and K-562 human leukemia cells (GI50 > 50 μg/mL) (Ivanova et al. 2007; Ivanova et al. 2013). These results may suggest that Pseudomonas sp. 99-Hex extract could contain the active component of antiproliferative substances in low concentration, pointing out that a pure extract could be more effective against tumor cells. Changes in the culture parameters should afford better conditions for the production of antiproliferative compounds.
Little is known about the activity of antimicrobials against protozoan parasites (Vizioli and Salzet 2002). Assays indicate that antimicrobials could represent an effective appliance for the development of novel drugs to fight the parasite in the vertebrate host (Hancock 2000; Zasloff 2002). The antiparasitic test was performed against T. cruzi, the causative agent of Chagas disease or American trypanosomiasis, which is a chronic tropical infectious disease endemic in Latin America (World Health Organization 2013). The protozoan parasite T. cruzi occurs throughout the American continent and is transmitted by the triatomine bug insect vector, infecting a variety of mammals, including humans (Coura 2015). Antiparasitic activity of Pseudomonas sp. 99-Hex extract was sufficiently promising in primary screening at single concentration; however more studies are necessary for further confirmation.
Pseudomonas sp. 99 is closely related to Pseudomonas sp. BTN1 (Online Resource 9), isolated from Ross Sea sediment, Antarctica (Tedesco et al. 2016). This strain showed an antibacterial activity against different strains of the Burkholderia cepacia complex (Bcc) and Minimal Bactericidal Concentration (MBC) of 100 μg/mL against S. aureus, roughly similar to our result of 200 μg/mL in MIC test, considering that we tested the crude extract while Tedesco and co-workers tested the pure compound. Moreover, the authors achieved the isolation and identification of the antimicrobial compounds from Pseudomonas sp. BTN1, which corresponded to three rhamnolipids. Although the active compound in this study may not be same as the ones produced by Pseudomonas sp. BTN1, these results give us a clue on how to start the identification of the molecule (Tedesco et al. 2016).
Despite the great potential that cold environments hold for revealing diverse products and processes which may have numerous industrial applications, our knowledge from cold-adapted organisms remains limited. Moreover, as pharmaceutical concerns for new products arise, biological tools are increasingly replacing old means of processing materials and even show promise to meet societal needs. It is imperative that we continue investigating ways in which natural products can offer economic alternatives to the traditional processes (Margesin 2008).
In the present study, 326 bacteria were identified by 16S rRNA gene sequencing and 45 may represent unknown species. A deeper taxonomic survey is further necessary to fully characterize those isolates that were not clustered with recognized species in the phylogenetic trees (ESM). Results of antimicrobial, antiproliferative, and antiparasitic substance screening demonstrated that there is an untapped wealth in Antarctic environments for bioprospecting compounds with pharmaceutical potential application (O’Brien et al. 2004; Rojas et al. 2009). However, the small number of antibiotic-producing isolates obtained and the weakness of their inhibition halos corroborated previous findings suggesting that cold-loving bacteria from Antarctica are not as good as their relatives from mesophilic environments for antimicrobial prospecting (Kennedy et al. 2009; Romanenko et al. 2013). Nonetheless, antiproliferative and antiparasitic results observed are promising and further work is needed to elucidate the structure of the bioactive molecule produced by Pseudomonas sp. 99.
Change history
19 April 2018
During initial stage of copyediting, extra columns were spanned in Table 2; i.e., 6 and 7 and 10 and 11 resulting from automation. This produced an extra space between columns, which went unnoticed following the proofing stage. There was no request for a correction until publication. The publisher sincerely apologizes to the authors for the inconvenience caused. The original article has been corrected.
References
Anan`Ina LN, Yastrebova OV, Demakov VA, Plotnikova EG (2011) Naphthalene-degrading bacteria of the genus Rhodococcus from the Verkhnekamsk salt mining region of Russia. Antonie van Leeuwenhoek, Int J Gen Mol Microbiol 100:309–316. https://doi.org/10.1007/s10482-011-9580-3
Anthony BF, Giuliano DM, Oh W et al (1972) Nursery outbreak of staphylococcal scalded skin syndrome. Am J Dis Child 124:41. https://doi.org/10.1001/archpedi.1972.02110130043006
Bakermans C, Ayala-del-Río HL, Ponder MA et al (2006) Psychrobacter cryohalolentis sp. nov. and Psychrobacter arcticus sp. nov., isolated from Siberian permafrost. Int J Syst Evol Microbiol 56:1285–1291. https://doi.org/10.1099/ijs.0.64043-0
Belgini DRB, Dias RS, Siqueira VM et al (2014) Culturable bacterial diversity from a feed water of a reverse osmosis system, evaluation of biofilm formation and biocontrol using phages. World J Microbiol Biotechnol 30:2689–2700. https://doi.org/10.1007/s11274-014-1693-1
Białkowska AM, Cieśliński H, Nowakowska KM et al (2009) A new β-galactosidase with a low temperature optimum isolated from the Antarctic Arthrobacter sp. 20B: gene cloning, purification and characterization. Arch Microbiol 191:825–835. https://doi.org/10.1007/s00203-009-0509-4
Bowman JP (2004) Psychrophilic prokaryote structural-functional relationships, biogeography and evolution within marine sediment. Cell Mol Biol 50:503–515
Bowman JP, McCammon SA, Brown MV et al (1997) Diversity and association of psychrophilic bacteria in Antarctic sea ice. Appl Environ Microbiol 63:3068–3078
Bruntner C, Binder T, Pathom-aree W et al (2005) Frigocyclinone, a novel angucyclinone antibiotic produced by a Streptomyces griseus strain from Antarctica. J Antibiot (Tokyo) 58:346–349. https://doi.org/10.1038/ja.2005.43
Busse HJ, Wieser M (2014) The genus Arthrobacter. In: The Prokaryotes: Actinobacteria. pp 105–132
Carr SA, Vogel SW, Dunbar RB et al (2013) Bacterial abundance and composition in marine sediments beneath the Ross Ice Shelf, Antarctica. Geobiology 11:377–395. https://doi.org/10.1111/gbi.12042
Cavicchioli R, Siddiqui KS, Andrews D, Sowers KR (2002) Low-temperature extremophiles and their applications. Curr Opin Biotech. https://doi.org/10.1016/S0958-1669(02)00317-8
Chong CW, Convey P, Pearce DA, Tan IKP (2012) Assessment of soil bacterial communities on Alexander Island (in the maritime and continental Antarctic transitional zone). Polar Biol 35:387–399. https://doi.org/10.1007/s00300-011-1084-0
Coura JR (2015) The main sceneries of chagas disease transmission. The vectors, blood and oral transmissions—a comprehensive review. Mem Inst Oswaldo Cruz 110:277–282. https://doi.org/10.1590/0074-0276140362
Deming JW (2002a) Psychrophiles and polar regions. TL-5. Curr Opin Microbiol 5 VN-re:301–309. https://doi.org/10.1016/s1369-5274(02)00329-6
Deming JW (2002b) Psychrophiles and polar regions. Curr Opin Microbiol 5:301–309
Dsouza M, Taylor MW, Turner SJ, Aislabie J (2015) Genomic and phenotypic insights into the ecology of Arthrobacter from Antarctic soils. BMC Genomics 16:1–18. https://doi.org/10.1186/s12864-015-1220-2
Dworkin M (2006) The Prokaryotes: Symbiotic Associations, Biotechnology, Applied Microbiology. In: The Prokaryotes. pp 1–184
Eloff JN (1998) A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med 64:711–713. https://doi.org/10.1055/s-2006-957563
Engelhardt K, Degnes KF, Kemmler M et al (2010) Production of a new thiopeptide antibiotic, TP-1161, by a marine Nocardiovsis species. Appl Environ Microbiol 76:4969–4976. https://doi.org/10.1128/AEM.00741-10
Eythorsdottir A, Omarsdottir S, Einarsson H (2016) Antimicrobial activity of marine bacterial symbionts retrieved from shallow water hydrothermal vents. Mar Biotechnol 18:293–300. https://doi.org/10.1007/s10126-016-9695-7
Feller G, Zekhnini Z, Lamotte-Brasseur J, Gerday C (1997) Enzymes from cold-adapted microorganisms. The class C beta-lactamase from the antarctic psychrophile Psychrobacter immobilis A5. Eur J Biochem 244:186–191. https://doi.org/10.1111/j.1432-1033.1997.00186.x
Finnerty WM (1992) The biology and genetics of the genus Rhodococcus. Annu Rev Microbiol 46:193–218
Fong NJC, Burgess ML, Barrow KD, Glenn DR (2001) Carotenoid accumulation in the psychrotrophic bacterium Arthrobacter agilis in response to thermal and salt stress. Appl Microbiol Biotechnol 56:750–756. https://doi.org/10.1007/s002530100739
Ganzert L, Bajerski F, Mangelsdorf K et al (2011) Arthrobacter livingstonensis sp. nov. and Arthrobacter cryotolerans sp. nov., salt-tolerant and psychrotolerant species from antarctic soil. Int J Syst Evol Microbiol 61:979–984. https://doi.org/10.1099/ijs.0.021022-0
Gerday C, Aittaleb M, Arpigny JL et al (1997) Psychrophilic enzymes: a thermodynamic challenge. Biochim. Biophys Acta Protein Struct Mol Enzymol 1342:119–131
Graumann P, Schröder K, Schmid R, Marahiel MA (1996) Cold shock stress-induced proteins in Bacillus subtilis. J Bacteriol 178:4611–4619
Häggblom M, Margesin R (2005) Microbial life in cold ecosystems. FEMS Microbiol Ecol 53(1):1–2
Hancock RE (2000) Cationic antimicrobial peptides: towards clinical applications. Expert Opin Investig Drugs 9:1723–1729. https://doi.org/10.1517/13543784.9.8.1723
Harder T, Lau SCK, Dahms HU, Qian PY (2002) Isolation of bacterial metabolites as natural inducers for larval settlement in the marine polychaete Hydroides elegans (HASWELL). J Chem Ecol 28:2029–2043. https://doi.org/10.1023/A:1020702028715
Holmström C, Kjelleberg S, Baumann L et al (1999) Marine Pseudoalteromonas species are associated with higher organisms and produce biologically active extracellular agents. FEMS Microbiol Ecol 30:285–293. https://doi.org/10.1111/j.1574-6941.1999.tb00656.x
Huang JP, Swain AK, Thacker RW et al (2013) Bacterial diversity of the rock-water interface in an East Antarctic freshwater ecosystem, Lake Tawani(P). Aquat Biosyst 9:4. https://doi.org/10.1186/2046-9063-9-4
Ichikawa T, Date M, Ishikura T, Ozaki A (1971) Improvement of kasugamycin-producing strain by the agar piece method and the prototroph method. Folia Microbiol (Praha) 16:218–224. https://doi.org/10.1007/BF02884210
Im JK, Ang SJ, Ee CL (2013) An Organic Solvent-Tolerant Alkaline Lipase from Cold-Adapted Pseudomonas mandelii: cloning, Expression, and Characterization. Biosci Biotechnol Biochem 77:320–323. https://doi.org/10.1271/bbb.120733
Ivanova V, Kolarova M, Aleksieva K et al (2007) Microbiaeratin, a new natural indole alkaloid from a Microbispora aerata strain, isolated from Livingston Island, Antarctica. Prep Biochem Biotechnol 37:161–168. https://doi.org/10.1080/10826060701199122
Ivanova V, Laatsch H, Kolarova M, Aleksieva K (2013) Structure elucidation of a new natural diketopiperazine from a Microbispora aerata strain isolated from Livingston Island, Antarctica. Nat Prod Res 27:164–170. https://doi.org/10.1080/14786419.2012.665911
Jensen WB (2007) The origin of the soxhlet extractor. J Chem Educ 84:1913. https://doi.org/10.1021/ed084p1913
Kennedy J, Marchesi JR, Dobson AD (2008) Marine metagenomics: strategies for the discovery of novel enzymes with biotechnological applications from marine environments. Microb Cell Fact 7:27. https://doi.org/10.1186/1475-2859-7-27
Kennedy J, Baker P, Piper C et al (2009) Isolation and analysis of bacteria with antimicrobial activities from the marine sponge Haliclona simulans collected from irish waters. Mar Biotechnol 11:384–396. https://doi.org/10.1007/s10126-008-9154-1
Kim SJ, Shin SC, Hong SG et al (2012) Genome sequence of a novel member of the genus Psychrobacter isolated from antarctic soil. J Bacteriol 194:2403–2403
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–175
Leifert C, Li H, Chidburee S et al (1995) Antibiotic production and biocontrol activity by Bacillus subtilis CL27 and Bacillus pumilus CL45. J Appl Bacteriol 78:97–108. https://doi.org/10.1111/j.1365-2672.1995.tb02829.x
Li R, Jiang Y, Wang X et al (2013) Psychrotrophic Pseudomonas mandelii CBS-1 produces high levels of poly-β-hydroxybutyrate. Springerplus 2:335. https://doi.org/10.1186/2193-1801-2-335
Liu J-T, Lu X, Liu X et al (2013) Bioactive natural products from the antarctic and arctic organisms. Mini Rev Med Chem 13:617–626. https://doi.org/10.2174/1389557511313040013
Lo Giudice A, Bruni V, Michaud L (2007) Characterization of Antarctic psychrotrophic bacteria with antibacterial activities against terrestrial microorganisms. J Basic Microbiol 47:496–505. https://doi.org/10.1002/jobm.200700227
Lonhienne T, Mavromatis K, Vorgias CE et al (2001) Cloning, sequences, and characterization of two chitinase genes from the Antarctic Arthrobacter sp. Strain TAD20: isolation and partial characterization of the enzymes. J Bacteriol 183:1773–1779. https://doi.org/10.1128/JB.183.5.1773-1779.2001
Luque de Castro MD, Priego-Capote F (2010) Soxhlet extraction: past and present panacea. J Chromatogr A 1217:2383–2389
Mageswari A, Subramanian P, Ravindran V et al (2015) Synthesis and larvicidal activity of low-temperature stable silver nanoparticles from psychrotolerant Pseudomonas mandelii. Environ Sci Pollut Res 22:5383–5394. https://doi.org/10.1007/s11356-014-3735-5
Margesin R, Miteva V (2011) Diversity and ecology of psychrophilic microorganisms. Res Microbiol 162:346–361. https://doi.org/10.1016/j.resmic.2010.12.004
Margesin R, Gander S, Zacke G et al (2003) Hydrocarbon degradation and enzyme activities of cold-adapted bacteria and yeasts. Extremophiles 7:451–458. https://doi.org/10.1007/s00792-003-0347-2
Margesin R, Neuner G, Storey KB (2007) Cold-loving microbes, plants, and animals—fundamental and applied aspects. Naturwissenschaften 94:77–99. https://doi.org/10.1007/s00114-006-0162-6
Margesin R, Schinner F, Marx JC, Gerday C (2008) Psychrophiles: From biodiversity to biotechnology
Margesin R, Schumann P, Zhang DC et al (2012) Arthrobacter cryoconiti sp. nov., a psychrophilic bacterium isolated from alpine glacier cryoconite. Int J Syst Evol Microbiol 62:397–402. https://doi.org/10.1099/ijs.0.031138-0
Melo IS, Souza WR, Silva LJ et al (2016) Antifungal activity of Pseudomonas frederiksbergensis CMAA 1323 isolated from the antarctic hair grass Deschampsia antarctica. Br Microbiol Res J 14:1–11. https://doi.org/10.9734/BMRJ/2016/25314
Mojib N, Philpott R, Huang JP et al (2010) Antimycobacterial activity in vitro of pigments isolated from Antarctic bacteria. Antonie van Leeuwenhoek, Int J Gen Mol Microbiol 98:531–540. https://doi.org/10.1007/s10482-010-9470-0
Mojib N, Nasti TH, Andersen DT et al (2011) The antiproliferative function of violacein-like purple violet pigment (PVP) from an Antarctic Janthinobacterium sp. Ant5-2 in UV-induced 2237 fibrosarcoma. Int J Dermatol 50:1223–1233. https://doi.org/10.1111/j.1365-4632.2010.04825.x
Monks A, Scudiero D, Skehan P et al (1991) Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst 83:757–766. https://doi.org/10.1093/jnci/83.11.757
Moraes CB, Giardini MA, Kim H et al (2014) Nitroheterocyclic compounds are more efficacious than CYP51 inhibitors against Trypanosoma cruzi: implications for Chagas disease drug discovery and development. Sci Rep 4:4703. https://doi.org/10.1038/srep04703
Morita RY, Horikoshi K, Grant WD (1999) Extremophiles: microbial life in extreme environments. Bioscience 49:245. https://doi.org/10.2307/1313521
Muryoi N, Sato M, Kaneko S et al (2004) Cloning and expression of afpA, a gene encoding an antifreeze protein from the arctic plant growth-promoting rhizobacterium Pseudomonas putida GR12-2. J Bacteriol 186:5661–5671. https://doi.org/10.1128/JB.186.17.5661-5671.2004
Nakano MM, Zuber P (1990) Molecular biology of antibiotic production in Bacillus. Crit Rev Biotechnol 10:223–240. https://doi.org/10.3109/07388559009038209
Newman DJ, Hill RT (2006) New drugs from marine microbes: the tide is turning. J Indust Microbiol Biotechnol 33(7):539–544
Nichols D, Bowman J, Sanderson K et al (1999) Developments with Antarctic microorganisms: culture collections, bioactivity screening, taxonomy, PUFA production and cold-adapted enzymes. Curr Opin Biotechnol 10:240–246
Nichols CM, Bowman JP, Guezennec J (2005) Olleya marilimosa gen. nov., sp. nov., an exopolysaccharide-producing marine bacterium from the family Flavobacteriaceae, isolated from the Southern Ocean. Int J Syst Evol Microbiol 55:1557–1561. https://doi.org/10.1099/ijs.0.63642-0
O’Brien A, Sharp R, Russell NJ, Roller S (2004) Antarctic bacteria inhibit growth of food-borne microorganisms at low temperatures. FEMS Microbiol Ecol 48:157–167. https://doi.org/10.1016/j.femsec.2004.01.001
Pindi PK, Manorama R, Begum Z, Shivaji S (2010) Arthrobacter antarcticus sp. nov., isolated from an Antarctic marine sediment. Int J Syst Evol Microbiol 60:2263–2266. https://doi.org/10.1099/ijs.0.012989-0
Pitcher DG, Saunders NA, Owen RJ (1989) Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett Appl Microbiol 8:151–156. https://doi.org/10.1111/j.1472-765X.1989.tb00262.x
Prasad S, Manasa P, Buddhi S et al (2011) Antagonistic interaction networks among bacteria from a cold soil environment. FEMS Microbiol Ecol 78:376–385. https://doi.org/10.1111/j.1574-6941.2011.01171.x
Reasoner DJ, Geldreich EE (1985) A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol 49:1–7
Rezaee M, Assadi Y, Milani Hosseini M-R et al (2006) Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatogr A 1116:1–9. https://doi.org/10.1016/j.chroma.2006.03.007
Rojas JL, Martín J, Tormo JR et al (2009) Bacterial diversity from benthic mats of Antarctic lakes as a source of new bioactive metabolites. Mar Genomics 2:33–41. https://doi.org/10.1016/j.margen.2009.03.005
Romanenko LA, Zhukova NV, Rohde M et al (2003) Pseudoalteromonas agarivorans sp. nov., a novel marine agarolytic bacterium. Int J Syst Evol Microbiol 53:125–131. https://doi.org/10.1099/ijs.0.02234-0
Romanenko LA, Tanaka N, Kalinovskaya NI, Mikhailov VV (2013) Antimicrobial potential of deep surface sediment associated bacteria from the Sea of Japan. World J Microbiol Biotechnol 29:1169–1177. https://doi.org/10.1007/s11274-013-1276-6
Rosenberg E, Zuckerberg A, Rubinovitz C, Gutnick DL (1979) Emulsifier of Arthrobacter RAG-1: isolation and emulsifying properties. Appl Environ Microbiol 37:402–408
Rosenberg E, DeLong EF, Lory S, et al (2014) The Prokaryotes. In: The Prokaryotes: Actinobacteria. pp 1–1061
Sánchez LA, Gómez FF, Delgado OD (2009) Cold-adapted microorganisms as a source of new antimicrobials. Extremophiles 13:111–120. https://doi.org/10.1007/s00792-008-0203-5
Sant’Anna V, Correa APF, Motta A de S da, Brandelli A (2016) Liquid-liquid extraction of antimicrobial peptide P34 by aqueous two-phase and micellar systems. Prep Biochem Biotechnol https://doi.org/10.1080/10826068.2016.1141301
Schmidt F, Koch BP, Witt M, Hinrichs KU (2014) Extending the analytical window for water-soluble organic matter in sediments by aqueous Soxhlet extraction. Geochim Cosmochim Acta 141:83–96. https://doi.org/10.1016/j.gca.2014.06.009
Shekh RM, Singh P, Singh SM, Roy U (2011) Antifungal activity of Arctic and Antarctic bacteria isolates. Polar Biol 34:139–143. https://doi.org/10.1007/s00300-010-0854-4
Shivaji S, Prakash JSS (2010) How do bacteria sense and respond to low temperature? Arch Microbiol 192:85–95
Shivaji S, Begum Z, Shiva Nageswara Rao SS et al (2013) Antarctic ice core samples: culturable bacterial diversity. Res Microbiol 164:70–82. https://doi.org/10.1016/j.resmic.2012.09.001
Shoemaker RH (2006) The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer 6:813–823. https://doi.org/10.1038/nrc1951
Spellberg B, Powers JH, Brass EP et al (2004) Trends in antimicrobial drug development: implications for the future. Clin Infect Dis 38:1279–1286. https://doi.org/10.1086/420937
Spellberg B, Bartlett JG, Gilbert DN (2013) The future of antibiotics and resistance. N Engl J Med 368:299–302. https://doi.org/10.1056/NEJMp1215093
Taton A, Grubisic S, Brambilla E et al (2003) Cyanobacterial diversity in natural and artificial microbial mats of Lake Fryxell (McMurdo Dry Valleys, Antarctica): a morphological and molecular approach. Appl Environ Microbiol 69:5157–5169. https://doi.org/10.1128/AEM.69.9.5157-5169.2003
Tedesco P, Maida I, Esposito FP et al (2016) Antimicrobial activity of monoramnholipids produced by bacterial strains isolated from the Ross Sea (Antarctica). Mar Drugs. https://doi.org/10.3390/md14050083
Van Trappen S, Mergaert J, Van Eygen S et al (2002) Diversity of 746 heterotrophic bacteria isolated from microbial mats from ten Antarctic lakes. Syst Appl Microbiol 25:603–610. https://doi.org/10.1078/07232020260517742
Vizioli J, Salzet M (2002) Antimicrobial peptides versus parasitic infections? Trends Parasitol 18:475–476. https://doi.org/10.1016/S1471-4922(02)02428-5
Vyverman W, Verleyen E, Wilmotte A et al (2010) Evidence for widespread endemism among Antarctic micro-organisms. Polar Sci 4:103–113. https://doi.org/10.1016/j.polar.2010.03.006
Walker JE, Abraham EP (1970) Isolation of bacilysin and a new amino acid from culture filtrates of Bacillus subtilis. Biochem J 118:557–561
Wang F, Gai Y, Chen M, Xiao X (2009) Arthrobacter psychrochitiniphilus sp. nov., a psychrotrophic bacterium isolated from Antarctica. Int J Syst Evol Microbiol 59:2759–2762. https://doi.org/10.1099/ijs.0.008912-0
Webster NS, Bourne D (2007) Bacterial community structure associated with the Antarctic soft coral, Alcyonium antarcticum. FEMS Microbiol Ecol 59:81–94. https://doi.org/10.1111/j.1574-6941.2006.00195.x
White PL, Wynn-Williams DD, Russell NJ (2000) Diversity of thermal responses of lipid composition in the membranes of the dominant culturable members of an Antarctic fellfield soil bacterial community. Antarct Sci 12:386–393. https://doi.org/10.1017/S0954102000000432
Wilmotte A, Vyverman W, Willems A, et al (2012) Antarctic Microbial Biodiversity: The importance of geographical and ecological factors. Sci A Sustaunable Dev 1–2
World Health Organization (2013) Sustaining the drive to overcome the global impact of neglected tropical diseases: second WHO report on neglected tropical diseases
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415:389–395. https://doi.org/10.1038/415389a
Acknowledgements
We would like to thank Prof. Luis Henrique Rosa, coordinator of the MycoAntar Project (CNPq), and the Brazilian Antarctic Program for making the sampling feasible in the three expeditions OPERANTAR XXXII (summer 2013/2014), OPERANTAR XXXIII (summer 2014/2015), and OPERANTAR XXXIV (summer 2015/2016). The authors are also grateful to FAPESP for financial funding (process numbers 2014/17936-1; 2016/05640-6).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
The original version of this article has been revised due to a copyediting error where extra columns were spanned in Table 2; i.e., 6 and 7 and 10 and 11. The original article has been corrected. A correction to this article is available online at https://doi.org/10.1007/s00300-018-2322-5.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Silva, T.R., Duarte, A.W.F., Passarini, M.R.Z. et al. Bacteria from Antarctic environments: diversity and detection of antimicrobial, antiproliferative, and antiparasitic activities. Polar Biol 41, 1505–1519 (2018). https://doi.org/10.1007/s00300-018-2300-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-018-2300-y