Abstract
Antarctic notothenioid fish larvae and juveniles are pelagic and subject to oceanic transport, influencing species distribution and biogeography. The nature of notothenioid larval/juvenile diversity in the high-latitude McMurdo Sound is virtually unknown to-date. We report here a first assessment of this diversity and its contribution to species richness in the Sound. We collected 151 larvae and juveniles from under ice cover. To overcome uncertainties in identifying larvae by morphology, we used full-length mitochondrial ND2 gene sequences in phylogenetic reconstruction with reference adults and identified 13 species representing four families. Six are nototheniids whose adults are common in the Sound, and a seventh is a cryptic nototheniid that is likely Pagothenia brachysoma, previously unknown to the Sound. The rest included four icefishes, an artedidraconid, and a bathydraconid, all without prior adult record in the Sound. With seven of 13 species previously undocumented, larval/juvenile notothenioid diversity appears to double adult diversity. Published fish surveys show adults of these icefishes and the artedidraconid occur in the nearby Terra Nova Bay and/or western Ross Sea; thus, their pelagic larvae and juveniles could be transported by the Ross Sea circulation into the Sound. The bathydraconid, identified as Psilodraco breviceps, is reportedly endemic to S. Georgia. We found additional unpublished barcode sequences for specimens from the Dumont d’Urville Sea and Ross Sea, and they form a species clade with the McMurdo larval and Marguerite Bay adult P. breviceps in this study, indicating this species has a circumpolar distribution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antarctic waters are home to a largely benthic and highly endemic ichthyofauna, dominated by the members of the perciform suborder Notothenioidei that arose through nested adaptive radiations within the isolated Southern Ocean (Eastman 2005; Near et al. 2012; Lecointre et al. 2013). Five families—Artedidraconidae, Bathydraconidae, Channichthyidae, Harpagiferidae, and Nototheniidae—are traditionally recognized (Gon 1990), encompassing predominantly endemic Antarctic species (Eastman and Eakin 2015). In Antarctic coastal waters, notothenioids make up over 70 % of the species diversity and 91 % of the biomass (Eastman and Hubold 1999; Eastman 2005). At the southernmost marine habitat, McMurdo Sound (77°S), members of the genus Trematomus (family Nototheniidae) are particularly prevalent, with a few species from the other families occasionally captured (Eastman and DeVries 1982).
While nearly all Antarctic notothenioid species have benthic lifestyles (Eastman 1993) and limited home ranges as adults (Kawaguchi et al. 1989; Miyamoto and Tanimura 1999), many species have pelagic eggs and larvae (Kock 1992; Loeb et al. 1993) that could be dispersed by the Southern Ocean current systems. And for species with extended pelagic larval stages lasting from months to a year or longer (Kellerman 1990; Kock 1992), larvae passively entrained by currents may potentially be transported to distant habitats from their hatching sites. Such a dispersal hypothesis has been tested as contributing to species distributions and gene flow among populations around the Southern Ocean (Loeb et al. 1993; Matschiner et al. 2009; Damerau et al. 2012; Volckaert et al. 2012).
Understanding Antarctic notothenioid larval diversity and distribution should therefore be an integral component in understanding adult Antarctic fish biodiversity and biogeography. Additionally, comparing larval and adult diversity within the same locality would provide further insight into the extent of oceanic dispersal. However, larval diversity and distribution have been much less studied than older ages due to inherent logistical difficulties in collection, particularly at high-latitude locations where ice cover hampers sampling. Another challenge is larval species identification. Larvae and very young juvenile fishes of congeneric or confamilial species can often look similar superficially, making visual identification unreliable. Larval fishes are also fragile and easily damaged during capture, complicating identification by morphology. Moreover, commonly used larval morphological features such as pigmentation patterns can change with developmental age and thus may not provide definitive identification.
Among the most logistically challenging location for larval and juvenile fish sampling is the chronically ice-covered McMurdo Sound, the southern component of the Ross Sea and southern limit of Antarctic marine life. The frigid McMurdo Sound is in fact faunally rich, yielding a steady supply of adult fish specimens year after year. The adult fish fauna is well documented from decades of biological research conducted by the United States and the New Zealand Antarctic Programs. In contrast, thus far there are only three published studies on local or nearby larval fishes, namely the dragonfish Gymnodraco acuticeps Boulenger, 1902 and Trematomus borchgrevinki Boulenger, 1902 from McMurdo Sound, and Pleuragramma antarctica Boulenger, 1902 from Terra Nova Bay (Evans et al. 2005; Cziko et al. 2006; Evans et al. 2006). These studies pertained to spawning behavior, freeze avoidance, and energy metabolism, utilizing embryos discovered to be available by chance. The only dedicated studies of high-latitude ichthyoplankton diversity took place further north in Terra Nova Bay and the western Ross Sea in open waters during austral springs and summers by Italian expeditions with particular interests in the early life history and ecology of the Antarctic silverfish P. antarctica (Guglielmo et al. 1998; Vacchi et al. 1999; Granata et al. 2000, 2002). These studies identified a total of 38 notothenioid species representing four Antarctic families (absent Harpagiferidae) predominated by nototheniids, with P. antarctica being the overwhelmingly dominant species (Vacchi et al. 1999; Granata et al. 2002). Interestingly, species representation and relative abundance based on these larval and juvenile assemblages differ from those of adult notothenioids identified in Ross Sea surveys (Eastman and Hubold 1999; Hanchet et al. 2013), and are far more diverse than adult species known to McMurdo Sound though it is part of the Ross Sea system.
The larval and juvenile fish composition of McMurdo Sound is virtually unknown, as persistent sea ice cover precluded any mid-water sampling by trawls. We have made considerable non-trawling efforts to collect larval and juvenile fishes from under McMurdo Sound sea ice during various austral summer field seasons over a decade (2002–2012) to understand their diversity as a synergistic activity of our research program. Small juveniles were readily obvious as either red-blooded species or hemoglobinless icefish but small larvae proved more difficult, and definitive species identification by morphology was not possible for most specimens. We therefore took an alternate approach, using molecular phylogenetic analyses to identify species. We obtained full-length mitochondrial ND2 (NADH dehydrogenase 2) gene sequences from the larval/juvenile specimens, additional barcode gene sequences from a bathydraconid larva in the collection, and available vouchered adults from databases as reference, and reconstructed phylogenetic relationships to assign species identity. Our results allow for the first comparisons between the larval/juvenile and adult fish compositions in McMurdo Sound and a more comprehensive assessment of the species richness therein. We surveyed the literature and compiled catch data on adult species of McMurdo Sound, Terra Nova Bay, and Ross Sea to assess faunal connectivity of the species we identified. Additionally, we paired the identified larval/juvenile species and their adults with digital images as a photographic field guide that can be built upon with future collections and by the fish community.
Materials and methods
Specimen collection
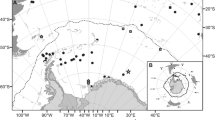
Juvenile and larval fishes were collected from various McMurdo Sound locations (approximately 77°S, 165°E) (Fig. 1, inset), Antarctica, during our austral summer seasons from 2002 through 2012 during the months of October through mid-January of the following year. We follow the definitions of larval and juvenile fish of Hubbs (1943); larvae refer to specimens in the prolarva or postlarva stages strikingly unlike juveniles in appearance, and juveniles resemble adults. Table 1 shows the sampling details including location of each sampling event, water depth, collection method, number collected at each site, and specimen total length (TL). Some specimens were collected alive by handheld dip nets under ice in shallow water (≤10 m) by our research divers, and others were recovered from overnight sets of plankton nets at about 400 m depth deployed using vertical set line through large ice holes drilled for adult fishing operations. In several instances, specimens were regurgitated by adult fishes during capture, or recovered from stomach content during dissection. Specimens were often obtained as a single individual, sometimes as several to a dozen individuals, and on a few occasions from a shoal of many larvae or young juveniles we chanced to encounter. Of a total of 151 specimens, 111 were collected in 2012, mostly from two shoals consisting of very young juvenile fishes. The remaining 40 specimens were collected during three earlier austral field seasons (2002–2003, 2003–2004, and 2004–2005) and consisted of mostly larvae and a few juveniles. Specimens were recorded and photographed, then preserved in 90 % ethanol and stored at −20 °C until analysis. An adult reference specimen of Psilodraco breviceps Norman, 1937 was collected from Marguerite Bay, West Antarctica Peninsula (Donnelly and Torres 2008), and provided to us as a generous gift by Jose Torres.
ND2 amplification and sequencing
Genomic DNA was isolated from fin clippings or a small bit of pectoral muscle using either standard lysis and chloroform–phenol extraction or the DNeasy Blood and Tissue kit (Qiagen, USA). The full-length (1047 nt) mitochondrial-encoded NADH dehydrogenase subunit 2 gene (ND2) was amplified using published ND2 primers (Kocher et al. 1995). The amplified ND2 products were treated with 1 U each shrimp alkaline phosphatase and Exonuclease I (NEB, USA), sequenced directly with ABI BigDye Terminator ver.3.1 chemistry (Applied Biosystems, USA) and read on an ABI 3730xl sequencer at the UIUC Keck Center for Comparative and Functional Genomics. Sequences were edited manually and full-length ND2 contigs assembled using ChromasPro ver.1.5 (Technelysium). ND2 sequences obtained in this study have been deposited with GenBank under accession numbers KR153329 to KR153480.
Phylogenetic analyses of ND2 to determine species identity
The full-length ND2 sequence of each unknown specimen was first used to query the GenBank database with BLASTN to identify the putative closest species. We then downloaded available ND2 sequences in the database for all members of the genus or family to which the “hit” species belong, plus additional ND2 reference sequences of various other species for full representation of the five Antarctic families and three basal non-Antarctic families in the dataset (Online Resource 1). The 77 adult references from the database (Online Resource 1) plus the Marguerite Bay P. breviceps adult reference, and the 151 larval/juvenile sequences, totaling 229 sequences, were aligned using Muscle (Edgar 2004). Because some of the database sequences lack the stop codon, it was trimmed from all sequences where present for a final length of 1044 nt using Mesquite (Maddison and Maddison 2011).
The best-fit sequence substitution model for the dataset was assessed with jModelTest ver.2.1.6 (Guindon and Gascuel 2003; Darriba et al. 2012) with the following likelihood settings: three substitution schemes (covering the 24 models implementable in Bayesian analyses using MrBayes), ML (maximum likelihood) optimized for base tree likelihood calculations, NNI (nearest neighbor interchange) for base tree topology search, and inclusion of both invariable sites (I) and rate variation about sites (gamma, or Γ). The GTR (general time reversible) + I + Γ model was found to best fit the dataset under all assessment criteria (−lnL, AIC, AICc, and BIC).
Phylogenetic trees were constructed using maximum-likelihood (ML) and Bayesian inference (BI) analyses incorporating the GTR + I + Γ model. ML analyses were run with RAxML ver.8 (Stamatakis 2014), and node supports of inferred trees were assessed with 1000 bootstrap replicates. BI analyses were run with MrBayes ver.3.2.2 (Ronquist et al. 2012) for 5,000,000 generations, using four chains, sampling every 100 generations, and with burn-in set to 25 %; 5,000,000 generations were sufficient to achieve stationarity as the standard deviation of the split frequency was below 0.01. Three outgroup species were used in these analyses: Bovichtus variegatus (family Bovichtidae) that is basal to all notothenioids, and two non-Antarctic notothenioids, Pseudaphritis urvillii (Valenciennes, 1832) and Eleginops maclovinus (Cuvier, 1830), representing their respective monotypic family (Pseudaphritidae and Eleginopsidae). Final consensus trees were visualized with FigTree ver.1.4.0.
COI and Rho sequencing for species confirmation of a larval bathydraconid
Sequencing of additional genes was performed to further support the species assignment by ND2 phylogenetic analyses of a McMurdo Sound bathydraconid larva as P. breviceps, which has no published record of adult occurrence in McMurdo Sound or the nearby Ross Sea regions. Full-length rhodopsin (Rho) and partial cytochrome oxidase subunit I (COI) sequences were amplified from this larva, the Marguerite Bay P. breviceps adult, and G. acuticeps (serving as outgroup). The intronless Rho sequences were amplified from genomic DNA using the primer pair NotoRh_5flnkF: 5′-GCTGATTGAAACCGCAAGCCGC-3′ and NotoRh_3flnkR1: 5′-GGATCATGGAGCCTGTGTCAACG-3′ designed from the flanking sequences of notothenioid rhodopsin genes from our in-house database. Partial COI sequences were amplified using universal barcode primers COI_FishF1t1: 5′-TGTAAAACGACGGCCAGTCGACTAATCATAAAGATATCGGCAC-3′ and COI_FishR1t1: 5′-TCAGGAAACAGCTATGACACCTCAGGGTGTCCGAARAAYCARAA-3′. Barcode Rho and COI partial sequences for reference adults (Online Resource 1), obtained from GenBank and/or BOLD (Barcode of Life Data System) databases, were aligned with the Marguerite Bay adult and the putative McMurdo larval P. breviceps sequences for neighbor-joining and maximum-likelihood phylogenetic analyses using various models and criteria and 1000 bootstrap replicates, implemented in MEGA6.0 (Tamura et al. 2013) to confirm species assignment. COI and Rho sequences obtained in this study have been deposited with GenBank under accession numbers KU647483 to KU647488.
Survey of documented adult notothenioids of McMurdo Sound, Terra Nova Bay, and the Ross Sea
To compare larval and adult notothenioid species composition between these three Ross Sea regions (Fig. 1) and assess faunal connectivity, we reviewed the literature extensively and compiled documented notothenioid catch data dating back to 1960, approximating the inception of modern Antarctic biological research on Ross Island and in the Ross Sea region.
Results
Phylogenetic analyses of McMurdo Sound larval and juvenile notothenioids to determine species identity
ND2 trees from ML and BI analyses depicting relationships between unknown larvae and juveniles and vouchered adult references are shown in abbreviated form for visual clarity in Figs. 2 and 3, respectively, and the full topology trees are given in Online Resource 2. Also for easy viewing, several genera of reference adult species were condensed as single taxonomic clades in the abbreviated trees (Figs. 2, 3). Both ML and BI analyses recovered the traditional family Nototheniidae as paraphyletic, the Notothenia/Paranotothenia clade as sister to the other four Antarctic families, and the Gobionotothen genus as sister to both, consistent with recent molecular phylogenies (Dettai et al. 2012; Near et al. 2012). Bathydraconidae is also paraphyletic, as has been reported in some phylogenetic studies, while the other families were monophyletic (Figs. 2, 3). Though family relationships were not completely resolved in the two trees, ND2 sequences from distinct individuals (unknown larva or juvenile, and the adult reference) of the same species form a strongly supported clade with negligible or no sequence divergence at the tip of a branch of the inferred trees, and thus were collapsible as a single taxon (insets in Figs. 2, 3 show an example of the subtree of a species clade). This is the criterion for our species assignment of the unknown larval or juvenile fish to be the same as the reference adult fish in the clade. With this criterion, both ML and BI analyses of the ND2 dataset yielded species identifications for all except one larval specimen, revealing 13 species with 100 % bootstrap support (except for 91 % for Pogonophryne scotti Regan, 1914) in the ML tree (Fig. 2), and strong posterior probability (0.9993–1) in the BI tree (Fig. 3). Species identifications agree between the two analyses. We paired the images of the identified larval or juvenile specimens with the corresponding adult species (Fig. 4). The very young Trematomus juveniles (TL 22–49 mm, Table 1; Fig. 4a–c) exhibit strong resemblance in coloration and pattern, and larval morphologies are distinctly unlike adults, illustrating the uncertainty in species identification by sight.
Maximum-likelihood phylogenetic tree for 151 unknown notothenioid larval/juvenile fish specimens (referred as “sample/s” or “unk” in bold text), 73 reference species, and three outgroup species. The tree was constructed using RAxML ver.8 (Stamatakis 2014) incorporating the substitution model GTR + I + Γ and 1000 bootstrap replicates. Bovichtus variegatus, Pseudaphritis urvillii, and Eleginops maclovinus served as outgroups. Bootstrap values >70 are indicated along the branches. Species clades containing larval/juvenile samples and their respective reference species have been collapsed (shown as triangles in the tree) for ease of viewing; the inset shows an example of the subtree in a collapsed species clade. Branches are scaled by the number of substitutions per site. Two basal branches have been abbreviated (indicated by hash marks) for clarity of presentation of the tree; the actual branch length for both branches was 1.1011
Bayesian inference phylogenetic tree for 151 unknown notothenioid larval/juvenile fish specimens (referred as “sample/s” or “unk” in bold text), 73 reference species, and three outgroup species. The analyses were run with MrBayes ver.3.2.2 (Ronquist et al. 2012) implementing the substitution model GTR + I + Γ, for 5,000,000 generations sampling every 100 generation, and a relative burn-in set at 0.25. Bovichtus variegatus, Pseudaphritis urvillii, and Eleginops maclovinus served as outgroups. Posterior probabilities above 0.90 are included. Species clades containing the larval/juvenile specimens and their respective species have been collapsed (shown as triangles in the tree) for ease of viewing; the inset shows an example of the subtree in a collapsed clade. Branches are scaled by the expected number of substitutions per site. Two basal branches have been abbreviated (indicated by hash marks) for clarity of presentation of the tree; the actual branch length for both branches was 1.7806
Select photographs of larval and juvenile specimens collected for this study from McMurdo Sound, Antarctica, paired with corresponding photos of adult specimens where possible. a Trematomus bernacchii; b Trematomus nicolai, c Trematomus pennellii, d Pleuragramma antarctica, e Trematomus borchgrevinki, f Pagothenia brachysoma (putative), g Psilodraco breviceps, h Pogonophryne scotti, i Chionodraco hamatus, j Neopagetopsis ionah, k Pagetopsis maculatus, l Chionodraco myersi. One identified juvenile, Trematomus newnesi, was not photographed. An adult specimen of Pagothenia brachysoma, the putative species identification of the larva in image f, was not available
The ND2-based species identities of the larvae and juveniles from each collection event, and the corresponding collection details, as well as specimen size (TL in millimeters) are shown in Table 1. Table 2 summarizes Table 1, listing the 13 identified larval and juvenile species, their family affiliation and the node supports in the ML and BI trees for species assignment. Of the 151 specimens, 142 (94.0 %) belong to the family Nototheniidae. The great majority (138 specimens) was identified as trematomids, in six species (Table 2; Fig. 4a–c, e, f; Trematomus newnesi Boulenger, 1902 larva was not photographed). The most prevalent trematomid (106 specimens) was T. bernacchii (Fig. 4a) mostly (86 individuals) derived from the 2012 cohort from the McMurdo jetty (Table 1), followed by T. borchgrevinki (Fig. 4e) (total 23 specimens; Table 2). The other four nototheniid specimens were identified to be the pelagic Antarctic silverfish, P. antarctica (Table 2; Fig. 4d). The single unidentified trematomid species labeled “03unk01” (Fig. 4f) in the ND2 trees was recovered with 100 % node support in both trees as the closest sister species to the T. borchgrevinki species clade containing one reference and 23 larval specimens (Figs. 2, 3). “03unk01” shares only 94.73 % ND2 nucleotide sequence identity with the T. borchgrevinki adult reference, as opposed to 99.62–100 % identity shared by the reference and the 23 T. borchgrevinki larvae collected from three different locations in three different years. T. borchgrevinki ((Fig. 4e) and the “03unk01” larva (Fig. 4f) were comparable in age (≈20 mm) and morphologically very similar, except “03unk01” completely lacked the pigmentation patterns on the skull and the abdomen, and along the dorsal and ventral fin bases of T. borchgrevinki, supporting that it is a distinct species.
The remaining nine specimens were identified to comprise six species from three families, namely P. breviceps (Bathydraconidae) (Fig. 4g), P. scotti (Artedidraconidae) (Fig. 4h), and the icefish species (Channichthyidae) Chionodraco hamatus (Lönnberg, 1905) (Fig. 4i), Neopagetopsis ionah Nybelin, 1947 (Fig. 4j), Pagetopsis maculatus Barsukov and Permitin, 1958 (Fig. 4k), and Chionodraco myersi DeWitt and Tyler, 1960 (Fig. 4l) (Table 2; Figs. 2, 3).
Comparison of McMurdo Sound larval/juvenile diversity and adult diversity of McMurdo Sound, Terra Nova Bay, and the Ross Sea
Our literature search of reported adult fish diversity dating back to 1960 identified 14 notothenioid species caught from McMurdo Sound, 25 species from Terra Nova Bay, and 61 species from the Ross Sea (Table 3). The relative locations of these three regions are shown in Fig. 1. The adult notothenioid communities of McMurdo Sound and Terra Nova Bay represent non-identical but overlapping subsets of the species known to the larger Ross Sea. The one exception is Cryothenia amphitreta; thus far the holotype discovered in McMurdo Sound (Cziko and Cheng 2006) was the only individual caught (Table 3).
Of the 13 larval and juvenile species identified in this study, five are Trematomus species common to McMurdo Sound as adults, and the pelagic nototheniid P. antarctica that also occurs in the Sound. Species identity for the Trematomus larva “03unk01” (Fig. 4f) remains obscure in the absence of a reference adult sequence in the database that would form a species clade with this specimen. For the six non-nototheniid species, i.e., P. breviceps, P. scotti, C. hamatus, N. ionah, P. maculatus, and C. myersi, there are no reported adult captures from McMurdo Sound. However, three of the icefishes and P. scotti occur in Terra Nova Bay, and all four icefishes and P. scotti occur in the Ross Sea. There is no published record of capture of the bathydraconid P. breviceps as adult in any of the three regions to date (Table 3).
Species confirmation of larval P. breviceps
To confirm the accuracy of ND2-based species assignment of the P. breviceps larva (Fig. 4g), COI (cytochrome oxidase I) and Rho (rhodopsin) sequences from the larva and the Marguerite Bay adult were additionally analyzed. The full-length Rho sequence (1059 nt) and partial COI sequence (652 nt) between the two specimens share 100 % sequence identity (Online Resource 3), supporting that they are the same species. Maximum-likelihood and neighbor-joining analyses of partial sequences of Rho (738 nt) and COI (652 nt) sequences of the McMurdo Sound and Marguerite Bay specimens and the homologous sites in available published and unpublished barcode sequences of these two genes from other vouchered P. breviceps originating from other Southern Ocean locations (Online Resource 1) recovered all specimens in a fully supported (99–100 %) species clade (ML trees shown in Fig. 5), with the bathydraconid G. acuticeps serving as outgroup.
Maximum-likelihood phylogenetic tree using barcode gene sequences a 652 nt COI and b 738 nt Rho of Psilodraco breviceps from various Southern Ocean locations (given in branch labels where known along with GenBank accession number or BOLDsystems sequence ID), with G. acuticeps serving as outgroup. The ML analyses were run in MEGA6.0 incorporating the GTR + I + Γ model, and clade support was tested using 1000 bootstrap replications
Discussion
Species identification by phylogenetic analyses of ND2 sequences
In this study, we explored the larval/juvenile notothenioid diversity in the high-latitude McMurdo Sound waters, which was virtually unknown to date. To overcome uncertainties of identifying larvae by morphology, we used full-length ND2 sequences in phylogenetic reconstruction to obtain species identity.
The phylogenetic approach is enabled by the availability of full-length ND2 sequences for a substantial number of notothenioid species in the database, and adequate phylogenetic signal in ND2 sequences to provide resolution at the species level for most taxa in notothenioid molecular phylogenies (Near et al. 2003; Near and Cheng 2008). The utility of full-length ND2 sequences and robust ML and BI analyses surpasses the commonly used, much shorter barcoding COI (cytochrome oxidase I; ~650 nt) sequences and simpler distance-based Neighbor-Joining analyses that quite often fall short of resolving species (Rock et al. 2008; Dettai et al. 2011). While some familial relationships as well as species relationships of more recently diverging notothenioid subflocks such as the Trematomus and Pogonophryne genera remain not entirely resolved with ND2 sequences (also with other molecular markers), the near-unanimous 100 % species group supports for the unknown larva/juvenile with its vouchered reference in the ND2 trees (Figs. 2, 3) provides strong confidence for the accuracy of the species assignment. A slight uncertainty concerns P. scotti (lower bootstrap support of 91 in ML analysis, though posterior probability was 0.9993 in BI analysis). This arose more likely due to the incompleteness of adult references in the database for this speciose genus (27 species) than to the resolving power of ND2. Only 11 Pogonophryne species have available ND2 sequences, all of which are included in our dataset. However, only seven of these 11 are represented in the 14 species known to occur in the Ross Sea (Table 3). Thus, if the unknown Pogonophryne larva (Fig. 4h) originated from adults in the Ross Sea, it may be one of the seven other Ross Sea species whose ND2 sequences were unavailable, or any of the other remaining species that may occur in the Ross Sea but have eluded capture thus far.
Comprehensive availability of reference sequences of vouchered species is therefore a necessity regardless of the chosen molecular marker for phylogenetically identifying cryptic larvae or juveniles. Our larval specimen “03unk01” (Fig. 4f) illustrates this need. The species is clearly very closely related to, yet clearly not T. borchgrevinki (Fig. 2, 3, 4e). The only known congeneric species of Pagothenia borchgrevinki (prior name of T. borchgrevinki) is Pagothenia brachysoma. There were reports of its occurrence in the Ross Sea region (DeWitt et al. 1990), most recently of a small (4.3 cm, 2 g) specimen in the eastern Ross Sea (Donnelly et al. 2004). Thus, the cryptic trematomid larva “03unk01” may be P. brachysoma, but unfortunately no molecular sequences including barcoding COI of vouchered specimens are available to date. Definitive species verification will therefore await future vouchered specimens suitable for gene amplification and sequencing. Despite some current limitations (but which will only improve as the notothenioid species representation in DNA database increases), phylogenetic reconstruction using full-length ND2 serves as an effective approach for identifying cryptic notothenioid larva and juvenile species, especially for specimens in poor physical conditions that preclude morphological identification. In all planktonic fish diversity studies, there were unidentifiable damaged specimens, specimens unrecognizable beyond the familial level by morphology, and/or specimens assigned with provisional species names (DeWitt 1970; Loeb et al. 1993; Guglielmo et al. 1998; Vacchi et al. 1999; Granata et al. 2002). Incorporating reconstruction of phylogenetic relationships to identify species as a routine procedure will add power in fully and accurately capturing notothenioid faunal diversity and biogeography.
McMurdo Sound larval versus adult diversity
Antarctic notothenioids dominate the shelf waters of high-latitude McMurdo Sound and the Ross Sea similar to other Southern Ocean coastal locations. In waters north of Ross Island and in the western Ross Sea, notothenioid dominance was evident in catch biomass (77–91 %), with members of the family Nototheniidae—especially Trematomus species—particularly prevalent, accounting for over 50 % of all individuals collected (Eastman and Hubold 1999). Species of three other Antarctic families—Artedidraconidae, Bathydraconidae, and Channichthyidae—also occur, but were caught at lower frequencies than nototheniids (Table 3 and references therein). The known adult notothenioid diversity of McMurdo Sound follows this general pattern, but with Trematomus nototheniids overwhelmingly dominant (Eastman and DeVries 1982). However, the overall McMurdo Sound diversity (14 species) represents only a small subset of the Ross Sea fauna (61 species) (Table 3).
Assessment of the notothenioid diversity of McMurdo Sound is severely constrained by the limited and localized fishing methods (hook and line, or traps) that could be deployed through holes drilled through fast sea ice, and thus is very likely a biased underestimation. The apparent greater larval/juvenile diversity found in this study reflects this bias. While we did recover five common Trematomus species (and in catch numbers consistent with Trematomus dominance) as well as P. antarctica whose adults occur in the Sound, the other seven identified species (over half of 13) have not been collected as adults from McMurdo Sound. It is possible that these species in fact occur in the Sound but have eluded capture from lack of appropriate fishing methods due to chronic ice cover. During research diving in the Sound between 2002 and 2012, we have encountered and collected several adult individuals of the icefish Pagetopsis macropterus at about 33 m depth (Cheng et al. 2003), and at shallower depths (~20 m) specimens of the artedidraconid Histiodraco velifer (Near et al. 2004) and the new nototheniid C. amphitreta (Cziko and Cheng 2006). However, our line and trap fishing in the Sound over five decades of austral summer field seasons had not yielded a single specimen of these three species, indicating fishing method is decidedly a factor in underestimating McMurdo Sound notothenioid species richness.
Apparent mismatch between larval and adult diversity is also seen at the nearby Terra Nova Bay (TNB) and the western Ross Sea regions (Fig. 1). As summarized in Granata et al. (2002), the four spring–summer ichthyoplankton surveys netted 394,453 fish larvae, of which P. antarctica overwhelmingly dominated. This is consistent with the dominance of P. antarctica as a mid-water species that it constitutes 92 % by number of the mid-water fish fauna on the Ross Sea shelf (DeWitt 1970). The prevalence and abundance of postlarval P. antarctica, particularly near TNB, were also due to the sub-ice layer around TNB being the spawning and hatching ground of this species, the surface water conditions favorable as feeding grounds, and the ichthyoplankton surveys timed to the hatching time in November and December. By virtue of the sheer massive total sampling size, larvae of notothenioid species not previously documented to occur in the Ross Sea were also caught. These included Lepidonotothen nudifrons (Lönnberg, 1905), Chionodraco rastrospinosus DeWitt and Hureau, 1979, Pseudochaenichthys georgianus Norman, 1937, Parachaenichthys charcoti (Vaillant, 1906), and Artedidraco mirus Lönnberg, 1905, plus additional unidentified specimens. Identifications were based on morphology in these studies, and ideally, they should be coupled with genetic analyses to support or authenticate species assignments when in doubt. Assuming species accuracy, the apparent discrepancy between larval and adult diversities in TNB and Ross Sea (as well as McMurdo Sound in this study) speaks to multiple factors including fishing methods, the scale of sampling, fishing locations, time of the year and developmental stage, and morphologically unidentifiable specimens, collectively at play affecting the assessment of faunal diversity.
Geographic source of McMurdo Sound larval species
Six of the seven identified larval/juvenile species have no records of adults being captured in McMurdo Sound, namely the four icefishes, P. scotti and the putative P. brachysoma, but they have been documented in Ross Sea regions further north (Table 3). The icefishes C. hamatus, C. myersi, and N. ionah, and the artedidraconid P. scotti occur in TNB; these as well as P. maculatus and P. brachysoma also occur in the greater Ross Sea. Therefore, if these species are indeed absent in the McMurdo Sound adult fish community, the closest geographic source of their larvae would be TNB and/or the Ross Sea. The clockwise-flowing Ross Gyre spans the Ross Sea sector of the Southern Ocean, effecting a general east–west circulation across the Ross Sea shelf between the southern section of the gyre and the continent (Rickard et al. 2010). McMurdo Sound receives Ross Sea inflow, which moves southwards along its eastern side, and eventually exits northward along its western side (Barry and Dayton 1988; Hunt et al. 2003). These current circulation patterns could be responsible for passive transport of planktonic larvae and pelagic juveniles of TNB and Ross Sea origin into the Sound.
The final larval specimen with no known adults in McMurdo Sound, identified as P. breviceps, was the least-expected and most intriguing in where its origin may be. The specimen was regurgitated from an adult Trematomus hansoni collected with hook and line from about 30 m bottom of Winter Quarters Bay by the ice pier of McMurdo Station. Both ML and BI trees based on ND2 sequences show 100 % node support for the species clade containing the larva with two references (Pbrev_HQ170128; PbrevB_HQ170129) and the Marguerite Bay adult (Donnelly and Torres 2008) in this study, leaving little doubt that the larva is P. breviceps. The 100 % nucleotide identity in the full-length Rho (rhodopsin) and partial COI sequences shared by the Marguerite Bay adult and the McMurdo Sound larva (Online resource 3) further supports the species assignment. Earlier reports of P. breviceps distribution indicated it is endemic to the shelf waters of South Georgia Island (Gon 1990). The capture of an adult from Marguerite Bay (Donnelly and Torres 2008) still puts the species at a huge distance from McMurdo Sound. To resolve the enigma of the origin of the larval P. breviceps, we searched and found a published barcode COI sequence (HQ712804) (Dettai et al. 2011) and a unpublished Rho sequence (EATF154-10Rho) of a vouchered specimen captured from Dumont d’Urville Sea, as well as unpublished barcode COI sequences (JN641130, JN641131) of two specimens caught from the Ross Sea. The fully supported species clade in ML trees constructed using these barcode COI and Rho sequences and those we generated for the Marguerite Bay adult and the putative McMurdo Sound larval P. breviceps (Fig. 5) confirm that all specimens are of the same species. The species should quite certainly be P. breviceps, as it is very unlikely that three different expert ichthyologists would have misidentified their respective specimen/s independently. The formation of a species clade consisting of specimens from Marguerite Bay, Dumont d’Urville Sea, Ross Sea, and McMurdo Sound also indicates that P. breviceps is not endemic to S. Georgia as previously proposed (Gon 1990), but very likely circum-Antarctic in distribution.
In one of the Italian planktonic fish surveys, seven larval P. breviceps specimens were collected from Terra Nova Bay (Guglielmo et al. 1998). This collection, in addition to the fact a local, sedentary T. hansoni in McMurdo Sound could chance to encounter and ingest a P. breviceps larva, and that two adult specimens were reportedly caught in the Ross Sea, collectively support that a regional/local adult P. breviceps population must exist that serves as the source of the planktonic larva collected in this study and in TNB (Guglielmo et al. 1998).
Conclusion
This study illustrates the importance of understanding larval/juvenile diversity as an integral part of comprehensively understanding Antarctic notothenioid diversity and biogeography in Southern Ocean locations. It also illustrates the power of the phylogenetic approach using full-length mitochondrial ND2 gene sequences in identifying larvae and very young juveniles that are difficult to identify by morphology with certainty. As a resource to the community, the first full list of reported adult notothenioid species as adults in McMurdo Sound, Terra Nova Bay, and the Ross Sea that we compiled for this study would facilitate future studies on gene flow and population connectivity of the McMurdo fishes to other Southern Ocean regions. Lastly, the incipient library of digital color images of notothenioid larvae and juveniles paired with adults, while not sufficiently robust for morphological identification, is an idea that can be improved upon in future collections, to generate an “in-life” field guide for aiding larval and juvenile species identification.
References
Balushkin AV, Spodareva VV (2013) Pogonophryne sarmentifera sp. nov. (Artedidraconidae; Notothenioidei; Perciformes)—the deep-water species of Antarctic plunderfishes from the Ross Sea (Southern Ocean). Trudy Zoologicheskogo Instituta 317:275–281
Balushkin AV, Petrov AF, Prutko VG (2010) Pogonophryne brevibarbata sp. nov. (Artedidraconidae, Notothenioidei, Perciformes)—a new species of toadlike plunderfish from the Ross Sea, Antarctica. Trudy Zoologicheskogo Instituta 314:381–386
Barry JP, Dayton PK (1988) Current patterns in McMurdo Sound, Antarctica and their relationship to local biotic communities. Polar Biol 8:367–376
Buckley PA (2013) Rapid change in shallow water fish species composition in a historically stable Antarctic environment. Antarct Sci 25:676–680
Cheng C-HC, Chen L, Near TJ, Jin Y (2003) Functional antifreeze glycoprotein genes in temperate-water New Zealand nototheniid fish infer an Antarctic evolutionary origin. Mol Biol Evol 20:1897–1908
Clark MR, Dunn MR, McMillan PJ, Pinkerton MH, Stewart A, Hanchet SM (2010) Latitudinal variation of demersal fish assemblages in the western Ross Sea. Antarct Sci 22:782–792
Cziko PA, Cheng C-HC (2006) A new species of nototheniid (Perciformes: Notothenioidei) fish from McMurdo Sound, Antarctica. Copeia 4:752–759
Cziko PA, Evans CW, Cheng C-HC, DeVries AL (2006) Freezing resistance of antifreeze-deficient larval Antarctic fish. J Exp Biol 209:407–420
Damerau M, Matschiner M, Salzburger W, Hanel R (2012) Comparative population genetics of seven notothenioid fish species reveals high levels of gene flow along ocean currents in the southern Scotia Arc. Antarct Polar Biol 35:1073–1086
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772
Dettai A et al (2011) The actinopterygian diversity of the CEAMARC cruises: barcoding and molecular taxonomy as a multi-level tool for new findings. Deep Sea Res Part II 58:250–263. doi:10.1016/j.dsr2.2010.05.021
Dettai A, Berkani M, Lautredou A-C, Couloux A, Lecointre G, Ozouf-Costaz C, Gallut C (2012) Tracking the elusive monophyly of nototheniid fishes (Teleostei) with multiple mitochondrial and nuclear markers. Mar Genomics 8:49–58
DeWitt HH (1970) The character of the midwater fish fauna of the Ross Sea, Antarctica. In: Holdgate W (ed) Antarctic ecology. Academic Press, London, pp 305–315
DeWitt HH, Tyler JC (1960) Fishes of the Stanford Antarctic Biological Research Program, 1958–1959. Stanford Ichthyol Bull 7:162–199
DeWitt HH, Heemstra PC, Gon O (1990) Nototheniidae. In: Heemstra PC, Gon O (eds) Fishes of the Southern Ocean. JLB Smith Institute of Ichthyology, Grahamstown, pp 279–331
Donnelly J, Torres JJ (2008) Pelagic fishes in the Marguerite Bay region of the West Antarctic Peninsula continental shelf. Deep Sea Res Part II 55:523–539. doi:10.1016/j.dsr2.2007.11.015
Donnelly J, Torres JJ, Sutton TT, Simoniello C (2004) Fishes of the eastern Ross Sea, Antarctica. Polar Biol 27:637–650
Eakin RR, Eastman JT (1998) New species of Pogonophryne (Pisces, Artedidraconidae) from the Ross Sea, Antarctica. Copeia 4:1005–1009
Eastman JT (1985) Pleuragramma antarcticum (Pisces, Nototheniidae) as food for other fishes in McMurdo Sound, Antarctica. Polar Biol 4:155–160
Eastman JT (1993) Antarctic fish biology: evolution in a unique environment. Academic Press Inc, San Diego
Eastman JT (2005) The nature of the diversity of Antarctic fishes. Polar Biol 28:93–107
Eastman JT, DeVries AL (1982) Buoyancy studies of notothenioid fishes in McMurdo Sound, Antarctica. Copeia 2:385–393
Eastman JT, Eakin RR (2015) Notothenioid classification and list of species. Ohio University. http://www.oucom.ohiou.edu/dbms-eastman/index.htm
Eastman JT, Hubold G (1999) The fish fauna of the Ross Sea, Antarctica. Antarct Sci 11:293–304
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Evans CW, Cziko P, Cheng C-HC, DeVries AL (2005) Spawning behaviour and early development in the naked dragonfish Gymnodraco acuticeps Antarctic. Science 17:319–327
Evans CW, Pace L, Cziko PA, Marsh AG, Cheng C-HC, DeVries AL (2006) Metabolic energy utilization during development of Antarctic naked dragonfish (Gymnodraco acuticeps). Polar Biol 29:519–525
Gon O (1990) Bathydraconidae. In: Gon O, Heemstra PC (eds) Fishes of the Southern Ocean. J. L. B. Smith Institute of Ichthyology, Grahamstown, pp 364–380
Granata A, Guglielmo L, Greco S, Vacchi M, Sidoti O, Zagami G, La Mesa M (2000) Spatial distribution and feeding habits of larval and juvenile Pleuragramma antarcticum in the Western Ross Sea (Antarctica). In: Faranda F, Guglielmo L, Lanora A (eds) Ross Sea ecology. Springer, Berlin, pp 369–393
Granata A, Cubeta A, Guglielmo L, Sidoti O, Greco S, Vacchi M, La Mesa M (2002) Ichthyoplankton abundance and distribution in the Ross Sea during 1987–1996. Polar Biol 25:187–202
Guglielmo L, Granata A, Greco S (1998) Distribution and abundance of postlarval and juvenile Pleuragramma antarcticum (Pisces, Nototheniidae) off Terra Nova Bay (Ross Sea, Antarctica). Polar Biol 19:37–51
Guindon S, Gascuel O (2003) A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Syst Biol 52:696–704
Hanchet SM, Stewart AL, McMillan PJ, Clark MR, O’Driscoll RL, Stevenson ML (2013) Diversity, relative abundance, new locality records, and updated fish fauna of the Ross Sea region. Antarct Sci 25:619–636. doi:10.1017/S0954102012001265
Hubbs CL (1943) Terminology of early stages of fishes. Copeia 4:260
Hunt BM, Hoefling K, Cheng C-HC (2003) Annual warming episodes in seawater temperatures in McMurdo Sound in relationship to endogenous ice in notothenioid fish. Antarct Sci 15:333–338. doi:10.1017/S0954102003001342
Kawaguchi K, Ishikawa S, Matsuda O, Naito Y (1989) Tagging experiment of nototheniid fish, Trematomus bernacchii Boulenger under the coastal fast ice in Lützow-Holm Bay, Antarctica. Proc NIPR Symp Polar Biol 2:111–116
Kellerman A (1990) Catalogue of early life stages of Antarctic notothenioid fishes. Berichte für. Polarforschung 67:45–136
Kocher TD, Conroy JA, McKaye KR, Stauffer JR, Lockwood SF (1995) Evolution of NADH dehydrogenase subunit 2 in east African cichlid fish. Mol Genet Evol 4:420–432
Kock K-H (1992) Development of eggs and larvae. Antarctic fish and fisheries. University Press, Cambridge, pp 96–100
La Mesa M, Cattaneo-Vietti R, Vacchi M (2006) Species composition and distribution of the Antarctic plunderfishes (Pisces, Artedidraconidae) from the Ross Sea off Victoria Land. Deep-Sea Res II 53:1061–1070
Lecointre G et al (2013) Is the species flock concept operational? The Antarctic shelf case. PLoS One 8:e68787
Loeb V, Kellerman A, Koubbi P, North A, White M (1993) Antarctic larval fish assemblages—a review. Bull Mar Sci 53:416–449
Maddison WP, Maddison DR (2011) Mesquite: a modular system for evolutionary analysis, version 2.75, 2.75 edn. http://mesquiteproject.org
Matschiner M, Hanel R, Salzburger W (2009) Gene flow by larval dispersal in the Antarctic notothenioid fish Gobionotothen gibberifrons. Mol Ecol 18:2574–2587
Miyamoto Y, Tanimura A (1999) Behavior of the Antarctic fish Trematomus bernacchii (Pisces, Nototheniidae) beneath the sea ice near the Antarctic station Syowa using acoustic biotelemetry. Fish Sci 65:315–316
Moylan TJ, Sidell BD (2000) Concentrations of myoglobin and myoglobin mRNA in heart ventricles from Antarctic fishes. J Exp Biol 203:1277–1286
Near TJ, Cheng C-HC (2008) Phylogenetics of notothenioid fishes (Teleostei: Acanthomorpha): Inferences from mitochondrial and nuclear gene sequences. Mol Phylogenet Evol 47:832–840
Near TJ, Pesavento JJ, Cheng C-HC (2003) Mitochondrial DNA, morphology, and the phylogenetic relationships of Antarctic icefishes (Notothenioidei: Channichthyidae). Mol Phylogenet Evol 28:87–98
Near TJ, Pesavento JJ, Cheng C-HC (2004) Phylogenetic investigations of Antarctic notothenioid fishes (Perciformes: Notothenioidei) using complete gene sequences of the mitochondrial encoded 16S rRNA. Mol Phylogenet Evol 32:881–891
Near TJ et al (2012) Ancient climate change, antifreeze, and the evolutionary diversification of Antarctic fishes. Proc Natl Acad Sci 109:3434–3439. doi:10.1073/pnas.1115169109
Rickard GJ, Roberts MJ, Williams MJM, Dunn A, Smith MH (2010) Mean circulation and hydrography in the Ross Sea sector, Southern Ocean: representation in numerical models. Antarct Sci 22:533–558
Rock J, Costa FO, Walker DI, North AW, Hutchinson WF, Carvalho GR (2008) DNA barcodes of fish of the Scotia Sea, Antarctica indicate priority groups for taxonomic and systematics focus. Antarct Sci 20:253–262
Ronquist F et al (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Shandikov GA, Eakin RR (2013) Pogonophryne neyelovi, a new species of Antarctic short-barbeled plunderfish (Perciformes, Notothenioidei, Artedidraconidae) from the deep Ross Sea. ZooKeys 296:59–77
Shandikov GA, Eakin RR, Usachev S (2013) Pogonophryne tronio, a new species of Antarctic short-barbeled plunderfish (Perciformes: Notothenioidei: Artedidraconidae) from the deep Ross Sea with new data on Pogonophryne brevibarbata. Polar Biol 36:273–289
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313
Takahashi M, Nemoto T (1984) The food of some Antarctic fish in the western Ross Sea in summer 1979. Polar Biol 3:237–239
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729
Vacchi M, La Mesa M, Greco S (1999) Summer distribution and abundance of larval and juvenile fishes in the western Ross Sea. Antarct Sci 11:54–60
Vacchi M, La Mesa M, Greco S (2000) The coastal fish fauna of Terra Nova Bay, Ross Sea, Antarctica. In: Faranda FM, Guglielmo L, Ianora A (eds) Ross Sea ecology: Italiantartide expeditions (1987–1995). Springer, Berlin, pp 457–468
Volckaert FAM, Rock J, Van de Putte AP (2012) Connectivity and molecular ecology of Antarctic fishes. In: di Prisco G, Verde C (eds) Adaptation and evolution in marine environments, vol 1., The impacts of global change on biodiversitySpringer, Berlin, pp 75–96
Acknowledgments
We would like to thank K. Hoefling, P. A. Cziko, and L. G. Fields for their efforts in catching larval and juvenile specimens by diving, as well as in photographing them for this study. Additional thanks to D. Downie for his participation on some of the PCR and DNA sequencing reactions. We thank J. J. Torres for the gift of the Marguerite Bay adult P. breviceps used in this study. This work was funded by the US National Science Foundation Division of Polar Programs grant ANT-1142158 to C.-H. C. Cheng and A. L. DeVries, and by the Herbert Holdsworth Ross Memorial Fund from the Illinois Natural History Survey to K. R. Murphy.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
List of GenBank accession numbers and BOLD sequence IDs for reference sequences used in this study (DOCX 26 kb)
Online Resource 2
Full topology trees from both Maximum Likelihood and Bayesian analyses of ND2 sequences (PDF 8127 kb)
Online Resource 3
Alignment of partial COI and full length Rho sequences of putative McMurdo Sound larval P. breviceps and vouchered adult from Marguerite Bay, W. Antarctic Peninsula (DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Murphy, K.R., Kalmanek, E.A. & Cheng, CH.C. Diversity and biogeography of larval and juvenile notothenioid fishes in McMurdo Sound, Antarctica. Polar Biol 40, 161–176 (2017). https://doi.org/10.1007/s00300-016-1939-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-016-1939-5