Abstract
Antarctic fishes were sampled with 41 midwater and 6 benthic trawls during the 1999–2000 austral summer in the eastern Ross Sea. The oceanic pelagic assemblage (0–1,000 m) contained Electrona antarctica, Gymnoscopelus opisthopterus, Bathylagus antarcticus, Cyclothone kobayashii and Notolepis coatsi. These were replaced over the shelf by notothenioids, primarily Pleuragramma antarcticum. Pelagic biomass was low and concentrated below 500 m. The demersal assemblage was characteristic of East Antarctica and included seven species each of Artedidraconidae, Bathydraconidae and Channichthyidae, ten species of Nototheniidae, and three species each of Rajidae and Zoarcidae. Common species were Trematomus eulepidotus (36.5%), T. scotti (32.0%), Prionodraco evansii (4.9%), T. loennbergii (4.7%) and Chaenodraco wilsoni (4.3%). Diversity indices were highest for tows from 450 to 517 m (H′=1.90–2.35). Benthic biomass ranged from 0.7 to 3.5 t km−2. It was generally higher in tows from 450 to 517 m (0.9–2.0 t km−2) although the highest biomass occurred at an inner-shelf station (238 m) due to large catches of T. eulepidotus, T. scotti and P. evansii.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pelagic and demersal fish assemblages are an important part of coastal marine ecosystems. The offshore pelagial in Antarctica is dominated by a few fish families (Bathylagidae, Gonostomatidae, Myctophidae and Paralepididae) with faunal diversity decreasing south from the Antarctic Polar Front to the continent (Everson 1984; Kock 1992; Kellermann 1996). South of the Polar Front, the majority of meso- and bathypelagic fishes have circum-Antarctic distributions (McGinnis 1982; Gon and Heemstra 1990). Taken collectively, the fishes are significant contributors to the pelagic biomass and are important trophic elements, both as predators and prey (Rowedder 1979; Hopkins and Torres 1989; Lancraft et al. 1989, 1991; Duhamel 1998). Over the continental slope and shelf, notothenioids dominate the ichthyofauna (DeWitt 1970). Most members of this group are primarily benthic as adults but some species have become pelagic to varying degrees (Andriashev 1970; Eastman 1991, 1993). Larval and juvenile notothenioids are part of the coastal pelagic ecosystem and some species occur to a lesser extent in oceanic waters (Kellermann and Kock 1984; Kellermann and Slósarczyk 1984; Williams 1985; Tabeta and Komaki 1986). The only wholly pelagic notothenioid genus known to occur regularly in oceanic waters as an adult is Dissostichus (Andriashev 1964; Yukhov 1971; Kock 1992).
Studies on the composition and distribution of coastal assemblages led to the zoogeographic classification of Antarctic fish into East Antarctic and West Antarctic Provinces (see reviews by Andriashev 1965, 1987; DeWitt 1971). More recently, Kock (1992) proposed a modified classification scheme with the intent of incorporating both the oceanic and coastal fish fauna. In his scheme, the West Antarctic Province (as well as the South Georgia Province of the Glacial Subregion and all of the Kerguelen Subregion) is designated the Seasonal Pack-Ice Zone, and the East Antarctic Province is designated the High-Antarctic Zone. For either scheme, data on species distribution and abundance provide the basis for characterizing regional assemblages and delineating zoogeographic boundaries.
Over the past two decades, our understanding of Antarctic fish biology and ecology has steadily increased through new literature on pelagic and benthic ichthyofauna (Daniels and Lipps 1982; Kock et al. 1984; Hubold and Ekau 1987; Williams 1988; Ekau 1990; Hureau et al. 1990; Piatkowski et al. 1990; White and Piatkowski 1993; Zimmerman 1997; Duhamel 1998; Eastman and Hubold 1999; Vacchi et al. 1999; Ruhl et al. 2003) and new species (DeWitt and Hureau 1979; Stein and Tomkins 1989; Miya 1994; Skóra 1995; Balushkin and Eakin 1998; Eakin and Balushkin 1998, 2000; Eakin and Eastman 1998; Balushkin 1999; Eastman and Eakin 1999; Chernova and Eastman 2001). However, except for range information on Dissostichus mawsoni (Andriashev 1964; Yukhov 1971), there are no fish data from the eastern Ross Sea region. This area is far removed from any permanent scientific research stations and has high year-round ice cover (Gloersen et al. 1992), making ichthyological sampling quite difficult. Regardless of the zoogeographical scheme or terminology one chooses, the eastern Ross Sea area is one of potential faunal transition and, in the words of Andriashev (1987), has been “mare incognito” for fishes.
Materials and methods
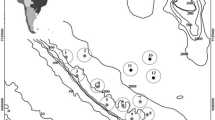
As part of the Antarctic pack-ice seal (APIS) study within the eastern Ross Sea, (68–79°S, 128–179°W), we conducted midwater and benthic trawling on board the RVIB Nathaniel B. Palmer during the 1999–2000 austral summer (NBP99-09, December 1999–February 2000). Midwater sampling was done using three different trawls: (1) a 4-m2 mouth area, five-net MOCNESS trawl with 4-mm mesh nets and 1-mm-mesh cod-end bags; (2) a 9-m2 mouth area Tucker trawl with a 4-mm-mesh main net tapering to a 1-mm-mesh tail section and terminating in a jug-type cod-end with a 1-mm mesh liner; and (3) a 14-m footrope-length balloon fish trawl (Pierce and Mahmoudi 2001) with a 9 m effective mouth width and 3 m height. The net consisted of a 10-cm-mesh front section, a 5-cm-mesh mid-section and terminating in a 3.8-cm-mesh end section fitted with a 0.6-cm-mesh liner. The net was spread using two 1.2×1.8 m steel “V” doors and fishing depth limited by adjusting the towing point on the yoke. Half-meter, 163-m-mesh plankton nets were nested inside the MOCNESS and Tucker nets (any fish collected were added to the totals for the main net). Volume filtered was estimated using both TSK dial-type flowmeters (MOCNESS and Tucker nets) and General Oceanics torpedo-type flowmeters (nested plankton nets). No flow volumes were recorded for the fish trawl. MOCNESS tows were over both 0–500 and 0–1,000 m depth ranges; Tucker and pelagic fish trawls were towed obliquely over 0–500 m. Trawling depth for both the Tucker and fish trawls was estimated from the amount of wire out and wire angle and recorded with a time-depth recorder. Benthic sampling was done using the fish trawl with bottom time ranging from 10 to 15 min. Trawling speed was 2.0–2.5 knots for all MOCNESS, Tucker and bottom tows. Midwater tows with the fish trawl were done at 3.0–3.4 knots. Sampling took place in three different coastal zones: (1) offshore, water depths >2,500 m; (2) continental slope, water depths ~500–2,000 m; and (3) continental shelf, water depths ≤500 m. Ice cover varied from ~2/10 to 10/10 within each zone throughout the sampling period.
Fresh specimens were identified, counted, and measured, then either preserved in 10% formalin or frozen. When not determined directly after capture, wet weight was calculated from length-weight regressions or estimated from preserved specimen weights (assuming a 20% loss from fresh weight). In cases where large numbers of individuals were collected, a sub-sample was kept and the remainder weighed, measured, and discarded. Species identifications were based on taxonomic keys in Gon and Heemstra (1990), supplemented with works by Miya (1994), Schneppenheim et al. (1994), Balushkin and Eakin (1998), Eastman and Eakin (1999, 2000), and La Mesa et al. (2002).
Integrated abundance (number m-2) for each species in the water column was calculated as number per volume filtered multiplied by the vertical range (in meters) of the tow, then summing all tows vertically. Integrated biomass (g WW m−2) was calculated following the same protocol. This procedure was used for the 0–500 and 0–1,000 m depth ranges for zone 1 and the 0–500 m depth range for zones 2 and 3. For bottom tows, integrated abundance and biomass for each species was determined by dividing the number or weight value by each tow’s swept area (km2). Swept area was estimated by multiplying towing speed (in knots, where 1 knot=1,850.7 m h−1)×time on bottom (in hours)×effective mouth width (in meters) (Sparre et al. 1989). Following Eastman and Hubold (1999), we considered species numerically dominant if they comprised ≥5% of the catch. Diversity (H′, Shannon and Weaver 1949), evenness (J′, Pielou 1966) and species’ richness (SR, Margalef 1958) indices were calculated for all bottom trawls. Hydrographic data were collected from CTD casts taken near our trawling locations.
Results
General overview
Temperature profiles within each zone showed little variability (Jacobs et al. 2002). Representative CTD traces of temperature for the three zones are shown in Fig. 1. For zone 1, a roughly isothermal surface layer with temperatures of −1.4 to −1.8°C extended to between 50 and 150 m. Temperatures increased rapidly over the next 200 m, reaching a maximum of 1.6–1.8°C, then gradually decreased with depth to approximately 1.5°C at 500 m and 1.0°C at 1,000 m. For zone 2, the only significant changes were a deepening of the surface layer to around 250–380 m and a depression of the temperature maximum to 400–500 m. For zone 3, surface temperatures extended to below 400 m. Bottom temperatures at our benthic trawling sites ranged from 0.5 to −1.7°C. Complete hydrographic results for the present study area can be found in Jacobs et al. (2002).
Trawl information and locations are shown in Table 1 and Fig. 2. We did 22 MOCNESS trawls (18 in zone 1, 4 in zone 2), 14 Tucker trawls (6 in zone 1, 3 in zone 2, 5 in zone 3) and 11 fish trawls (3 in zone 1, 1 in zone 2, 7 in zone 3). Fish were caught in 30 of the 47 tows (64%). Forty-one pelagic tows were done within all three zones while six bottom tows were done in zone 3. For abundance and biomass analyses, the bottom tows were combined into a shallow group (238–277 m depth, three tows) and a deep group (450–517 m depth, three tows). One bottom tow (no. 5) resulted in a severely damaged net, near the end of the tow and toward the front of the net, with no sample loss evident. A replacement fish trawl net was used for subsequent tows. The short time period spent on the bottom significantly reduced our incidence of major net damage, yet still resulted in considerable numbers of specimens collected.
Pelagic collections
Twenty-five of 41 pelagic tows and 4 of 6 bottom tows yielded 756 specimens of pelagic fishes, 70 from zone 1 (8 species), 46 from zone 2 (2 species) and 640 from zone 3 (2 species). Five common mesopelagic species were represented (Bathylagus antarcticus, Cyclothone kobayashii, Electrona antarctica, Gymnoscopelus opisthopterus and Notolepis coatsi), as well as four notothenioids (Aethotaxis mitopteryx, Pagothenia brachysoma, Pleuragramma antarcticum and Racovitzia glacialis) (Table 2). Within zone 1, 44 of 55 specimens collected in MOCNESS tows were caught between 500 and 1,000 m and only 4 were caught shallower than 200 m. One 41-mm SL P. brachysoma was collected in a 0- to 50-m net, one 64-mm SL R. glacialis was collected in a 0- to 100-m net and 2 P. antarcticum (59, 66 mm SL) were collected in a 100- to 200-m net. Fifteen specimens were collected in zone 1 in 0- to 500-m oblique tows so their exact depth of capture is uncertain. This group included one B. antarcticus (138 mm SL), four E. antarctica (46–79 mm SL), two juvenile N. coatsi (46, 71 mm SL) and eight larval N. coatsi (7–13 mm SL). In light of the discrete-depth MOCNESS collections, if we consider that only the larval N. coatsi might have been caught shallower than 200 m, then 12 specimens (17%) were collected from 0 to 200 m, 14 specimens (20%) from 200 to 500 m and 44 specimens (63%) from 500 to 1,000 m.
Moving across the continental slope toward the shelf, the five mesopelagic species quickly disappeared from our net samples. One Gymnoscopelus opisthopterus and 45 Pleuragramma antarcticum were caught in zone 2 while the nototheniids Aethotaxis mitopteryx and P. antarcticum were the sole pelagic representatives over the shelf. A single A. mitopteryx and 511 of the 639 P. antarcticum collected from zone 3 were captured in bottom trawls but it is likely that all of these specimens were caught in the water column, consistent with a pelagic lifestyle (DeWitt et al. 1990). With the exception of one 27-mm SL individual (Tucker trawl no. 6, zone 3), all of the P. antarcticum specimens caught with pelagic nets from all 3 zones were from 47–78 mm SL (2y; Hubold 1985) while the specimens caught in the benthic tows were larger, ranging from 61 to 202 mm SL (≥2y).
Integrated abundance and biomass values for the 0–500 m (all zones) and 0–1,000 m (zone 1 only) layers are shown in Table 3. Values per m2 for both zone 2 and zone 3 are either overestimated or incalculable since some or all of the specimens from these areas were collected with the fish trawl from which there are no volume-filtered data.
Benthic collections
Six bottom tows collected 1985 fish representing 6 families and 37 species (Table 4). Four common notothenioid families represented the overwhelming majority (98.8%) of the catch, with seven species each of Artedidraconidae, Bathydraconidae and Channichthyidae, and ten species of Nototheniidae. Three species each of Rajidae and Zoarcidae were also collected. Trematomus eulepidotus and T. scotti were most abundant, composing 36.5 and 32.0% of the total catch, respectively. Both these species occurred in all six trawls, however their high frequency of occurrence values are mainly due to single catches of many individuals. Three other species, P. evansii (4.9%), T. loennbergii (4.7%) and C. wilsoni (4.3%), occurred at abundances just under the 5% dominance criterion. The moderately high abundance of those three species was mainly due to single large catches as well, particularly for P. evansii and C. wilsoni. The effect of large single species’ catches is evident in the diversity indices for each trawl (Table 5), especially for trawl no. 9 where P. evansii, T. eulepidotus and T. scotti account for 90% of the catch, with T. eulepidotus alone responsible for 58% of the total.
In overall benthic biomass, Trematomus eulepidotus (47.9%) and T. scotti (10.0%) again composed the largest percentage, followed by Chaenodraco wilsoni (8.0%), Bathyraja maccaini (4.0%), Chionodraco hamatus (3.7%), T. loennbergii (3.4%) and Neopagetopsis ionah (3.1%). Integrated abundance and biomass values for the benthic fish are shown in Table 6. Sampling coverage was roughly equivalent for the three shallow and three deep tows with slightly more individuals and biomass collected shallow. Both the Artedidraconidae and Channichthyidae were more abundant, more speciose, and had greater biomass in the deeper tows. The Bathydraconidae were more abundant in the shallow tows, principally as a consequence of the large catch of Prionodraco evansii in tow no. 9. For this family, the deeper tows were slightly more speciose while biomass was evenly distributed. The family Nototheniidae dominated in terms of numbers (79%) and biomass (67%) and were well represented in both the shallow and deep tows. Numbers of species and individuals, as well as total biomass, were greater in the shallow group of tows. The Rajidae were a minor component of the total catch, their only notable contribution was in trawl no. 9 where one large Bathyraja maccaini accounted for 10% of the biomass for that tow. Zoarcids were only collected in the three deep trawls, with the majority of individuals (55%) in the deepest tow. Total benthic biomass ranged from 0.7 to 3.5 t km2 in the shallow tows and from 0.9 to 2.0 t km2 in the deep tows (Table 4).
Discussion
Pelagic assemblage
The five non-notothenioid species collected in this study (Electrona antarctica, Gymnoscopelus opisthopterus, Bathylagus antarcticus, Cyclothone kobayashii and Notolepis coatsi) are typical of a high-Antarctic oceanic assemblage. Notably absent were G. braueri and C. microdon, two common circum-Antarctic mesopelagic species (Hulley et al. 1989; Lancraft et al. 1989, 1991; White and Piatkowski 1993; Piatkowski et al. 1994). Low diversity in the pelagic fish fauna is expected for a High-Antarctic area (Kock 1992; Kellermann 1996), and even with the large number of midwater tows, the number of pelagic specimens caught in the eastern Ross Sea and their total biomass were quite low. Quantitative comparisons are limited due to the lack of similar data. E. antarctica, Gymnoscopelus spp. and B. antarcticus were collected routinely in the Indian and Atlantic sectors (Prydz Bay region, Hulley et al. 1989) but no abundance data were reported. High numbers of E. antarctica, G. opisthopterus, and B. antarcticus were collected over the continental slope in the eastern Weddell Sea (Hubold and Ekau 1987) using a 100-m2 krill trawl, but the total catch was expressed only in terms of fishing time. In another eastern Weddell Sea study (White and Piatkowski 1993), larvae and juvenile specimens of E. antarctica, G. opisthopterus, B. antarcticus and N. coatsi were common, with numerical abundances reported per 1,000 m3 of volume filtered but only for those nets in which these fish were present. The only data available for direct comparison come from studies at the marginal ice zone in the southern Scotia Sea/northern Weddell Sea (Lancraft et al. 1989, 1991). Abundance (and biomass) for juvenile and adult E. antarctica, Gymnoscopelus spp., B. antarcticus, N. coatsi and C. microdon integrated over the 1,000-m water column from those studies ranged from 60–102 ind. 100 m−2 (325–435 gWW 100 m−2) compared to 43 ind. 100 m−2 and 74 g WW 100 m−2 for this study.
The occurrence of juvenile Pleuragramma antarcticum, Pagothenia brachysoma and Racovitzia glacialis in the oceanic surface layers is consistent with previous observations. Juveniles of several notothenioid species have been found from various regions off the shelf, most often associated with krill (Rembizewski et al. 1978; Slósarczyk and Rembizewski 1982; Slósarczyk 1983; Kellermann and Kock 1984; Hubold 1985). Of the six specimens caught offshore in this study, only Pagothenia brachysoma was with concentrations of Euphausia superba (0.1–0.7 ind. m−3). The four juvenile (2y) P. antarcticum were from the thermocline (70–200 m) with only a few adult E. crystallorophias that also occurred in nets sampling the overlying 0–50 m and 50–100 m layers (0.1–0.5 ind. m3). Juvenile Pleuragramma antarcticum, however, are not solely dependent on krill since they feed on a variety of prey other than euphausiids (DeWitt and Hopkins 1977; Kellermann 1986). Neither the specific net in which the juvenile R. glacialis was collected nor the nets sampling the over- and underlying layers contained any notable abundance of micronekton species. This suggests that either the association of juvenile notothenioids with krill in oceanic waters may not be absolute for all species, or if it is, then our nets failed to sample the accompanying krill aggregation.
The horizontal distribution pattern of pelagic species in this study mirrors that observed by DeWitt (1970) in the eastern Ross Sea and by Hubold and Ekau (1987) and White and Piatkowski (1993) in the Weddell Sea: typical oceanic mesopelagic species disappear across the slope and are replaced by Pleuragramma antarcticum on the shelf. As noted in the earlier studies, the faunal change coincides with the deepening of the cold Antarctic Surface Water layer as one moves onto the shelf. Although P. antarcticum was the only fish collected in our pelagic trawls over the shelf, the inclusion of Aethotaxis mitopteryx in the pelagic assemblage is justifiable based on both reported catch records (Hureau 1985; Kunzmann and Zimmermann 1992; White and Piatkowski 1993) and physiological data (DeVries and Eastman 1981; Eastman 1981, 1985; Eastman and DeVries 1982). Individuals of other species may have been caught in the water column by our benthic tows since the net was open to and from the bottom. Adult Trematomus newnesi have been reported from pelagic, cryopelagic, and benthic habitats (Andriashev 1965, 1970; DeVries and Eastman 1981; Tomo 1981). T. eulepidotus, T. lepidorhinus, and T. loennbergii are considered epibenthic (Ekau 1991; Eastman 1993), the diet of T. eulepidotus at least consisting primarily of euphausiids and pelagic amphipods (Permitin and Tarverdieva 1978; Kock et al. 1984; Roshchin 1991). Adult Gerlachea australis are reported to feed primarily on pelagic hyperiids and euphausiids (Kock et al. 1984; Gon and Heemstra 1990) and many channichthyids also make regular pelagic feeding excursions (Kock 1992; Eastman 1993). In particular, adult specimens of Chionodraco myersi and C. hamatus have been collected in the Weddell Sea (Plötz et al. 2001) and adult Neopagetopsis ionah have been collected considerably off the bottom from several locations (Permitin 1969, 1970; DeWitt 1970; Hubold and Ekau 1987). While the occurrence of these species in the water column, whether merely episodic or more regular, does not alter the numerical predominance of P. antarcticum in coastal pelagic assemblages, it does underscore their potentially important contribution to the total pelagic biomass.
The paucity of specimens in the upper 500 m is likely a consequence of the overall low fish density within the study area and the season. Of the five oceanic species collected, Electrona antarctica and Notolepis coatsi are commonly found in the upper 500 m while Bathylagus antarcticus, Cyclothone kobayashii and Gymnoscopelus opisthopterus are more common in deeper water (Hulley 1981; Hulley et al. 1989; Lancraft et al. 1989, 1991). Additionally, except for C. kobayashii, the oceanic species collected are all vertical migrators (Torres and Somero 1988; Lancraft et al. 1989, 1991), and thus their vertical distribution may be deepened by the extended daylength of the Antarctic summer.
Benthic assemblage
The benthic trawls conducted in this study provided the first collections of coastal fishes from the eastern Ross Sea and, while limited in number, all six tows sampled characteristic bottom habitats at typical shelf depths. We caught 38 species (excluding Aethotaxis mitopteryx and Pleuragramma antarcticum) representing 6 families, numbers quite similar to those of previous studies from other Antarctic regions (Iwami and Abe 1981; Ekau 1990; Zimmermann 1997; Eastman and Hubold 1999). Ninety percent of the species we caught have either established or suspected circum-Antarctic distributions (Anderson 1990; DeWitt et al. 1990; Eakin 1990; Gon 1990; Iwami and Kock 1990; Stehmann and Bürkel 1990). The artedidraconids, Artedidraco orianae and Histiodraco velifer, the bathydraconid, Bathydraco macrolepis and the nototheniid, Trematomus nicolai are relatively uncommon and have only been collected from East Antarctica, and the zoarcid, Lycodichthys dearborni is only known from McMurdo Sound and the western Ross Sea (Anderson 1990; Eastman and Hubold 1999).
The predominance of the nototheniid genus Trematomus together with a large contingent of artedidraconids, bathydraconids and channichthyids is indicative of an East Antarctic assemblage (Kock 1992; Eastman 1993). The absence of genera such as Notothenia, Champsocephalus, Chaenocephalus, and Harpagifer also suggests that there were no discernible faunal contributions from West Antarctica in the eastern Ross Sea. In fact, the benthic assemblage from our study area essentially duplicates that found by Eastman and Hubold (1999) in the western Ross Sea. Thirty-two of the 46 species caught in the western Ross Sea were also caught in our study, with 10 of the remaining 14 species (Paraliparis spp., Trematomus bernacchii, Artedidraco glareobarbatus, A. shackletoni, Pogonophryne spp., Akarotaxis nudiceps and Dacodraco hunteri) from depths shallower or deeper than we sampled. At comparable depths, they caught four species absent from our samples (Muraenolepis microps, Ophthalmolycus bothriocephalus, Pogonophryne cerebropogon and Vomeridens infuscipinnis). Excluding the pelagic Aethotaxis mitopteryx and cryopelagic Pagothenia brachysoma, we caught six species not present in their samples (Chaenodraco wilsoni, Lepidonotothen squamifrons, T. hansoni, T. newnesi, T. nicolai, and T. tokarevi).
Abundance values were often influenced by large single catches of particular species. This was the case for Chaenodraco wilsoni, Prionodraco evansii, Trematomus eulepidotus and T. scotti in the present study, for T. scotti in the western Ross Sea study (Eastman and Hubold 1999) and for several species from studies in the Weddell Sea (Kock et al. 1984; Ekau 1990). Isolated aggregations of particular species underscore the variable nature of benthic assemblages and may reflect real preferences by species in response to local hydrographic, habitat or trophic conditions (Ekau 1990; Ekau and Gutt 1991; Gutt and Ekau 1996; Brenner et al. 2001). Differences in species composition with depth in our study are generally consistent with findings from the western Ross Sea (Eastman and Hubold 1999) and Weddell Sea (Ekau 1990).
With one exception, all diversity indices were higher in the three deep tows (Table 5). Diversity and evenness values for these deep tows are similar to high-end values reported from the Scotia Sea islands (Targett 1981), Antarctic Peninsula (Daniels and Lipps 1982), Weddell Sea (Hubold 1991), Lazarev Sea (Zimmermann 1997), and western Ross Sea (Eastman and Hubold 1999). Our deep tows were done at typical mid-shelf depths and fell within the 300- to 600-m depth range found to contain the highest biomass and species diversity (DeWitt 1971; Andriashev 1987).
Considering the variable nature of benthic communities, it is doubtful that the slightly higher biomass estimates in the present study area reflect a significant increase over other East Antarctic shelf regions. However, it is interesting to note that biomass estimates for East Antarctica in general tend to be higher for areas with narrow shelves (e.g., Vestkapp region of Weddell Sea, Lazarev Sea, present study area) than areas with wide shelves (e.g., Gould Bay region of Weddell Sea, western Ross Sea). Whether or not this is the case, one thing is for certain: the biomass of benthic fish on the shelf in the eastern Ross Sea contrasts sharply with that observed for the water column, both on and off the shelf. Consequently, for predators capable of foraging deep enough, benthic fish assemblages provide an abundant food source.
References
Anderson ME (1990) Zoarcidae. In: Gon O, Heemstra PC (eds) Fishes of the Southern Ocean. JLB Smith Institute of Ichthyology, Grahamstown, pp 256–278
Andriashev AP (1964) On the composition and origin of the Antarctic pelagic fish fauna. In: Carrick R, Holdgate M, Prévost J (eds) Biologie Antarctique. Hermann, Paris, pp 271–272
Andriashev AP (1965) A general review of the Antarctic fish fauna. In: van Mieghem I, Van Oye P (eds) Biogeography and ecology in Antarctica. Junk, The Hague, pp 491–550
Andriashev AP (1970) Cryopelagic fishes of the Arctic and Antarctic and their significance in polar ecosystems. In: Holdgate MW (ed) Antarctic ecology I. Academic, London, pp 297–304
Andriashev AP (1987) A general review of the Antarctic bottom fish fauna. In: Kullander SO, Fernholm B (eds) Proceedings of the fifth congress of European ichthyologists, Stockholm, 1985. Swedish Museum of Natural History, Stockholm, pp 357–372
Balushkin AV (1999) Pogonophryne eakini sp. nova (Artedidraconidae, Notothenioidei, Perciformes): a new species of plunderfish from the Antarctica. J Ichthyol 39:799–802
Balushkin AV, Eakin RR (1998) A new toad plunderfish Pogonophryne fusca sp. Nova (Fam. Artedidraconidae: Notothenioidei) with notes on species composition and species groups in the genus Pogonophryne Regan. J Ichthyol 38:574–579
Brenner M, Buck BH, Cordes S, Dietrich L, Jacob U, Mintenbeck K, Schröder A, Brey T, Knust R, Arntz W (2001) The role of iceberg scours in niche separation within the Antarctic fish genus Trematomus. Polar Biol 24:502–507
Chernova NV, Eastman JT (2001) Two new species of snailfish genus Paraliparis (Pisces:Liparidae) from the Ross Sea, Antarctica. J Fish Biol 59:92–104
Daniels RA, Lipps JH (1982) Distribution and ecology of fishes of the Antarctic Peninsula. J Biogeogr 9:1–9
DeVries AL, Eastman JT (1981) Physiology and ecology of notothenioid fishes of the Ross Sea. J R Soc NZ 11:329–340
DeWitt HH (1970) The character of the midwater fish fauna of the Ross Sea, Antarctica. In: Holdgate MW (ed) Antarctic ecology I. Academic, London, pp 305–314
DeWitt HH (1971) Coastal and deep-water fishes of the Antarctic. In: Bushnell VC (ed) Antarctic map folio series, Folio 15. American Geophysical Society, New York, pp 1–10
DeWitt HH, Hopkins TL (1977) Aspects of the diet of the Antarctic silverfish, Pleuragramma antarcticum. In: Llano GA (ed) Proceedings of the third SCAR symposium on Antarctic biology, Adaptations within Antarctic ecosystems. Smithsonian Institution, Washington DC, pp 557–567
DeWitt HH, Hureau J-C (1979) Fishes collected during “Hero” Cruise 72–2 in the Palmer Archipelago, Antarctica, with the description of two new genera and three new species. Bull Mus Natl Hist Nat (4)1A(3):775–820
DeWitt HH, Heemstra PC, Gon O (1990) Nototheniidae. In: Gon O, Heemstra PC (eds) Fishes of the Southern Ocean. JLB Smith Institute of Ichthyology, Grahamstown, pp 279–331
Duhamel G (1998) The pelagic fish community of the Polar Frontal Zone off the Kerguelen Islands. In: di Prisco G, Pisano E, Clarke A (eds) Fishes of Antarctica. A biological overview. Springer, Rome, pp 63–74
Eakin RR (1990) Artedidraconidae. In: Gon O, Heemstra PC (eds) Fishes of the Southern Ocean. JLB Smith Institute of Ichthyology, Grahamstown, pp 332–356
Eakin RR, Balushkin AV (1998) A new species of toadlike plunderfish Pogonophryne orangiensis sp. Nova (Artedidraconidae, Notothenioidei) from the Weddell Sea, Antarctica. J Ichthyol 38:800–803
Eakin RR, Balushkin AV (2000) A new species of Pogonophryne (Pisces: Perciformes: Artedidraconidae) from East Antarctica. Proc Biol Soc Wash 113:264–268
Eakin RR, Eastman JT (1998) New species of Pogonophryne (Pisces, Artedidraconidae) from the Ross Sea, Antarctica. Copeia 1998:1005–1009
Eastman JT (1981) Morphological specializations in Antarctic fishes. Antarct J US 16:146–147
Eastman JT (1985) The evolution of neutrally buoyant notothenioid fishes: their specializations and potential interactions in the Antarctic food web. In: Siegfried WR, Condy PR, Laws RM (eds) Antarctic nutrient cycles and food webs. Springer, Berlin Heidelberg NewYork, pp 430–436
Eastman JT (1991) Evolution and diversification of Antarctic notothenioid fishes. Am Zool 31:93–109
Eastman JT (1993) Antarctic fish biology: evolution in a unique environment. Academic, San Diego
Eastman JT, DeVries AL (1982) Buoyancy studies of notothenioid fishes in McMurdo Sound, Antarctica. Copeia 1982:385–393
Eastman JT, Eakin RR (1999) Fishes of the genus Artedidraco (Pisces, Artedidraconidae) from the Ross Sea, Antarctica, with the description of a new species and a colour morph. Antarct Sci 11:13–22
Eastman JT, Eakin RR (2000) An updated species list for notothenioid fish (Perciformes; Notothenioidei), with comments on Antarctic species. Arch Fish Mar Res 48:11–20
Eastman JT, Hubold G (1999) The fish fauna of the Ross Sea, Antarctica. Antarct Sci 11:293–304
Ekau W (1990) Demersal fish fauna of the Weddell Sea, Antarctica. Antarct Sci 2:129–137
Ekau W (1991) Morphological adaptations and mode of life in High Antarctic fish. In: di Prisco G, Maresca B, Tota B (eds) Biology of Antarctic fish. Springer, Berlin Heidelberg New York, pp 23–39
Ekau W, Gutt J (1991) Notothenioid fishes from the Weddell Sea and their habitat, observed by underwater photography and television. Proc NIPR Symp Polar Biol 4:36–49
Everson I (1984) Fish Biology. In: Laws RM (ed) Antarctic Ecology II. Academic, London, pp 491–532
Gloersen P, Campbell WJ, Cavilieri DJ, Comiso JC, Parkinson CL, Zwally HJ (1992) Arctic and Antarctic sea ice 1978–87: satellite passive microwave observations and analysis. NASA SP-511. National Aeronautics and Space Administration, Washington
Gon O (1990) Bathydraconidae. In: Gon O, Heemstra PC (eds) Fishes of the Southern Ocean. JLB Smith Institute of Ichthyology, Grahamstown, pp 364–380
Gon O, Heemstra PC (eds) (1990) Fishes of the Southern Ocean. JLB Smith Institute of Ichthyology, Grahamstown
Gutt J, Ekau W (1996) Habitat partitioning of dominant high Antarctic demersal fish in the Weddell Sea and Lazarev Sea. J Exp Mar Biol Ecol 206:25–37
Hopkins TL, Torres JJ (1989) Midwater food web in the vicinity of a marginal ice zone in the western Weddell Sea, March 1986. Deep Sea Res 36:543–560
Hubold G (1985) The early life-history of the high-Antarctic silverfish Pleuragramma antarcticum. In: Siegfried WR, Condy PR, Laws RM (eds) Antarctic nutrient cycles and food webs. Springer, Berlin Heidelberg New York, pp 445–451
Hubold G (1991) Ecology of notothenioid fish in the Weddell Sea. In: diPrisco G, Maresca B, Tota B (eds) Biology of Antarctic fish. Springer, Berlin Heidelberg New York, pp 3–22
Hubold G, Ekau W (1987) Midwater fish fauna of the Weddell Sea, Antarctica. In: Kullander SO, Fernholm B (eds) Proceedings of the fifth congress of European ichthyologists, Stockholm, 1985. Swedish Museum of Natural History, Stockholm, pp 391–396
Hulley PA (1981) Results of the research cruises of FRV ‘Walther Herwig’ to South America. LVIII. Family Myctophidae (Osteichthyes, Myctophiformes). Arch Fischereiwiss 31:1–300
Hulley PA, Camus P, DuHamel G (1989) Ichthyological results of cruise MD-42/SIBEX-II. Part 1. Fishes from RMT-8 stations, with additional records of Lanternfishes (Myctophidea: Osteichthyes) from the Indian and Atlantic sectors of the Southern Ocean. Cybium 13:83–89
Hureau J-C (1985) Nototheniidae. In: Fischer W, Hureau J-C (eds) FAO species identification sheets for fishery purposes. Southern Ocean (Fishing Areas 48, 58 and 88) (CCAMLR Convention area). FAO, Rome, pp 323–385
Hureau J-C, Balguerìas E, DuHamel G, Kock K-H, Ozouf-Costaz C, White M (1990) Fish fauna of the eastern Weddell Sea. Ber Polarforsch 68:130–138
Iwami T, Abe T (1981) The collection of the fishes trawled in the Ross Sea. Antarct Rec Natl Inst Polar Res 71:130–141
Iwami T, Kock K-H (1990) Channichthyidae. In: Gon O, Heemstra PC (eds) Fishes of the Southern Ocean. JLB Smith Institute of Ichthyology, Grahamstown, pp 381–399
Jacobs SS, Mele PA, O’Hara SH, Ardai JL Jr (2002) Oceanographic observations in the eastern Ross Sea, APIS NB Palmer Cruise 99–09, Dec 1999–Feb 2002. LDEO-2002-1. Lamont-Doherty Earth Observatory, New York
Kellermann A (1986) On the biology of early life stages of notothenioid fishes (Pisces) off the Antarctic Peninsula. Ber Polarforsch 31:1–149
Kellermann A (1996) Midwater fish ecology. In: Ross RM, Hofmann EE, Quetin LB (eds) Foundations for ecological research west of the Antarctic Peninsula. Antarct Res Ser 70:231–256
Kellermann A, Kock K-H (1984) Postlarval and juvenile notothenioids (Pisces, Perciformes) in the Southern Scotia Sea and Northern Weddell Sea during FIBEX 1981. Meeresforschung 30:82–93
Kellermann A, Slósarczyk W (1984) Distribution of postlarval and juvenile Notothenioidei in the Atlantic sector of the Southern Ocean during FIBEX 1981. BIOMASS Rep Ser 36:1–27
Kock K-H (1992) Antarctic fish and fisheries. Cambridge University Press, Cambridge
Kock K-H, Schneppenheim R, Siegel V (1984) A contribution to the fish fauna of the Weddell Sea. Arch Fischereiwiss 34:103–120
Kunzmann A, Zimmermann C (1992) Aethotaxis mitopteryx, a high-Antarctic fish with a benthopelagic mode of life. Mar Ecol Prog Ser 88:33–40
La Mesa M, Vacchi M, Iwami T, Eastman JT (2002) Taxonomic studies of the Antarctic icefish genus Cryodraco Dollo, 1900 (Notothenioidei: Channichthyidae). Polar Biol 25:384–390
Lancraft TM, Torres JJ, Hopkins TL (1989) Micronekton and macrozooplankton in the open waters near Antarctic ice-edge zones (AMERIEZ 1983 and 1986). Polar Biol 9:225–233
Lancraft TM, Hopkins TL, Torres JJ, Donnelly J (1991) Oceanic micronektonic/macrozooplanktonic community structure and feeding in ice covered Antarctic waters during the winter (AMERIEZ 1988). Polar Biol 11:157–167
Margalef R (1958) Information theory in ecology. Gen Syst 3:36–71
McGinnis RF (1982) Biogeography of lanternfishes (Myctophidae) south of 30 S. In: Pawson DL (ed) Antarctic Research Series, vol 35. Biology of the Antarctic Seas XII. American Geophysical Union, Washington DC, pp 1–110
Miya M (1994) Cyclothone kobayashii, a new gonostomatid fish (Teleostei: Stomiiformes) from the Southern Ocean with notes on its ecology. Copeia 1994:191–204
Permitin YuYe (1969) New data on species composition and distribution of fishes in the Scotia Sea, Antarctica (second communication). Prob Ichthyol 9:167–181
Permitin YuYe (1970) The consumption of krill by Antarctic fishes. In: Holdgate MW (ed) Antarctic ecology I. Academic, London, pp 177–182
Permitin YuYe, Tarverdieva MI (1978) Feeding of fishes of the families Nototheniidae and Channichthyidae in the South Orkney Islands. Sov J Mar Biol 4:619–622
Piatkowski U, White M, Dimmler W (1990) Micronekton of the Weddell Sea: distribution and abundance. Ber Polarforsch 68:73–81
Piatkowski U, Rodhouse PG, White MG, Bone D, Symon C (1994) Nekton community of the Scotia Sea as sampled by the RMT 25 during austral summer. Mar Ecol Prog Ser 112:13–28
Pielou EC (1966) Shannon’s formula as a measure of specific diversity: its use and disuse. Am Nat 100:463–465
Pierce DJ, Mahmoudi B (2001) Nearshore fish assemblages along the central west coast of Florida. Bull Mar Sci 68:243–270
Plötz J, Bornemann H, Knust R, Schröder A, Bester M (2001) Foraging behavior of Weddell seals, and its ecological implications. Polar Biol 24:901–909
Rembizewski JM, Krzeptowski M, Linkowski TB (1978) Fishes as by-catch in fisheries of krill Euphausia superba Dana (Euphausiacea-Crustacea). Pol Arch Hydrobiol 25:677–695
Roshchin EA (1991) Aspects of the life cycle of Trematomus eulepidotus (Nototheniidae) in the Indian Ocean sector of the Antarctic. J Ichthyol 31:1–11
Rowedder U (1979) Feeding ecology of the myctophid Electrona antarctica (Gunther, 1978) (Teleostei). Meeresforschung 27:252–263
Ruhl HA, Hastings PA, Zarubick LA, Jensen RM, Zdzitowiecki K (2003) Fish populations of Port Foster, Deception Island, Antarctica and vicinity. Deep Sea Res II 50:1843–1858
Schneppenheim R, Kock K-H, Duhamel G, Janssen G (1994) On the taxonomy of the Lepidonothen squamifrons group (Pisces, Perciformes, Notothenioidei). Arch Fish Mar Res 42:137–148
Shannon CE, Weaver W (1949) The mathematical theory of communication. University of Illinois Press, Urbana
Skóra KE (1995) Acanthodraco dewitti gen. et sp. n. (Pisces, Bathydraconidae) from Admiralty Bay (King George Island, South Shetland Islands, Antarctica). Arch Fish Mar Res 42:286–289
Slósarczyk W (1983) Juvenile Trematomus bernachii and Pagothenia brachysoma (Pisces, Nototheniidae) within krill concentrations off Balleny Islands (Antarctic). Pol Polar Res 4:57–69
Slósarczyk W, Rembizewski JM (1982) The occurrence of juvenile Notothenioidei (Pisces) within krill concentrations in the region of the Bransfield Strait and the southern Drake Passage. Pol Polar Res 3:299–312
Sparre P, Ursin E, Venema SC (1989) Introduction to tropical fish stock assessment, Part 1. Manual. FAO Fisheries Technical Paper, no. 306/1. FAO, Rome
Stehmann M, Bürkel DL (1990) Rajidae. In: Gon O, Heemstra PC (eds) Fishes of the Southern Ocean. JLB Smith Institute of Ichthyology, Grahamstown, pp 86–97
Stein DL, Tomkins LS (1989) New species and new records of rare Antarctic Paraliparis fishes (Scorpaeniformes: Liparididae). Ichthyol Bull JLB Smith Inst Ichthyol 53:1–8
Tabeta O, Komaki Y (1986) Distribution and abundance of pelagic fishes in the epipelagic layers of the Indian sector of the Southern Ocean in summer, 1983–84. Mem Natl Inst Polar Res 40 Spec Issue:316–322
Targett TE (1981) Trophic ecology and structure of coastal Antarctic fish communities. Mar Ecol Prog Ser 4:243–263
Tomo AP (1981) Contribuciòn al conocimiento de la fauna ictiològica del sector Antàrtico Argentino. Inst Antart Argent 14:7–241
Torres JJ, Somero G (1988) Vertical distribution and metabolism in Antarctic mesopelagic fishes. Comp Biochem Physiol 90B:521–528
Vacchi M, La Mesa M, Greco S (1999) The coastal fish fauna of Terra Nova Bay, Ross Sea, Antarctica. In: Faranda F, Guglielmo L, Ionova A (eds) Ross Sea ecology: italiantartide expedtions (1987–1995). Springer, Berlin Heidelberg New York, pp 457–468
White MG, Piatkowski U (1993) Abundance, horizontal and vertical distribution of fish in eastern Weddell Sea micronekton. Polar Biol 13:41–53
Williams R (1985) The potential impact of a krill fishery upon pelagic fish in the Prydz Bay area of Antarctica. Polar Biol 5:1–4
Williams R (1988) The inshore fishes of the Vestfold Hills region, Antarctica. Hydrobiologia 165:161–167
Yukhov VL (1971) The range of Dissostichus mawsoni Norman and some features of its biology. J Ichthyol 11:8–18
Zimmermann C (1997) On the demersal fish fauna of the Lazarev Sea (Antarctica): composition and community structure. In: Battaglia B, Valenicia P, Walton DWH (eds) Antarctic communities: species, structure and survival. Cambridge University Press, Cambridge, pp 26—32
Acknowledgements
We thank Captain J. Borkowski and the crew of the RVIB Nathaniel B. Palmer, as well as K. Newyear and the team of marine technicians from Raytheon Polar Services Company for their expertise and immeasurable help with trawling operations. Thanks also go to K. Daly for her assistance at sea, both on deck and in the laboratory. This research was supported by NSF grant OPP9815973 and NSF grant OPP9910100, both to J.J.T.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Donnelly, J., Torres, J.J., Sutton, T.T. et al. Fishes of the eastern Ross Sea, Antarctica. Polar Biol 27, 637–650 (2004). https://doi.org/10.1007/s00300-004-0632-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-004-0632-2