Abstract
Current trends of fish communities in the interior Arctic Ocean are largely unknown, whereas more fishes of boreal origin are reported from the Chukchi and Barents Seas recently. To assess variability in species composition and spatiotemporal occurrence in ichthyoplankton in the southeast Beaufort Sea, we sampled larval and juvenile fish using square-conical nets in the upper water column (<100 m) from June to September between 2002 and 2011. Gadidae consisting of Boreogadus saida and Arctogadus glacialis numerically accounted for >75 % of total catches every month. Cottidae and Liparidae usually followed Gadidae, together representing 9–94 % of non-gadid species in number. The majority of dominant and subdominant species occurred ubiquitously through the sampling area, whereas Gymnocanthus tricuspis (Cottidae), Liparis gibbus (Liparidae), and Leptoclinus maculatus (Stichaeidae) occurred abundantly on the Mackenzie Shelf. In contrast, Triglops nybelini (Cottidae) was frequently found in the Amundsen Gulf, which was characterized by higher salinities (>25). Exceptional species composition was observed in September 2011, when Ammodytes hexapterus (Ammodytidae) numerically accounted for 67 % of non-gadid species. In the southeast Beaufort Sea, summer ichthyoplankton are characterized by the overwhelming dominance of Arctic gadids as well as the frequent occurrence of Arctic cottids and liparids. However, the sudden and frequent occurrence of A. hexapterus may be a first sign of significant changes in fish communities in the interior Arctic Ocean.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sea surface warming combined with increasing river discharge and changing ocean currents will strongly impact the Arctic marine ecosystem within the next half a century (ACIA 2005). Although fish constitute the main energy channel from invertebrates to seabirds, seals, and whales in the Arctic Ocean (Bradstreet and Cross 1982; Welch et al. 1992), fish communities have mostly been studied in the main gateways to the Arctic Ocean, such as the Chukchi Sea (Mecklenburg et al. 2007; Norcross et al. 2010; Lin et al. 2012), Barents Sea (Byrkjedal and Høines 2007; Eriksen et al. 2011, 2012) and Baffin Bay (Munk et al. 2003; Jørgensen et al. 2011). Recently, more fishes of boreal origin occur in these gateways, as many species are extending their distribution ranges northward (Perry et al. 2005; Fleischer et al. 2007; Mueter and Litzow 2008). Given that such biological invasions are threatening fishes of Arctic origin (Christiansen et al. 2014; Falardeau et al. 2014), current trends of fish communities should be investigated not only in the gateways but also in the interior Arctic Ocean, which is not directly influenced by Pacific or Atlantic waters (Carmack and Wassmann 2006).
The southeast Beaufort Sea is characterized by all topographic features that typically characterize the interior Arctic Ocean: large estuarine system, shallow continental shelf, and deep ocean basin (Carmack and Wassmann 2006). The Mackenzie River plume dominates the surface water layer over the Mackenzie Shelf, sometimes extending to the Canada Basin over the Beaufort Slope (Macdonald and Yu 2006). Following the first interdisciplinary study in the 1980s (Northern Environmental Protection Branch 1985), several large-scale research programs have been conducted in this area (Fortier et al. 2008; Barber et al. 2012). These research programs have accumulated baseline information about fish communities in coastal waters (Chiperzak et al. 1990, 2003a, b, c; Majewski et al. 2006, 2009, 2011, 2013) as well as for the dominant fish species, polar cod Boreogadus saida (Benoit et al. 2008, 2010; Bouchard and Fortier 2011; Bouchard et al. 2013, 2014; Geoffroy et al. 2011; Walkusz et al. 2011, 2012; Falardeau et al. 2014). Recent studies reported that the Mackenzie River plume dictates the distribution of ichthyoplankton communities on the Mackenzie Shelf (Paulic and Papst 2012; Wong et al. 2013). However, little or no information is available concerning subdominant fishes, especially in offshore waters.
As a first step for investigations into current trends of fish communities in the southeast Beaufort Sea, the present study focused on larval and juvenile fish in the upper water column (hereafter, ichthyoplankton). Physical and biological sampling was conducted in summer between 2002 and 2011. We examined (1) interannual changes in species composition and (2) variability in the spatiotemporal occurrence of dominant and subdominant species.
Materials and methods
Study region
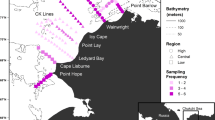
The southeast Beaufort Sea is comprised of the Mackenzie Shelf, the Beaufort Slope, and the Amundsen Gulf (Fig. 1). The Mackenzie Shelf is a shallow rectangular shelf (520 km × 120 km), bordered by the Mackenzie Trough to the west, the Amundsen Gulf to the east, and the Beaufort Slope to the north (shelf break depth, ca. 100 m). The Mackenzie River, the fourth largest river flowing into the Arctic Ocean, delivers a large amount of fresh water and sediments to the Mackenzie Shelf mainly from May to September (Macdonald and Yu 2006). Three water layers of distinctive origins co-occur in the sea: the Polar Mixed Layer (<50 m), the Pacific Halocline (50–200 m), and the Atlantic Layer (>200 m) (Carmack et al. 1989; Macdonald et al. 1989). The Polar Mixed Layer consists of sea ice melt and river discharge as well as Pacific or Atlantic waters that have been mixed sufficiently to have lost their original identity. In summer, changeable wind forcing primarily dictates water movement on the Mackenzie Shelf (Carmack and Macdonald 2002; Williams and Carmack 2008), whereas off the shelf relatively constant currents exist: the Beaufort shelf break jet flowing eastward along the Beaufort Slope and the Beaufort Gyre flowing westward in the southern Canada Basin (Pickart 2004; Steele et al. 2004).
Field sampling
Physical and biological sampling was conducted in the southeast Beaufort Sea from June to September between 2002 and 2011 onboard Canadian Coast Guard icebreakers. Vertical profiles of temperature and salinity were obtained at 1-m intervals with a rosette-type oceanographic profiler equipped with a Seabird CTD. Ichthyoplankton were sampled using a double square-net (DSN) sampler that consisted of a rectangular frame carrying two square-conical nets (1 m2 opening, 6 m long; Bouchard et al. 2014). As ichthyoplankton increased in size during the sampling season, the mesh size was changed from 200 or 500 μm to 750 or 1600 μm. The DSN sampler was towed obliquely in the surface layer (<100 m) at a speed of ca. 1 m s−1. The maximum sampling depth was determined in accordance with bottom depth at each station. The volume of water filtered was calculated from ship speed and towing duration, due to the frequent failure of flow meters in frigid waters. Biological sampling stations were selected among physical sampling stations in each year. The selected stations were arranged throughout the southeast Beaufort Sea in 2004 and 2008, whereas in 2009 and 2010, they were concentrated around the shelf break (Fig. 1). In addition to oblique tows using the DSN sampler, several water layers were sampled separately using a EZNet multi-layer sampler (2–9 layers; Bouchard et al. 2014) to assess the vertical distribution of ichthyoplankton in July 2004. Square-conical nets (1 m2 opening, 200 or 333 μm mesh) mounted on the EZNet sampler were opened sequentially and towed obliquely at a speed of ca. 1 m s−1. The number and depth of water layers sampled were set in accordance with bottom depth at each station. The volume of water filtered was calculated from a flow meter attached to the EZNet sampler. Ichthyoplankton specimens were enumerated and most were measured for fresh standard length (SL) onboard before individual preservation in 95 % ethanol.
Laboratory analysis
All ichthyoplankton specimens were enumerated, identified morphologically to the lowest taxonomic level possible, and measured for preserved SL. Fresh SL of individuals not measured at sea was estimated from their preserved SL using family-specific relationships obtained from individuals measured at sea. The morphological identification was realized following relevant literature (e.g., Able et al. 1986; Matarese et al. 1989; Fahay 2007a, b; Blood and Matarese 2010), whereas scientific names followed Mecklenburg et al. (2011). Families were listed in accordance with Nelson (2006), and species were listed alphabetically within each family. The two gadid species B. saida and A. glacialis were pooled in Gadidae because of close similarities in morphology during their early life stages. As genetic (Nelson et al. 2013) and otolithometric (Bouchard et al. 2013) analysis have recently enabled identification of the two gadid species, their respective early life histories have been compared and published elsewhere (Bouchard and Fortier 2011; Bouchard et al. 2014). Identification of Ammodytes hexapterus was confirmed by genetic analysis (Falardeau et al. 2014).
Results
Both Amundsen Gulf and Beaufort Slope were characterized by consistently higher salinities (>25) in contrast to variable salinities off the mouth of the Mackenzie River (Fig. 2). The river plume was visible in 2004 with the distribution of higher temperatures and lower salinities in surface waters (>4 °C and <25 respectively). The river plume was also observed at least partially in 2008 and 2009, whereas in other years, it was not detected within the area observed. Spatial differences in temperature and salinity were less marked in subsurface waters (not shown).
Gadidae numerically accounted for >75 % of monthly catches in each year (Fig. 3). Besides Gadidae, five families, 11 genera, and 13 species were identified (Table 1). Cottidae and Liparidae usually followed Gadidae, together representing 9–94 % of non-gadid species in number. In Cottidae, Gymnocanthus tricuspis and Triglops nybelini were the dominant species. Liparis fabricii was more abundant than Liparis gibbus in Liparidae. Other subdominant species included Leptoclinus maculatus (Stichaeidae), Stichaeus punctatus (Stichaeidae), Aspidophoroides olrikii (Agonidae), and A. hexapterus (Ammodytidae). Although A. hexapterus larvae and juveniles were caught only in 2010 and 2011, they numerically accounted for 67 % of non-gadid species in 2011.
Growth during a prolonged planktonic period was reflected by temporal shifts in SL frequency distributions of T. nybelini, L. fabricii, L. gibbus, and A. olrikii, from June to September (Fig. 4). In these species, SL increased from 10 mm in June to >30 mm in September at an average growth rate of >0.2 mm day−1. In contrast, early settlement after a shorter planktonic period was suggested in G. tricuspis and S. punctatus as their occurrence was restricted both in terms of size and season: G. tricuspis, <20 mm SL in July; S. punctatus, <25 mm SL in September. Leptoclinus maculatus of various sizes (12–50 mm SL) occurred from June to September, with no clear pattern in its SL frequency distribution. Ammodytes hexapterus occurred abundantly only in September 2011 (12–53 mm SL).
The spatial occurrence of dominant and subdominant species was classified into three groups: ubiquitous through the sampling area, abundant on the shelf, and abundant off the shelf (Fig. 5). The ubiquitous distribution was evident in Gadidae and L. fabricii, whereas it was less evident in S. punctatus, A. olrikii, and A. hexapterus. Generally, G. tricuspis, L. gibbus, and L. maculatus occurred more abundantly on the Mackenzie Shelf. In contrast, T. nybelini occurred more abundantly off the shelf, specifically in the Amundsen Gulf. While peak abundance of most species corresponded with the plankton bloom in June and July (Tremblay et al. 2012), higher densities of S. punctatus were observed in September.
Spatial occurrence of dominant and subdominant ichthyoplankton species caught by the double square-net sampler in the southeast Beaufort Sea in summer between 2002 and 2011 (pooled years). Monthly occurrence is shown for Gadidae (a), Cottidae (b), Liparidae (c), Stichaeidae (d), and others (e). Gadidae consists of B. saida and A. glacialis. Note that the scale of density may differ among plots
In July 2004, the majority of ichthyoplankton were distributed in the Polar Mixed Layer (<50 m), independent of bottom depth (30–490 m, Online Resource 1). The number of larval and juvenile fish caught by the EZNet sampler was 293 (26 tows), 201 (84 tows), and 10 (54 tows) in depth layers <10, 10–50, and >50 m, respectively. Gadidae numerically accounted for >75 % of catches in all depth layers. These results corroborated the validity of the regular sampling method employed in the present study (i.e., oblique tows in the upper water column).
Discussion
Ichthyoplankton in the interior Arctic Ocean
Geographic isolation from Pacific and Atlantic waters, combined with large estuarine system, shallow continental shelf, and deep ocean basin, characterizes the interior Arctic Ocean (i.e., the Beaufort, East Siberian, Laptev, and Kara Seas; Carmack and Wassmann 2006). The fish species composition described here, with an overwhelming dominance of Gadidae, a subdominance of Cottidae and Liparidae of Arctic origin, and frequent occurrence of Agonidae and Stichaeidae, can be considered to be characteristic of summer ichthyoplankton in the interior Arctic Ocean. The two Arctic gadids B. saida and A. glacialis represented >75 % of the ichthyoplankton in the present study, irrespective of sampling depth or year. Between the two species, B. saida have been shown to outnumber A. glacialis by a factor of 12 in the southeast Beaufort Sea (Bouchard et al. 2014). The two Arctic cottids G. tricuspis and T. nybelini, and the two Arctic liparids L. fabricii and L. gibbus frequently occurred in our samples and are likely widespread elsewhere in the interior Arctic Ocean. In contrast to coastal and estuarine waters (Chiperzak et al. 1990, 2003a, b, c; Majewski et al. 2006, 2009, 2011, 2013; Paulic and Papst 2012; Wong et al. 2013), no diadromous or estuarine species, such as Pacific herring Clupea palasii palasii and whitefishes Coregonus spp., were found in our study area. Fish species composition similar to ours was reported from the adjacent southwest Beaufort and Chukchi Seas, although in these seas fishes of Arctic origin are occasionally replaced by fishes of boreal origin, including capelin Mallotus villosus, yellowfin sole Limanda aspera, or Bering flounder Hippoglossoides robustus (Jarvela and Thorsteinson 1999; Norcross et al. 2010; Lin et al. 2012). On the other hand, an overwhelming dominance of fishes of boreal origin, such as sand lance Ammodytes spp., Atlantic herring Clupea herengus, and Atlantic cod Gadus morhua, was reported for ichthyoplankton in the Barents Sea and Baffin Bay (Munk et al. 2003; Eriksen et al. 2011, 2012).
Potential effects of climate change on Arctic ichthyoplankton
Although the spatiotemporal resolution of our sampling was not sufficient to correlate ichthyoplankton densities to environmental parameters, some general patterns of spatial occurrence can, nonetheless, be drawn. For example, G. tricuspis, L. gibbus, and L. maculatus occurred abundantly on the Mackenzie Shelf, indicating early life histories associated with shallow waters, where the river plume frequently brings higher temperatures and lower salinities in summer. Whereas T. nybelini occurred abundantly in the Amundsen Gulf, many other species were found ubiquitously through the southeast Beaufort Sea. In temporal patterns, the majority of dominant and subdominant species exhibited gradual growth during a longer planktonic period, although early settlement after a shorter planktonic period was suggested in G. tricuspis and S. punctatus as their occurrence was restricted both in terms of size and season (Brown and Green 1976).
In the interior Arctic Ocean, ichthyoplankton species would be impacted by ongoing climate change differently in response to their respective early life histories. Shelf-associated species are more vulnerable to changes in river discharge, whereas variability in water temperature and ocean currents is more likely to affect species with an extended planktonic period (cf. ACIA 2005). Besides such direct impacts, environmental changes could affect Arctic ichthyoplankton indirectly through trophic relationships. Sea ice retreat will likely increase light availability and wind-driven upwelling to enhance phytoplankton production over continental shelves, whereas in ocean basins sea surface freshening and warming probably strengthen stratification and prevent the replenishment of nutrients available for phytoplankton (Carmack and McLaughlin 2011; Tremblay et al. 2012). According to this scenario, consumers might benefit from bottom-up effects of increasing phytoplankton production only on continental shelves. Such spatial heterogeneity should be addressed in further investigations into Arctic ichthyoplankton relative to their changing environment.
Ichthyoplankton diversity and abundance can serve as an indicator of changing ocean conditions (e.g., Brodeur et al. 2008). The high abundance of L. maculatus in June 2008 and of A. hexapterus in September 2011 represents significant invasions of fishes of boreal origin in our study area. The substantial presence of these species, rarely found in ichthyoplankton in the southeast Beaufort Sea (Chiperzak et al. 1990, 2003a, b, c; Paulic and Papst 2012; Wong et al. 2013), most likely results from recent environmental changes in this area (e.g., sea surface warming and sea ice loss; Wood et al. 2013). Although there is a possibility of aberrant drift from the northern Bering Sea (Berline et al. 2008), significant reproduction of A. hexapterus in the Beaufort Sea in 2011 is strongly suggested by its unimodal size/age frequency distributions including small/young individuals (<20 mm SL or <10 days old; Falardeau et al. 2014). A similar inference about L. maculatus can be drawn from its SL frequency distribution (cf. Meyer Ottesen et al. 2011). As such, ichthyoplankton may act as sentinels of climate change, detecting significant reproduction of new species and forecasting biological invasions in a given area. Moreover, ichthyoplankton species observed in the present study have a benthic (12 species) or bentho-pelagic (B. saida, A. glacialis, and A. hexapterus) adult stage and therefore characterized by different vulnerability to standard fishing gear such as bottom or pelagic trawls during the adult stage. Intense bottom trawl surveys conducted on certain Arctic shelves also bring concerns about habitat destruction (Christiansen et al. 2014). Ichthyoplankton surveys thus constitute a powerful tool to assess the response of fish communities to environmental changes in the interior Arctic Ocean.
References
Able KW, Fahay MP, Markle DF (1986) Development of larval snailfishes (Pisces: Cyclopteridae: Liparidinae) from the western North Atlantic. Can J Zool 64:2294–2316
ACIA (2005) Arctic climate impact assessment. Cambridge University Press, Cambridge
Barber DG, Tjaden T, Leitch D, Barber L, Chan W (2012) On the edge: from knowledge to action during the fourth international polar year circumpolar flaw lead system study (2007–2008). Prolific Printing, Winnipeg
Benoit D, Simard Y, Fortier L (2008) Hydroacoustic detection of large winter aggregations of Arctic cod (Boreogadus saida) at depth in ice-covered Franklin Bay (Beaufort Sea). J Geophys Res 113:C06S90
Benoit D, Simard Y, Gagné J, Geoffroy M, Fortier L (2010) From polar night to midnight sun: photoperiod, seal predation, and the diel vertical migrations of polar cod (Boreogadus saida) under landfast ice in the Arctic Ocean. Polar Biol 33:1505–1520
Berline L, Spitz YH, Ashjian CJ, Campbell RG, Maslowski W, Moore SE (2008) Euphausiid transport in the Western Arctic Ocean. Mar Ecol Prog Ser 360:163–178
Blood DM, Matarese AC (2010) Larval development and identification of the genus Triglops (Scorpaeniformes: Cottidae). NOAA Professional Paper NMFS 10, National Marine Fisheries Service, NOAA, Seattle
Bouchard C, Fortier L (2011) Circum-arctic comparison of the hatching season of polar cod Boreogadus saida: a test of the freshwater winter refuge hypothesis. Prog Oceanogr 90:105–116
Bouchard C, Robert D, Nelson RJ, Fortier L (2013) The nucleus of the lapillar otolith discriminates the early life stages of Boreogadus saida and Arctogadus glacialis. Polar Biol 36:1537–1542
Bouchard C, Mollard S, Suzuki K, Robert D, Fortier L (2014) Contrasting the early life histories of sympatric Arctic gadids Boreogadus saida and Arctogadus glacialis in Canadian Beaufort Sea. Polar Biol. doi:10.1007/s00300-014-1617-4
Bradstreet MSW, Cross WE (1982) Trophic relationships at high Arctic ice edges. Arctic 35:1–12
Brodeur RD, Peterson WT, Auth TD, Soulen HL, Parnel MM, Emerson AA (2008) Abundance and diversity of coastal fish larvae as indicators of recent changes in ocean and climate conditions in the Oregon upwelling zone. Mar Ecol Prog Ser 366:187–202
Brown J, Green JM (1976) Territoriality, habitat selection, and prior residency in underyearling Stichaeus punctatus (Pisces: Stichaeidae). Can J Zool 54:1904–1907
Byrkjedal I, Høines Å (2007) Distribution of demersal fish in the south-western Barents Sea. Polar Res 26:135–151
Carmack EC, Macdonald RW (2002) Oceanography of the Canadian Shelf of the Beaufort Sea: a setting for marine life. Arctic 55:29–45
Carmack E, McLaughlin F (2011) Towards recognition of physical and geochemical change in subarctic and Arctic Seas. Prog Oceanogr 90:90–104
Carmack E, Wassmann P (2006) Food webs and physical-biological coupling on pan-Arctic shelves: unifying concepts and comprehensive perspectives. Prog Oceanogr 71:446–477
Carmack EC, Macdonald RW, Papadakis JE (1989) Water mass structure and boundaries in the Mackenzie shelf estuary. J Geophys Res 94:18043–18055
Chiperzak DB, Hopky GE, Lawrence MJ, Lacho G (1990) Marine ichthyoplankton data from the Canadian Beaufort Sea Shelf, July and September, 1984. Can Data Rep Fish Aquat Sci 779:1–45
Chiperzak DB, Hopky GE, Lawrence MJ, Schmid DF, Reist JD (2003a) Larval and post-larval fish data from the Canadian Beaufort Sea Shelf, July to September 1985. Can Data Rep Fish Aquat Sci 1119:1–116
Chiperzak DB, Hopky GE, Lawrence MJ, Schmid DF, Reist JD (2003b) Larval and post-larval fish data from the Canadian Beaufort Sea Shelf, July to September 1986. Can Data Rep Fish Aquat Sci 1120:1–153
Chiperzak DB, Hopky GE, Lawrence MJ, Schmid DF, Reist JD (2003c) Larval and post-larval fish data from the Canadian Beaufort Sea Shelf, July to September 1987. Can Data Rep Fish Aquat Sci 1121:1–84
Christiansen JS, Mecklenburg CW, Karamushko OV (2014) Arctic marine fishes and their fisheries in light of global change. Glob Chang Biol 20:352–359
Eriksen E, Bogstad B, Nakken O (2011) Ecological significance of 0-group fish in the Barents Sea ecosystem. Polar Biol 34:647–657
Eriksen E, Prokhorova T, Johannesen E (2012) Long term changes in abundance and spatial distribution of pelagic Agonidae, Ammodytidae, Liparidae, Cottidae, Myctophidae and Stichaeidae in the Barents Sea. In: Ali M (ed) Diversity of ecosystems. In Tech, Rijeka, pp 109–126
Fahay MP (2007a) Early stages of fishes in the western North Atlantic Ocean (Davis Strait, Southern Greenland and Flemish Cap to Cape Hatteras): Acipenseriformes through Syngnathiformes, vol 1. The Northwest Atlantic Fisheries Organization, Dartmouth
Fahay MP (2007b) Early stages of fishes in the western North Atlantic Ocean (Davis Strait, Southern Greenland and Flemish Cap to Cape Hatteras): Scorpaeniformes through Tetraodontiformes, vol 2. The Northwest Atlantic Fisheries Organization, Dartmouth
Falardeau M, Robert D, Fortier L (2014) Could the planktonic stages of polar cod and Pacific sand lance compete for food in the warming Beaufort Sea? ICES J Mar Sci 71:1956–1965
Fleischer D, Schaber M, Piepenburg D (2007) Atlantic snake pipefish (Entelurus aequoreus) extends its northward distribution range to Svalbard (Arctic Ocean). Polar Biol 30:1359–1362
Fortier L, Barber D, Michaud J (2008) On thin ice: a synthesis of the Canadian Arctic Shelf Exchange Study (CASES). Aboriginal Issue Press, Winnipeg
Geoffroy M, Robert D, Darnis G, Fortier L (2011) The aggregation of polar cod (Boreogadus saida) in the deep Atlantic layer of ice-covered Amundsen Gulf (Beaufort Sea) in winter. Polar Biol 34:1959–1971
Jarvela LE, Thorsteinson LK (1999) The epipelagic fish community of Beaufort Sea coastal waters, Alaska. Arctic 52:80–94
Jørgensen OA, Hvingel C, Treble MA (2011) Identification and mapping of bottom fish assemblages in northern Baffin Bay. J Northwest Atl Fish Sci 43:65–79
Lin L, Liao Y, Zhang J, Zheng S, Xiang P, Yu X, Wu R, Shao K (2012) Composition and distribution of fish species collected during the fourth Chinese National Arctic Research Expedition in 2010. Adv Polar Sci 23:116–127
Macdonald RW, Yu Y (2006) The Mackenzie Estuary of the Arctic Ocean. Handb Environ Chem 5:91–120
Macdonald RW, Carmack EC, McLaughlin FA, Iseki K, Macdonald DM, O’Brien MC (1989) Composition and modification of water masses in the Mackenzie Shelf estuary. J Geophys Res 94:18057–18070
Majewski AR, Reist JD, Sareault JE (2006) Fish catch data from offshore sites in the Mackenzie River Estuary and Beaufort Sea during the open water season, August 2004 aboard the CCGS Nahidik. Can Manuscr Rep Fish Aquat Sci 2771:1–37
Majewski AR, Reist JD, Park BJ, Lowdon MK (2009) Fish catch data from offshore sites in the Mackenzie River Estuary and Beaufort Sea during the open water season, August 2006 aboard the CCGS Nahidik. Can Data Rep Fish Aquat Sci 1218:1–37
Majewski AR, Lowdon MK, Reist JD, Park BJ (2011) Fish catch data from Herschel Island, Yukon Territory, and other offshore sites in the Canadian Beaufort Sea, July and August 2007, aboard the CCGS Nahidik. Can Data Rep Fish Aquat Sci 1231:1–50
Majewski AR, Lynn BR, Lowdon MK, Williams WJ, Reist JD (2013) Community composition of demersal marine fishes on the Canadian Beaufort Shelf and at Herschel Island, Yukon Territory. J Mar Syst 127:55–64
Matarese AC, Kendall AW Jr, Blood DM, Vinter BM (1989) Laboratory guide to early life history stages of Northeast Pacific fishes. NOAA Technical Report NMFS 80, National Marine Fisheries Service, NOAA
Mecklenburg CW, Stein DL, Sheiko BA, Chernova NV, Mecklenburg TA, Holladay BA (2007) Russian–American long-term census of the Arctic: benthic fishes trawled in the Chukchi Sea and Bering Strait, August 2004. Northwest Nat 88:168–187
Mecklenburg CW, Møller PR, Steinke D (2011) Biodiversity of arctic marine fishes: taxonomy and zoogeography. Mar Biodiv 41:109–140
Meyer Ottesen CA, Hop H, Christiansen JS, Falk-Petersen S (2011) Early life history of the daubed shanny (Teleostei: Leptoclinus maculatus) in Svalbard waters. Mar Biodiv 41:383–394
Mueter FJ, Litzow MA (2008) Sea ice retreat alters the biogeography of the Bering Sea continental shelf. Ecol Appl 18:309–320
Munk P, Hansen BW, Nielsen TG, Thomsen HA (2003) Changes in plankton and fish larvae communities across hydrographic fronts off West Greenland. J Plankton Res 25:815–830
Nelson JS (2006) Fishes of the world, 4th edn. Wiley, Hoboken
Nelson RJ, Bouchard C, Madsen M, Praebel K, Rondeau E, Schalburg K, Leong JS, Jantzen S, Sandwith Z, Puckett S, Messmer A, Fevolden SE, Koop BF (2013) Microsatellite loci for genetic analysis of the arctic gadids Boreogadus saida and Arctogadus glacialis. Conserv Genet Resour 5:445–448
Norcross BL, Holladay BA, Busby MS, Mier KL (2010) Demersal and larval fish assemblages in the Chukchi Sea. Deep Sea Res II 57:57–70
Northern Environmental Protection Branch (1985) Beaufort environmental monitoring project 1983–1984 final report. Indian North Aff Dev Can Environ Stud no. 34, Northern Environmental Protection Branch, Indian and Northern Affairs Canada
Paulic JE, Papst MH (2012) Larval and early juvenile fish distribution and assemblage structure in the Canadian Beaufort Sea during July–August, 2005. J Mar Syst 127:46–54
Perry AL, Low PJ, Ellis JR, Reynolds JD (2005) Climate change and distribution shifts in marine fishes. Science 308:1912–1915
Pickart RS (2004) Shelfbreak circulation in the Alaskan Beaufort Sea: mean structure and variability. J Geophys Res 109:C04024
Steele M, Morison J, Ermold W, Rigor I, Ortmeyer M, Shimada K (2004) Circulation of summer Pacific halocline water in the Arctic Ocean. J Geophys Res 109:C02027
Tremblay JE, Robert D, Varela DE, Lovejoy C, Darnis G, Nelson RJ, Sastri AR (2012) Current state and trends in Canadian Arctic marine ecosystems: I. Primary production. Clim Chang 115:161–178
Walkusz W, Paulic JE, Williams WJ, Kwasniewski S, Papst MH (2011) Distribution and diet of larval and juvenile Arctic cod (Boreogadus saida) in the shallow Canadian Beaufort Sea. J Mar Syst 84:78–84
Walkusz W, Majewski A, Reist JD (2012) Distribution and diet of the bottom dwelling Arctic cod in the Canadian Beaufort Sea. J Mar Syst 127:65–75
Welch HE, Bergmann MA, Siferd TD, Martin KA, Curtis MF, Crawford RE, Conover RJ, Hop H (1992) Energy flow through the marine ecosystem of the Lancaster Sound region, Arctic Canada. Arctic 45:343–357
Williams WJ, Carmack EC (2008) Combined effect of wind-forcing and isobath divergence on upwelling at Cape Bathurst, Beaufort Sea. J Mar Res 66:645–663
Wong S, Hanson W, Hanson M, Papst MH (2013) The influence of the Mackenzie River plume on distribution and diversity of marine larval fish assemblages on the Canadian Beaufort Shelf. J Mar Syst 127:36–45
Wood KR, Overland JE, Salo SA, Bond NA, Williams WJ, Dong X (2013) Is there a “new normal” climate in the Beaufort Sea? Polar Res 32:19552
Acknowledgments
The authors are grateful to the officers and crew of the Canadian Coast Guard icebreakers Amundsen, Pierre Radisson, and Sir Wilfrid Laurier for their technical assistance under the extreme conditions of the Arctic Ocean. We also express gratitude to A. Forest for his valuable suggestions on CTD data processing. Laboratory technicians L. Létourneau, C. Aubry, and H. Cloutier analyzed ichthyoplankton samples attentively. The present study was partly supported by a grant to L. Fortier from the Natural Science and Engineering Research Council of Canada. K. Suzuki benefited from the scholarship program of le Fonds de recherche du Québec-Nature et technologies (FRQNT). This article is a contribution to the Canadian Arctic Shelf Exchange Study (CASES), ArcticNet, Québec-Océan, and the Canada Research Chair on the response of marine arctic ecosystems to climate warming.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Suzuki, K.W., Bouchard, C., Robert, D. et al. Spatiotemporal occurrence of summer ichthyoplankton in the southeast Beaufort Sea. Polar Biol 38, 1379–1389 (2015). https://doi.org/10.1007/s00300-015-1701-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1701-4