Abstract
Lichens as symbiotic associations consisting of a fungus (the mycobiont) and a photosynthetic partner (the photobiont) dominate the terrestrial vegetation of continental Antarctica. The photobiont provides carbon nutrition for the fungus. Therefore, performance and protection of photosystem II is a key factor of lichen survival. Potentials and limitations of photobiont physiology require intense investigation to extend the knowledge on adaptation mechanisms in the lichen symbiosis and to clarify to which extent photobionts benefit from symbiosis. Isolated photobionts and entire lichen thalli have been examined. The contribution of the photobiont concerning adaptation mechanisms to the light regime and temperature conditions was examined by chlorophyll a fluorescence and pigment analysis focusing on the foliose lichen Umbilicaria decussata from North Victoria Land, continental Antarctica. No photoinhibition has been observed in the entire lichen thallus. In the isolated photobionts, photoinhibition was clearly temperature dependent. For the first time, melanin in U. decussata thalli has been proved. Though the isolated photobiont is capable of excess light protection, the results clearly show that photoprotection is significantly increased in the symbiotic state. The closely related photobiont of Pleopsidium chlorophanum, a lichen lacking melanin, showed a higher potential of carotenoid-based excess light tolerance. This fact discriminates the two photobionts of the same Trebouxia clade. Based on the results, it can be concluded that the successful adaptation of lichens to continental Antarctic conditions is in part based on the physiological potential of the photobionts. The findings provide information on the success of symbiotic life in extreme environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The vegetation of continental Antarctica is sparse and consists of highly specialized organisms (Robinson et al. 2003; Hughes et al. 2006; Convey 2010). In this harsh environment, lichens dominate the terrestrial macrovegetation (Kappen 2000). These symbiotic consortia of a fungus (mycobiont) and a photosynthetic partner (photobiont) can, in general, be described as primary colonizers of extreme environments (Chen et al. 2000). The poikilohydric lifestyle of lichens is characterized by anabiosis, the physiologically inactivated state of dehydrated lichen thalli. In particular, in the state of anabiosis, many lichen species show considerable tolerance against abiotic stressors, enabling them to survive under environmental conditions which might be lethal to other organisms (Meeßen et al. 2013). The conditions consist of sustainable life in hot and cold deserts, in alpine regions, but also survival under most extreme parameters such as ultraviolet (UV) irradiation (Sánchez et al. 2014), freezing (Kappen and Lange 1972; Harańczyk et al. 2012), space exposure (Onofri et al. 2012) and even acetone rinsing (Gauslaa and Solhaug 2004).
Lichens are highly resistant to extreme temperatures, a feature that substantially supports the success of these organisms in cold environments such as the ice-free areas of the Antarctic continent and the adjacent islands (Kappen and Lange 1970, 1972; Kappen 1973; Schlensog et al. 2003). While lichens are often highly resistant to temperature extremes in the anabiotic state, photosynthetic activity of polar lichens has been detected at subzero temperatures (Kappen et al. 1996).
Lichen colonization occurs in microniches on the Antarctic continent. The microclimatical conditions of these sites are determined by abiotic factors such as rock topography (Jahns and Fritzler 1982). Such microniches for colonization by various lichen species can be minimum sized rock surface fissures. Microniches in rock fissures are characterized by meltwater supply and therefore, at least temporarily improve water availability. The occurrence of meltwater depends on topography of the respective site, substrate structure and the amount of snow and ice close by. Meltwater availability may be erratic compared to the diurnal change of air humidity (Schroeter et al. 2011). In general, the lichen Umbilicaria decussata colonizes rock sites of different size characterized by fissures and especially at continental sites supported by meltwater. Water availability strictly controls lichen activity due to the poikilohydric nature of these organisms (Green and Lange 1995). At continental Antarctic sites, meltwater supply is especially crucial for lichen activity (Kappen et al. 1998). Metabolic activity and especially photosynthetic primary production in continental Antarctica are primarily limited by water availability (Kappen 1985; Gjessing and Øvstedal 1989; Kappen and Breuer 1991; Schroeter et al. 1992).
At habitats of continental Antarctica, lichens live under the conditions of a frigid desert (Huiskes et al. 2006). The physiologically active period of lichens can be characterized by high light intensity (Kappen et al. 1998; Schroeter et al. 2010). Photoprotective mechanisms of the lichen symbiosis are crucial to avoid photodamage which would reduce photosynthetic productivity in the short vegetation period. Lichens of maritime Antarctica mainly depend on air humidity and dehydrate during times of excess insolation (Schlensog et al. 2003). Photoprotection in the hydrated state seems to be a prerequisite for the meltwater-activated lichens at continental sites.
For metabolically active, moist thalli of the continental Antarctic lichen species Umbilicaria aprina, considerable tolerance to high light intensities was detected by Kappen et al. (1998). Schlensog et al. (2003) proposed that the reflectance and absorbance of the U. aprina thallus cortex are the key factors of irradiation tolerance in this lichen. The shading capacity of lichen cortices reduces the light intensity experienced by the symbiotic photobionts substantially, compared to the ambient light (Ertl 1951; Büdel and Lange 1994).
Green algae of the genus Trebouxia, which are the most common among lichen photobionts, are specialized to a lichenized lifestyle. It has still not been clarified to which extent they can survive aposymbiotically under natural conditions (Wornik and Grube 2010). Meanwhile, there is a lot of experience in cultivation of lichen symbionts, especially Trebouxia photobionts (Schaper and Ott 2003; Sadowsky and Ott 2012). To compare the performance of photobionts in the lichen thallus to their isolated cultured state is a prerequisite for the knowledge on special adaptation mechanisms of the photobiont. Photobiont adaptations are especially interesting when low photobiont diversity is detected in a lichen community of a definite environment. Special characteristics of photobionts might be based on adaptation mechanisms to the respective environment (Sadowsky and Ott 2012; Domaschke et al. 2013). Clade S Trebouxia photobionts (Helms et al. 2001) dominate in macrolichens throughout Antarctica (Romeike et al. 2002).
The photosynthetic response of various isolated Antarctic photobionts to drought and cold showed different patterns. These differences between photobionts could be correlated with the environmental conditions of the corresponding lichen species rather than to their phylogenetic position in the genus Trebouxia (Sadowsky and Ott 2012). The environmental conditions inside the thallus normally differ considerably from those outside the thallus. In particular, hydration and light regime for the photobiont are determined by thallus structure and secondary lichen compounds (SLCs) inside the thallus (Sadowsky et al. 2012; Meeßen et al. 2013). Environmental adaptations of lichen symbionts can be regarded as adaptations toward the symbiotic lifestyle. Besides other factors, especially the physiological potential of the isolated symbionts determines the prerequisites for a successful symbiotic lifestyle in respective environments. Therefore, to clarify possible benefits of lichen symbiosis, the performance of symbiotic and aposymbiotic forms should be compared with experiments, as it has been done in the present study.
Materials and methods
Lichen material
Samples of Umbilicaria decussata (Vill.) Zahlenbr. (1942) and Pleopsidium chlorophanum (Wahlenb.) Zopf (1855) were collected during the 2009/2010 German Antarctic North Victoria Land Expedition (GANOVEX) X in Terra Nova Bay, North Victoria Land, continental Antarctica. Both lichens grew on a sun-exposed rock surface.
All lichen thalli were air-dried and deep-frozen (−25 °C) until photobiont isolation. Both photobionts belong to clade S Trebouxia (Brandt 2011; taxonomy after Helms et al. 2001).
Frozen lichen thalli were reactivated prior to the chlorophyll fluorescence analysis. The reactivation procedure was as follows: Entire lichen thalli were sprayed with water and stored at 12 °C in sealed vials (to prevent dehydration) in the dark for 24 h, followed by 14 h of light (20 µmol photons m2 s−1, fluorescent light source) at the same temperature.
Symbiont isolation and cultivation
Photobionts were isolated from lichen thalli according to Yoshimura et al. (2002) from thallus fragments. Axenic photobiont cultures on Trebouxia organic medium agar (TOM) with 1 % glucose (Ahmadjian 1967) were kept at low light intensity (20 µmol photons m2 s−1; diurnal cycle with 10 h of darkness) and 12 °C in a growth chamber (Rubarth Apparate GmbH, Germany). For pigment isolation and chlorophyll fluorescence experiments, the photobionts were transferred to 1 cm2 nitrocellulose filter disks on TOM-agar growing in the growth chamber for 4 weeks. Mycobionts were isolated from ascospores and cultivated on malt–yeast (MY) medium under the same conditions as the photobionts.
Chlorophyll fluorescence
Chlorophyll a fluorescence (chl f) was determined by using a Mini-PAM (pulse-amplitude modulated) fluorimeter (Walz Mess-und Regeltechnik, Germany) according to Maxwell and Johnson (2000). The parameter (F m − F 0)/F m = F v/F m (maximum quantum yield of photosystem II) was measured in the dark after 20 min of dark acclimation of the samples by applying a saturating light pulse. Quantum yield of photosystem II (\(\text{d}F/F_{\rm m}^\prime\)) in the light-acclimatized state was calculated as \((F_{\rm m}^{\prime}-F_t)/F_{\rm m}^{\prime}\), and non-photochemical quenching (NPQ) of maximum fluorescence was calculated as \((F_{\rm m}-F_{\rm m}^{\prime})/F_{\rm m}^{\prime}\). Slow chl f induction was performed by applying constant illumination on the dark-acclimatized samples for 12 min, followed by 20 min of dark relaxation. Eight samples of photobionts and, due to material limitations, four samples of lichen thalli were used per treatment. The effects of temperature and light intensity on quantum yield and NPQ were analyzed by two-way ANOVA (α = 0.05). A post hoc test (Tukey–Kramer multiple comparisons) was applied to detect significant reduction in photosystem II quantum yield after illumination and recovery.
Maximum quantum yield of freshly reactivated lichens was compared between the two species Umbilicaria decussata and Pleopsidium chlorophanum by an unpaired two-sided t test (α = 0.05). The checked null hypothesis H0 was that there were no differences between the lichen species.
Pigment analysis
Photobiont pigments were determined from acetone extracts by HPLC as described in Sadowsky and Ott (2012). The de-epoxidation status of the xanthophyll pool (DEPS) was calculated from the xanthophyll concentrations (zeaxanthin + antheraxanthin)/(violaxanthin + antheraxanthin + zeaxanthin) according to Vráblikóvá et al. (2004). The effects of temperature and photobiont origin on DEPS were analyzed by two-way ANOVA (α = 0.05; n = 4). A Tukey–Kramer multiple comparisons post hoc test was applied to detect pairs of significantly differing values.
Photospectrometric determination of Umbilicaria decussata thallus and mycobiont pigments from acetone, methanol and dimethyl sulfoxide (DMSO) extracts as well as testing on melanin was performed according to Meeßen et al. (2013). Synthetic melanin (>97 %, Sigma-Aldrich) was used as a reference. Despite the test on acetone solubility, the homogenized samples were pre-rinsed with acetone prior to the melanin testing. Mycobiont pigment extraction in DMSO was performed with native and acetone pre-rinsed mycobiont material.
Microclimate at lichen sites
Air, substrate and thallus temperature as well as relative air humidity and light intensity (photosynthetically active photon flux density, PPFD) were measured with sensors attached closely to lichen thalli near the Gondwana station at Terra Nova Bay, North Victoria Land. Air humidity and temperature were measured with combined temperature/humidity probes (HMP 35 A/TH, Vaisala, Finland). Substrate temperature was measured by thermistors (FF-U-V5-0 and FM-SU-VS5-0, Grant, UK). PPFD was measured using a quantum Sensor (Li-190SA, Licor, USA). Data were recorded on loggers (Grant and Eltek, 1000 series). The measurement was taken from January 4 to February 3 at a site of Umbilicaria decussata.
Results
Microclimate
Air, substrate and thallus temperature (Fig. 1) at the site of Umbilicaria decussata followed the same time course and showed similar values. At this site, the air temperature slightly exceeded the substrate and thallus temperature. Thallus temperature exceeded the substrate temperature during the daily temperature maximum. The diurnal rhythm was naturally correlated with the changing insolation (Fig. 2). Maximum photosynthetic photon flux density (PPFD) of c. 1600 µmol photons m−2 s−1 from sunlight was reached at c. 1 p.m. during the time when the measurement site was snow free. Maximum temperature >20 °C was reached about 2 h later. In the middle of the night, when PPFD was as low as c. 50 µmol photons m−2 s−1, temperatures of air, substrate and U. decussata thalli dropped to 0 °C. Air humidity was generally too low for lichen rehydration but reached c. 60 % relative humidity during the night. Air humidity during daytime was generally <20 % (Fig. 2). The measurement site was snow covered from January 22 until the end of the measurement. During snow cover, thallus and substrate temperatures were around or slightly below 0 °C (Fig. 1a). PPFD was reduced to a daily maximum of c. 200–400 µmol photons m−2 s−1. Relative air humidity (measured above the snow) rose up to c. 90 % (Fig. 2a).
Microclimate of an Umbilicaria decussata site: temperatures. X axis represents the time; a whole measurement period from January 4 to February 3, 2010; b detail (January 16/17). Snow coverage of the site occurred from January 22 to the end of the measurement. The sensor for air temperature remained snow free
Microclimate of an Umbilicaria decussata site: relative air humidity and photosynthetically active photon flux density (PPFD). X axis represents the time; a whole measurement period from January 4 to February 3, 2010; b detail (January 16/17). Snow coverage of the site occurred from January 22 to the end of the measurement
Chlorophyll fluorescence
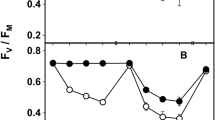
Entire lichen thalli (n = 4) were successfully reactivated and showed maximum quantum efficiencies (F v/F m) of 0.61 ± 0.04 (Umbilicaria decussata) and 0.66 ± 0.07 (Pleopsidium chlorophanum), respectively. No significant difference of the F v/F m values of these two lichens could be detected directly after the reactivation procedure (unpaired two-sided t test, p = 0.19, α = 0.05).
The reaction of reactivated U. decussata thalli to continuous illumination at 4, 12 and 20 °C is shown in Fig. 3, Tables 3 and 4. Actual quantum yield \(\text{d}F/F_{\rm m}^{\prime}\) reached a steady state after <5 min at any applied light intensity (20, 200 and 1000 µmol photons m−2 s−1) or temperature except the combination of low temperature and low light intensity (4 °C and 20 µmol photons m−2 s−1, Fig. 3a). Saturation of NPQ occurred more slowly. In particular, at 4 °C and 200 µmol photons m−2 s−1 (Fig. 3b), NPQ did not reach a steady state within the illumination time. By two-factor ANOVA, a significant effect of light intensity and temperature on the steady-state level of quantum yield was detected (Table 1). The effect of light was considerably greater than the effect of temperature.
Quantum yield of photosystem II and non-photochemical quenching (NPQ) derived from slow chlorophyll fluorescence induction in reactivated, dark-acclimatized entire Umbilicaria decussata thalli. Actinic light was switched on in minute 2 and switched off in minute 13. Applied temperatures: 4 °C (a, b); 12 °C (c–e); 20 °C (f–h). Applied light intensities (photosynthetically active photon flux density, PPFD): 20 µmol photons m−2 s−1 (a, c, f); 200 µmol photons m−2 s−1 (b, d, g); 1000 µmol photons m−2 s−1 (e, h). Values are presented as mean ± SD. Please note the higher NPQ scale in e and h
No significant effect of temperature and light intensity on the regeneration potential of NPQ and PS II quantum yield was detected by two-factor ANOVA (Table 2). The dark relaxation time seemed to be sufficient to regenerate F v/F m at any condition applied, which is indicated by the lack of statistically significant differences (Tukey–Kramer post hoc test) between the initial F v/F m and the quantum yield values after the light treatment followed by 20 min of dark relaxation (Fig. 3).
The photosynthetic characteristics of the isolated photobionts (Fig. 4; Tables 3, 4) were more effected by the conditions applied as described above, compared to the entire lichen thalli, indicated by lower steady-state levels of \(\text{d}F/F_{\rm m}^{\prime}\) and higher NPQ levels during illumination. In particular, at moderate PPFD combined with 4/12 °C (Fig. 4b, d), and at high PPFD (Fig. 4e, h), NPQ exceeded the values obtained from the lichen thalli and was not saturated after 12 min of illumination. NPQ was not fully regenerated within 20 min at any applied condition except the combination of 20 °C and low light intensity.
Quantum yield of photosystem II and non-photochemical quenching (NPQ) derived from slow chlorophyll fluorescence induction in dark-acclimatized isolated, cultured Umbilicaria decussata photobionts. Actinic light was switched on in minute 2 and switched off in minute 13. Applied temperatures: 4 °C (a, b); 12 °C (c–e); 20 °C (f–h). Applied light intensities (photosynthetically active photon flux density, PPFD): 20 µmol photons m−2 s−1 (a, c, f); 200 µmol photons m−2 s−1 (b, d, g); 1000 µmol photons m−2 s−1 (e, h). Values are presented as mean ± SD. Please note the higher NPQ scale in b, d, e and h. Asterisks indicate significant differences (Tukey–Kramer multiple comparisons test) between recovered and unstressed quantum yield values: **p < 0.01; ***p < 0.001
By two-factor ANOVA, significant effects of temperature and light intensity variation could be detected, including a pronounced stronger effect of light intensity (Table 1). Additionally, the interactive effect of the two factors was significant.
The quantum yield of PS II reached the initial unstressed value after illumination at up to 200 µmol photons m−2 s−1 at 12 °C, while at lower temperatures, quantum yield of PS II was significantly reduced after illumination at 200 and 1000 µmol photons m−2 s−1 (Table 1; Fig. 4). After treatment at 20 °C and 200 µmol photons m−2 s−1 and a subsequent recovery, quantum yield was only slightly but significantly reduced from 0.57 ± 0.05 to 0.47 ± 0.19. Two-factor ANOVA (Table 2) indicated that only light intensity, not the temperature, had a significant effect on dark recovered quantum yield values. By contrast, both factors as well as their interaction significantly affected the variation of NPQ recovery. Again, the light intensity contributed more to the parameter variation than temperature did.
Pigment analysis
NPQ of the photobionts was correlated with activation of the violaxanthin cycle as indicated by increased de-epoxidation state of the xanthophyll pool (DEPS) under moderate illumination (Fig. 5; Table 6). DEPS did not reach the relaxed initial state after 20 min of dark relaxation. By two-factor ANOVA, it could be shown that the illumination treatment and the photobiont origin (species level) both had a significant effect on the DEPS values. However, the contribution of light intensity was greater than that of photobiont species (Table 5). Furthermore, Tukey–Kramers post hoc test revealed that the photobiont of Pleopsidium chlorophanum showed significantly higher DEPS than the unstressed control after 5 min of irradiation. DEPS of the Ubmilicaria decussata photobiont was significantly elevated after 10 min (Fig. 5; Table 6).
De-epoxidation state of the xanthophyll pool (DEPS) of isolated photobionts of Pleopsidium chlorophanum and Umbilicaria decussata at 12 °C. The first pair of bars represents the unstressed DEPS of dark-acclimatized photobionts. The second and third pair of bars represent the DEPS values of photobionts illuminated at 200 µmol photons m−2 s−1 for 5 and 10 min, respectively. The last pair of values shows the DEPS after 10 min of illumination, followed by 20 min of dark acclimation. Values are presented as mean ± standard deviation. Asterisks indicate significant differences (Tukey–Kramer multiple comparisons test) from values obtained from the respective unstressed photobionts: *p < 0.05; **p < 0.01; ***p < 0.001
Brownish thallus extracts of Umbilicaria decussata revealed absorption maxima of chlorophylls in the blue and red light, but also absorption due to other substances in the blue light and UV spectrum (Fig. 6). In the DMSO extract, the spectrum is characterized by increasing absorption in the blue and UV region toward a maximum at c. 270 nm (Fig. 6a). Extracted compounds of the isolated mycobiont showed a similar spectrum in DMSO and a brown coloration. It could be shown by chemical testing (Table 7) that the absorbance increasing with lower wavelengths is due to melanin present in both the thallus and the cultivated mycobiont. A blue light-absorbing compound of the thallus and the isolated mycobiont could be detected in the acetone extract. Acetone pre-rinsed mycobiont material extracted in DMSO showed reduced absorbance in the blue and near UV spectrum. Acetone and methanol extracts (Fig. 6b, c) of the lichen and mycobiont showed absorption peaks at c. 320 nm, but no brown coloration.
Absorption spectra of Umbilicaria decussata lichen thallus and mycobiont extracts in different solvents. Pre-rinsed mycobiont material was rinsed with acetone prior to the DMSO extraction. Solvents are: a DMSO; b methanol; c acetone. Values are presented as arbitrary units, giving the highest absorption the value 1
Discussion
Pigmentation
Light-screening pigments are a diverse class of secondary lichen substances which are often located in the cortex (Dietz et al. 2000). Some are able to protect against excess visible (photosynthetically active radiation, PAR) light, but most are UVR screening substances (Solhaug et al. 2003). A known UVR screening compound in Umbilicaria decussata thalli is gyrophoric acid (Øvstedal and Lewis Smith 2001). Fungal melanins absorb PAR and especially UVR. Melanic compounds are also widespread among lichens (Beckett et al. 2012). Melanin-pigmented lichen cortices can serve as effective barriers against different harmful effects of excess insolation (Nybakken et al. 2004; Meeßen et al. 2013). This study presents the first proof of melanin in Umbilicaria decussata. The pigment may contribute substantially to the protection of the photobiont when the metabolically active U. decussata thallus is exposed to sunlight. The Antarctic endemic lichen Buellia frigida may also profit from its protective melanin-pigmented cortex. The B. frigida mycobiont, as that of U. decussata, also produces melanin in the isolated and cultured state, and it has been hypothesized that this pigmentation may protect the development of new-formed fungal tissue in B. frigida from UV radiation (Meeßen et al. 2013).
Microclimate
Continuous variations in air, substrate as well as thallus temperature measured at microsites of U. decussata for 24 h for 4 weeks from below 0 °C until about 20 °C occurred (Fig. 1). The relatively high temperatures are accompanied by increasing light intensities at noon (Fig. 2) when the thallus in general is dry (Ott in prep.). Because of low air humidity (Fig. 2), no dew fall occurred. Schlensog et al. (2013) analyzed a variety of plants and cryptogams of Léonie Island, Antarctic Peninsula and southern maritime Antarctic. In its more xeric microhabitat on Léonie Island, Marguerite Bay, U. decussata was moistened by precipitation of rain and snow during the measuring period exclusively, resulting in an erratic activity pattern and the shortest total activity time of that study.
As a cosmopolitan lichen (Øvstedal and Lewis Smith 2001), a broad ecological amplitude may be beneficial for U. decussata at habitats in North Victoria Land. Romeike et al. (2002) compared the hydration-dependent physiological activities of endemic and cosmopolitan lichen species across the maritime Antarctic until Alexander Island south of the maritime Antarctic, Antarctic Peninsula. In general, cosmopolitan species displayed broader ecological amplitudes. In particular, the bipolar lichen Stereocaulon alpinum showed adaptation toward high light intensity combined with high thallus humidity. As U. decussata, S. alpinum grows at cold, alpine sites in the northern hemisphere (Øvstedal and Lewis Smith 2001) and was found in meltwater-fed Antarctic sites. The similar distribution patterns of these two lichen species may cause similar adaption mechanisms on physiology.
Light energy conversion
Excess light energy can lead to the production of cytotoxic reactive oxygen species (ROS) in photosynthetic cells. Lichens can avoid excess light by anabiosis or by shading of the photobionts by a well-developed optically dense cortex. On the other hand, many lichens are highly susceptible to photoinhibition in the dry state. Susceptibility to photoinhibition is especially relevant during the drying process (Gauslaa et al. 2012). In the dry state, dissipation of excess energy can be induced by conformational changes of the photosynthetic apparatus (Heber 2008). As long dry periods with strong insolation may occur at Antarctic habitats, light energy quenching in the dry state may be crucial for lichen survival. While this mechanism can be very effective and is independent of pigment conversions, active photobiont cells can also tolerate excess light by xanthophyll-based NPQ reactions. Additionally, evidence for pigment-independent chlorophyll fluorescence quenching has been found in desiccated Antarctic lichen photobionts (Sadowsky and Ott 2012). In the illumination experiments concerning isolated and symbiotic photobionts (Figs. 3, 4), NPQ was more rapidly induced at higher temperatures (12 and 20 °C). In particular, in the isolated photobionts, NPQ reached significantly higher values when the temperature was higher at any given light intensity.
Reduction in photosynthetic capacity (photoinhibition) by light stress occurs especially at low temperatures. Active NPQ prevents the photosystem from oxidative damage and avoids long-term (chronic) photoinhibition (Schlensog et al. 2003). The isolated photobionts of Umbilicaria decussata (Fig. 4) displayed chronic photoinhibition (detected as incomplete recovery of F v/F m) at low temperature (4 °C), even if combined with low or moderate light intensity (20 and 200 µmol photons m−2 s−1, respectively). At 12 and 20 °C, chronic photoinhibition could only be observed under high light conditions (1000 µmol photons m−2 s−1), despite a minor reduction in quantum yield at 20 °C and 200 µmol photons m−2 s−1 (Fig. 4). Consequently, it can be hypothesized that the NPQ-based excess light protection of the isolated U. decussata photobiont can effectively prevent photodamage at temperatures of 12 or 20 °C combined with a light intensity which exceeds the cultivation PPFD (at 20 µmol photons m−2 s−1) tenfold. A PPFD of 200 µmol photons m−2 s−1 is in the range of maximum PPFD detected under snow cover in the measuring period. NPQ can be correlated with the de-epoxidation of the xanthophyll violaxanthin to zeaxanthin which quenches excess excitation energy from photosystem II (Havaux and Niyogi 1999).
The P. chlorophanum entire lichen thallus, unlike the U. decussata thallus (Meeßen et al. 2013, Fig. 6; Table 6), lacks melanin as a photoprotective metabolite. P. chlorophanum contains the UVR protective SLC rhizocarpic acid. Rhizocarpic acid shows little absorption of photosynthetically relevant wavelengths and can thus not be regarded as a protectant against excess PPFD (Hidalgo et al. 2002; Meeßen et al. 2013). Based on its pigmentation, the light-absorbing capacity of the P. chlorophanum thallus can be regarded as minor compared to U. decussata. The photobionts of U. decussata and of Pleopsidium chlorophanum belong to clade S Trebouxia. Despite the close phylogenetic position and despite the identical culture and experimental conditions of both isolated photobionts, the P. chlorophanum photobiont displayed faster activation of xanthophyll de-epoxidation. The photobiont originating from a less shady intrathalline environment may rely on more effective own photoprotection. The xanthophyll-based NPQ can be such a mechanism. Based on the NPQ-relevant xanthophyll composition of the isolated photobionts after illumination (Fig. 5), it can be concluded that the strategy of photoprotection was retained in the isolated, cultured state of the photobionts.
The entire lichen thalli of the study displayed no chronic photoinhibition at any light/temperature condition applied (Fig. 3). Barták et al. (2008) have demonstrated that in the umbilicate lichen Lasallia pustulata from a more temperate European site, photosynthetic processes were less influenced by light intensity than by the duration of irradiation. Laboratory experiments of this study indicated efficient use of light energy by moist, active Umbilicaria decussata thalli even at high PPFD and low temperatures, compared to the isolated photobiont. This is consistent with findings concerning lichens from across polar regions which show considerable adaptation to low temperatures (Kappen and Lange 1970, 1972). In the isolated state, the U. decussata photobiont, among other analyzed Trebouxia photobionts from various lichen species, even tolerates long-term freezing without taking serious damage to its photosynthetic capacity (Sadowsky and Ott 2012). NPQ was induced to a lesser extent in the entire U. decussata thalli, compared to the isolated photobionts. This may be due to reduced light intensity in the intrathalline algal layer of the lichen, especially caused by melanin pigmentation of the cortex. As the presented experiments show, the examined isolated photobionts are more likely stressed by excess light than by variations in temperature. Therefore, effective sun screening by the mycobiont can be a key feature of successful lichen colonization at continental Antarctic sites.
Abbreviations
- ANOVA:
-
Analysis of variance
- chl f :
-
Chlorophyll fluorescence
- DEPS:
-
De-epoxidation state of the xanthophyll pool
- DMSO:
-
Dimethyl sulfoxide
- GANOVEX:
-
German Antarctic North Victoria Land Expedition
- HPLC:
-
High-performance liquid chromatography
- MY:
-
Malt–yeast
- NPQ:
-
Non-photochemical quenching
- PAM:
-
Pulse-amplitude modulation
- PAR:
-
Photosynthetically active radiation
- PPFD:
-
Photosynthetically active photon flux density
- PS:
-
Photosystem
- ROS:
-
Reactive oxygen species
- SLC:
-
Secondary lichen compound
- TOM:
-
Trebouxia organic medium
- UVR:
-
Ultraviolet radiation
References
Ahmadjian V (1967) A guide to the algae occurring as lichen symbionts: isolation, culture, cultural physiology and identification. Phycologia 6:127–160
Barták M, Vráblíková-Cempírková H, Štepigová J, Hájek J, Váczi P, Večeřová K (2008) Duration of irradiation rather than quantity and frequency of high irradiance inhibits photosynthetic processes in the lichen Lasallia pustulata. Photosynthetica 46:161–169
Beckett RP, Minibayeva FV, Liers C (2012) Occurrence of high tyrosinase activity in the non-Peltigeralean lichen Dermatocarpon miniatum (L.) W. Mann. Lichenologist 44:827–832
Brandt A (2011) Genetische Diversität der Flechtenalgen von North Victoria Land, Antarktis. Diploma thesis, HHU Düsseldorf
Büdel B, Lange OL (1994) The role of cortical and epinecral layers in the lichen genus Peltula. Cryptogam Bot 4:262–269
Chen J, Blume HP, Beyer L (2000) Weathering of rocks induced by lichen colonization: a review. Catena 39:121–146
Convey P (2010) Terrestrial biodiversity in Antarctica: recent advances and future challenges. Polar Sci 4:135–147
Dietz S, Büdel B, Lange OL, Bilger W (2000) Transmittance of light through the cortex of lichens from contrasting habitats. Bibl Lichenol 75:171–182
Domaschke S, Vivas M, Sancho LG, Printzen C (2013) Ecophysiology and genetic structure of polar versus temperate populations of the lichen Cetraria aculeata. Oecologia 173:699–709
Ertl L (1951) Über die Lichtverhältnisse in Laubflechten. Planta 39:245–270
Gauslaa Y, Solhaug KA (2004) Photoinhibition in lichens depends on cortical characteristics and hydration. Lichenologist 36:133–144
Gauslaa Y, Coxson DS, Solhaug KA (2012) The paradox of higher light tolerance during desiccation in rare old forest cyanolichens than in more widespread co-occurring chloro- and cephalolichens. N Phytol 195:812–822
Gjessing Y, Øvstedal O (1989) Microclimate and water budget of alpine algae, lichens and a moss on some Nunataks in Queen Maud Land. Int J Biometeorol 33:272–281
Green TGA, Lange OL (1995) Photosynthesis in poikilohydric plants: a comparison of lichens and bryophytes. In: Schulze ED, Caldwell MM (eds) Ecophysiology of photosynthesis. Springer, Berlin, pp 319–341
Harańczyk H, Nowak P, Bacior M, Lisowska M, Marzec M, Florek M, Olech MA (2012) Bound water freezing in Antarctic Umbilicaria aprina from Schirmacher Oasis. Antarct Sci 24:342–352
Havaux M, Niyogi KK (1999) The violoxanthin cycle protects from photoxidative damage by more than one mechanism. Proc Natl Acad Sci 96:8762–8767
Heber U (2008) Photoprotection of green plants: a mechanism of ultra-fast thermal energy dissipation in desiccated lichens. Planta 228:641–650
Helms G, Friedl T, Rambold G, Mayrhofer H (2001) Identification of photobionts from the lichen family Physciaceae using algal specific ITS rDNA sequencing. Lichenologist 33:73–86
Hidalgo ME, Fernández E, Ponce M, Rubio C, Quilhot W (2002) Photophysical, photochemical, and thermodynamic properties of shikimic acid derivatives: calycin and rhizocarpic acid (lichens). J Photochem Photobiol B Biol 66:213–217
Hughes KA, Ott S, Bölter M, Convey P (2006) Colonisation processes. In: Bergstrom DM, Convey P, Huiskes AHL (eds) Trends in Antarctic terrestrial and limnetic ecosystems. Springer, Dordrecht, pp 35–54
Huiskes AHL, Convey P, Bergstrom DM (2006) Trends in Antarctic and terrestrial and limnetic ecosystems: Antarctica as a global indicator. In: Bergstrom DM, Convey P, Huiskes AHL (eds) Trends in Antarctic terrestrial and limnetic ecosystems. Springer, Dordrecht, pp 1–13
Jahns HM, Fritzler E (1982) Flechtenstandorte auf einer Blockhalde. Herzogia 6:243–270
Kappen L (1973) Response to extreme environments. In: Ahmadjian V (ed) The lichens. Academic Press, New York and London, pp 311–380
Kappen L (1985) Water relations and net photosynthesis of Usnea: a comparison between Usnea fasciata (maritime Antarctic) and Usnea sulphurea (continental Antarctic). In: Brown DH (ed) Lichen physiology and cell biology. Plenum Press, New York and London, pp 41–56
Kappen L (2000) Some aspects of the great success of lichens in Antarctica. Antarct Sci 12:314–324
Kappen L, Breuer M (1991) Ecological and physiological investigations in continental Antarctic cryptogams II. Moisture relations and photosynthesis of lichens near Casey Station, Wilkes Land. Antarct Sci 3:273–278
Kappen L, Lange OL (1970) The cold resistance of phycobionts from macrolichens of various habitats. Lichenologist 4:289–293
Kappen L, Lange OL (1972) Die Kälteresistenz einiger Makrolichenen. Flora 161:1–29
Kappen L, Schroeter B, Scheidegger C, Sommerkorn M, Hestmark G (1996) Cold resistance and metabolic activity of lichens below 0°C. Adv Space Res 18:119–128
Kappen L, Schroeter B, Green TGA, Seppelt RD (1998) Microclimatic conditions, meltwater moistening, and the distributional pattern of Buellia frigida on rock in a southern continental Antarctic habitat. Polar Biol 19:101–106
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence: a practical guide. J Exp Bot 51:659–668
Meeßen J, Sánchez FJ, Sadowsky A, de la Torre R, Ott S, de Vera JP (2013) Extremotolerance and resistance of lichens: comparative studies on five species used in astrobiological research II. Secondary lichen compounds. Orig Life Evol Biosph 43:501–526
Nybakken L, Solhaug KA, Bilger W, Gauslaa Y (2004) The lichens Xanthoria elegans and Cetraria islandica maintain a high protection against UV-B radiation in Arctic habitats. Oecologia 140:211–216
Onofri S, de la Torre R, de Vera JP, Ott S, Zucconi L, Selbmann L, Scalzi G, Venkateswaran KJ, Rabbow E, Sánchez Iñigo FJ, Horneck G (2012) Survival of rock-colonizing organisms after 1.5 years in outer space. Astrobiology 12:508–516
Øvstedal DO, Lewis Smith RI (2001) Lichens of Antarctica and South Georgia: a guide to their identification and ecology. Cambridge University Press, Cambridge
Robinson SA, Wasley J, Tobin AK (2003) Living on the edge–plants and global change in continental and maritime Antarctica. Glob Change Biol 9:1681–1717
Romeike J, Friedl T, Helms G, Ott S (2002) Genetic diversity of algal and fungal partners in four species of Umbilicaria (lichenized ascomycetes) along a transect of the Antarctic Peninsula. Mol Biol Evol 19:1209–1217
Sadowsky A, Ott S (2012) Photosynthetic symbionts in Antarctic terrestrial ecosystems: the physiological response of lichen photobionts to drought and cold. Symbiosis 58:81–90
Sadowsky A, Hussner A, Ott S (2012) Submersion tolerance in a habitat of Stereocaulon paschale (Stereocaulaceae) and Cladonia stellaris (Cladoniaceae) from the high mountain region Rondane, Norway. Nova Hedwig 94:323–334
Sánchez FJ, Meeßen J, Ruiz MG, Sancho LG, Ott S, Vílchez C, Horneck G, Sadowsky A, de la Torre R (2014) UV-C tolerance of symbiotic Trebouxia sp. in the space-tested lichen species Rhizocarpon geographicum and Circinaria gyrosa: role of the hydration state and cortex/screening substances. Int J Astrobiol 13:1–18
Schaper T, Ott S (2003) Photobiont selectivity and interspecific interactions in lichen communities. I. Culture experiments with the mycobiont Fulgensia bracteata. Plant Biol 5:441–450
Schlensog M, Schroeter B, Pannewitz S, Green TGA (2003) Adaptations of mosses and lichens to irradiance stress in maritime and continental habitats. In: Huiskes AHL, Gieskes WWC, Rozema J, Schorno RML, van der Vies SM, Wolff WJ (eds) Antarctic biology in a global context. Backhuys Publishers, Leiden, pp 161–166
Schlensog M, Green TGA, Schroeter B (2013) Life form and water source interact to determine active time and environment in cryptogams: an example from the maritime Antarctic. Oecologia 173:59–72
Schroeter B, Green TGA, Seppelt RD, Kappen L (1992) Monitoring photosynthetic activity of crustose lichens using a PAM-2000 fluorescence system. Oecologia 92:457–462
Schroeter B, Green TGA, Pannewitz S, Schlensog M, Sancho LG (2010) Fourteen degrees of latitude and a continent apart: comparison of lichen activity over two years at continental and maritime Antarctic sites. Antarct Sci 22:681–690
Schroeter B, Green TGA, Pannewitz S, Schlensog M, Sancho LG (2011) Summer variability, winter dormancy: lichen activity over 3 years at Botany Bay, 77 S latitude, continental Antarctica. Polar Biol 34:13–22
Solhaug KA, Gauslaa Y, Nybakken L, Bilger W (2003) UV induction of sun-screening pigments in lichens. New Phytol 158:91–100
Vráblikóvá H, Barták M, Wonisch A (2004) Changes in glutathione and xanthophyll cycle pigments in the high light-stressed lichens Umbilicaria antarctica and Lasallia pustulata. J Photo Biol 79:35–41
Wornik S, Grube M (2010) Joint dispersal does not imply maintenance of partnerships in lichen symbioses. Microb Ecol 59:150–157
Yoshimura I, Yamamoto Y, Nakano T, Finnie J (2002) Isolation and culture of lichen photobionts and mycobionts. In: Kranner I, Beckett R, Varma A (eds) Protocols in lichenology: culturing, biochemistry, ecophysiology and use in biomonitoring. Springer, Berlin, pp 3–33
Acknowledgments
Special thanks to Eva Posthoff for her substantial help with the photobiont cultures. Thanks are also due to the organization committee of the XIth SCAR Biology Symposium 2013, Barcelona. The first author thanks the Studienstiftung des Deutschen Volkes for financial support. The second author is grateful to the German Research Foundation (DFG) for financing the research project Ot 96/15–1 as part of the Antarctic Priority Program (SPP 1158). Special thanks are due to the BGR (Bundesanstalt für Geologie und Rohstoffe), Andreas Läufer and Detlef Damaske for inviting the second author to the expedition GANOVEX X and logistic support. The staff of the Gondwana Station is thanked for their invaluable help. The results are included in the doctoral thesis of Andres Sadowsky. Thanks are also due to the anonymous reviewers for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is an invited contribution on Life in Antarctica: Boundaries and Gradients in a Changing Environment as the main theme of the XIth SCAR Biology Symposium.
Rights and permissions
About this article
Cite this article
Sadowsky, A., Ott, S. Symbiosis as a successful strategy in continental Antarctica: performance and protection of Trebouxia photosystem II in relation to lichen pigmentation. Polar Biol 39, 139–151 (2016). https://doi.org/10.1007/s00300-015-1677-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1677-0