Abstract
The Antarctic silverfish, Pleuragramma antarctica, is ecologically important for connecting lower and upper trophic levels within the coastal marine Antarctic food web. In recent decades, populations of silverfish have exhibited a declining trend in some regions of the Antarctic, in particular the western Antarctic Peninsula. It is of paramount importance to elucidate its life history and to characterize the areas that are crucial for the reproduction of the species: spawning, hatching and nursery areas. Presently, the overall available knowledge is scant and spatially restricted. In this study, we assessed the spatial scales of variation in the distribution patterns of eggs and newly hatched larvae, and their vertical distribution within the platelet ice layer underlying fast ice at Terra Nova Bay. We found that (1) distribution patterns of eggs and larvae abundance significantly changed at a spatial scale of kilometers, while they did not at scale of tens of kilometers and hundreds/tens meters, and (2) eggs were not homogeneously distributed under the solid ice; in particular, the egg abundance was highest at −2.5 m within the platelet ice and dramatically declined more in depth. This study thus allowed shed light on distribution patterns of eggs and early-hatched larvae of the Antarctic silverfish. Such information will be useful to better understand the ecological processes possibly producing the patterns we have observed and then identify further reproduction and nursery areas around the Antarctic continent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to their usually large biomass, small pelagic fish are worldwide considered as crucial in marine ecosystems for connecting lower and upper trophic levels within food webs (Palomera et al. 2007). Small pelagic fish are also well known to display significant fluctuations of their populations, which may have relevant ecological (and sometimes socio-economical) implications (Chavez et al. 2003; Barange et al. 2009). Fish population dynamics, in fact, depend on a number of processes, like production and supply of eggs and larvae, recruitment intensity and mortality rates (Guidetti et al. 2013) as a consequence of natural changes, direct human impacts and climatic changes (Moline et al. 2008).
In the Antarctic coastal marine ecosystem, the abovementioned pivotal ecological role within the food web is fulfilled by the Antarctic silverfish Pleuragramma antarctica, according the taxonomic statement by Eschmeyer (2014), consistent also with the recently published Biogeographic Atlas of the Southern Ocean (Duhamel et al. 2014); in the present work, the specific name ‘antarctica’ is used instead of the former ‘antarcticum.’ This fish feeds upon planktonic organisms, and on the other hand, it is the primary food item for most Antarctic marine vertebrates including mammals, birds and other fishes (La Mesa et al. 2004; Eastman 2005; O’Driscoll et al. 2011). In the Ross and Weddell Seas, this fish may account for more than 90 % of the overall fish biomass (DeWitt 1970; Hubold and Ekau 1987).
Typically, the Antarctic silverfish is distributed in the coastal areas around the Antarctic continent, including the Scotia Arc and adjacent islands (Vacchi et al. 2012a). It spends most of the life (from juvenile to adult stages) in the water column (in open waters and areas covered by sea ice between 0 and 900 m depth, mostly thriving at mid-water levels; Gerasimchuk 1986; DeWitt et al. 1990; Fuiman et al. 2002). Consequently, it has developed specific adaptations to a holopelagic life cycle (e.g., streamlined body and forked tail, emphasized lipid storage and reduction in skeletal ossification; Eastman 1993, 1997; Albertson et al. 2010; La Mesa and Eastman 2012).
Due to the strong limitations to field work in Antarctica during the winter season (extremely low temperatures and strong katabatic winds), quite few data on sexual maturity, fecundity, spawning periods and hatching have been reported in the available literature (Faleyeva and Gerasimchuk 1990; Kellermann 1991; Kock and Kellermann 1991; Ferrando et al. 2010). Spawning events are thought to mostly occur at the end of the austral winter or the beginning of spring (Kellermann 1987; Hubold 1990; Eastman 1993; Ferrando et al. 2010; Vacchi et al. 2012a), while hatching should occur around November and December (Regan 1916; Kellermann 1989; Hubold 1990; Vacchi et al. 2004). Some authors suggest, also, the occurrence of temporal shifts for these events (DeWitt and Tyler 1960; Eastman 1993; Liu and Chen 1995).
Silverfish larvae and juveniles constitute the majority of ichthyoplankton in many locations around Antarctica (Hubold and Ekau 1987; Koubbi et al. 1997; Morales-Nin et al. 1998; Koubbi et al. 2011). In the western Ross Sea, they can account for more than 98 % of the ichthyoplankton biomass (Guglielmo et al. 1998; Vacchi et al. 1999; Granata et al. 2002).
Huge amounts of embryonated eggs of P. antarctica were detected for the first time in November 2002, trapped within the ice platelets under the consolidated sea ice at Terra Nova Bay (TNB), Ross Sea. This area has been thus identified as the first (and still remains the only one) hatching/nursery area of the Antarctic silverfish (Vacchi et al. 2004, 2012a). Since then, a regular monitoring has been carried out within the Italian National Program for Research in Antarctica (PNRA).
Due to the prominent ecosystem-wide role of P. antarctica within the coastal marine food web in Antarctica (La Mesa et al. 2004), it is crucial to improve our knowledge about the distribution patterns of eggs and larvae and, ultimately, about the areas that guarantee the population renewal of this fish.
Taking into account the wide distribution of this species, it is reasonable to hypothesize the presence of a number of spawning/hatching/nursery areas around the Antarctic continent. Distribution of eggs, until now, was found to be fairly heterogeneous in space (Vacchi et al. 2012a). This may imply that sites showing huge amounts of eggs may have specific ecological/hydrological characteristics making them particularly apt to host the eggs and, probably, reproductive aggregations of P. antarctica. From this perspective, the patterns of spatial variability across multiple scales in the distribution of early stages of P. antarctica (chiefly eggs and newly hatched larvae) have never been investigated before. This step, as for any other ecological study, is crucial to then infer about the underlying causal processes driving the observed patterns (Underwood et al. 2000). As different processes are likely to operate differently in space (and time), the identification of relevant scales of variation is a prerequisite before explanatory models can be proposed and tested (Andrew and Mapstone 1987). Assessing distribution patterns of eggs and early-hatched larvae in TNB can thus help predict how early stages of P. antarctica are distributed also in other reproductive areas possibly located along the Antarctic coast.
The present study, therefore, is aimed at assessing the distribution patterns of eggs and newly hatched larvae of P. antarctica (1) at different spatial scales (from tens kilometers to tens meters in TNB) and (2) within the platelet ice layer underlying the fast ice.
Materials and methods
General description of the study area
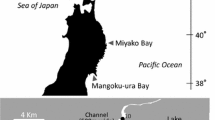
Terra Nova Bay (hereinafter TNB) is a coastal area making part of the Victoria Land (western Ross Sea; near 75°S, 164°E) approximately extending over an area of 6,000 km2 (Fig. 1). Its limits are represented by Cape Washington to the north and the floating Drygalski Ice Tongue to the south. The coastline is characterized by many bays, such as the Gerlache Inlet (where the Italian Mario Zucchelli Station (MZS) is located) and Silverfish Bay in its northern sector, and by the presence of a number of glaciers (e.g., Drygalski Ice Tongue, the Nansen Ice Sheet and the Campbell Glacier Tongue) flowing down from the continent into the TNB. The sea bottom is chiefly characterized by steep seabeds and by the Drygalski depression falling down to 1,100 m. Marine waters in the TNB are among the coldest at global scale, due to the latitude together with the effects of large floating masses of continental ice. The seawater temperature is commonly around its freezing point (FP, −1.91 °C) and seldom above −1.0 °C (Buffoni et al. 2002).
Water circulation in TNB shows a prevailing northward direction during summer in the upper layer close to the coast, with a clockwise rotation at depth. Coastal waters are generally characterized by higher temperature and salinity, while the lowest temperatures can be usually found in the central area of TNB, as a consequence of local eddies and upwelling processes caused by katabatic winds (Budillon and Spezie 2000; Buffoni et al. 2002). An extended 1,300 km2-wide polynya (sometimes achieving 5,000 km2) persists in winter due to a combination of frequent katabatic winds and a barrier effect of the Drygalski Ice Tongue on pack ice advection coming from the south/southwest (Kurtz and Bromwich 1983, 1985). The TNB polynya acts as a ‘ice factory,’ especially during winter (Van Woert 1999). These complex climatic features at TNB reflect into a seasonal sea-ice cover, bordering coastal areas for almost 9 months throughout the year.
The presence of platelet ice characterizes many portions of the study area. Platelet ice consists of various-sized flat plate-like crystals up to above 10 cm in diameter, randomly oriented. Even though the platelet ice can be incorporated into fast ice, more often it loosely aggregates under the solid ice, making up a semi-consolidated layer from a few centimeters to some meters in thickness (Vacchi et al. 2012b).
TNB is one of the most important biodiversity hotspots in the Ross Sea region, as acknowledged by the establishment of two ASPAs (Antarctic Specially Protected Areas) under the Antarctic Treaty System. The most recent one is the 73rd ASPA ‘Cape Washington & Silverfish Bay,’ established in 2013, that includes the Cape Washington Emperor penguins rookery area and a part of the reproductive area of the silverfish at Terra Nova Bay.
Samples collection and treatment
A first step of the study was represented by the assessment of spatial distribution patterns of eggs and newly hatched larvae of P. antarctica at multiple spatial scales in TNB. Silverfish early life stages were collected between early November and early December 2011 in the framework of the XXVII Expedition of the Italian National Antarctic Program (PNRA) at TNB, Ross Sea.
Eggs and newly hatched larvae were sampled within an area comprised between the Gerlache Inlet and Cape Washington (Fig. 1). Sampling was performed from the under-surface of sea ice by using a 15-cm-diameter hand-held auger at a number of sampling sites (see below details about the sampling design) reached either by snowmobile (at Gerlache Inlet) or by helicopter (at Silverfish Bay and Cape Washington). Samples of eggs and hatched larvae, floating in the sea water and platelet ice dislodged from the underside of the sea ice while drilling holes, were collected by means of a PVC cylindrical ‘spooner,’ 10.5 cm in diameter and 7 L in capacity. The sampler was lowered into the ice hole and retrieved when filled (see detail in Vacchi et al. 2012b). Two 7 L volume samples were taken from each hole for a total of 14 L of sea water and platelet ice filtered. Samples were rapidly stored in plastic bags to be transported to the laboratory at the MZS. The number of eggs and larvae from each 14 L sample was considered as a relative estimate of abundance. To do the counts, samples were filtered through a 500-μm mesh sieve to eliminate the water and then sorted: silverfish eggs and newly hatched larvae were separated from the ice platelets, examined and counted under a stereomicroscope within a few hours after sampling, before being preserved in 4 % paraformaldehyde in seawater for further analyses.
A second step of the study was represented by the assessment of the distribution patterns of P. antarticum eggs within the platelet ice layer. Photographic samples (images) were taken and analyzed by using an ad-hoc methodology. At the same sites where samples of eggs and larvae were taken, further holes, 15 cm diameter, were done across which a modular plastic tube, approximately 12 cm diameter and 6 m long, was lowered. The plastic tube was build up so to have a wide transparent window along the length axis. Once the plastic tube was secured at the level of the upper side of the consolidated sea ice cap, an aluminum rod about 6–7 m long, provided with a system on the top that allowed to host and secure a microcamera Hero-Go Pro, was lowered down inside the plastic tube. Starting from the upper side of the sea ice cap (approximately 2.5 m thick), footages were taken at nine predetermined depth levels for every 25 cm: −2.5, −2.75, −3.0, −3.25, −3.75, −4.0, −4.25, −4.5 and −4.75 m. Footages were not taken at the −3.5 m, this level corresponding to the point where the two modules of the plastic tube were connected. Images were got both within the platelet ice layer and in the water column. The variable taken into account was the number of eggs appearing within each photogram randomly extracted from the footages taken at each depth level considered.
Sampling design and data analyses
Samples aimed at assessing spatial distribution patterns of silverfish eggs and larvae at multiple scales were collected at three locations: Gerlache Inlet, Silverfish Bay and Cape Washington (Fig. 1). At each location, four sites were randomly selected (distances between sites within location comprised between about 2 and 8 km; geographic position determined in situ by GPS) and three holes within a radius of about 100–150 m (considered as replicates) were done at each site. This spatial sampling design was repeated at two times (time 1: November, 10–16 2011; time 2: November 27 to December 2, 2011).
The sampling design adopted thus comprised ‘location’ (Lo) as a random factor (with 3 levels) and ‘site’ (Si) as a random factor (with 4 levels) nested within Lo. Data from the two sampling times were analyzed separately as we could not exclude a priori the possibility of temporal dependence of data, having got the samples approximately at the same spots at time 1 and 2. Three replicated samples were taken at each site and time for a total of 72 samples collected and examined. A two-way ANOVA (analysis of variance) was performed to test for putative differences in spatial distribution patterns at different spatial scales of locations, sites and replicates (approximately tens kilometers, kilometers and hundreds-tens meters, respectively).
As far as the distribution of silverfish eggs within the platelet ice is concerned, four images (replicates) were randomly extracted from the profiles (i.e., footages) at each of the nine abovementioned depth levels, at each of the three study locations (Gerlache Inlet, Silverfish Bay and Cape Washington). The sampling design in this case comprised ‘location’ (Lo) as a random factor (with three levels), ‘depth level’ (De) as a fixed factor (with nine levels) orthogonal to Lo, and four replicated images were taken at each location and depth level, for a total of 108 images selected where the eggs were counted within a standard framework. A two-way ANOVA was performed to test for putative differences in the distribution patterns of silverfish eggs among locations and depth levels (Table 1).
ANOVAs were performed using the GMAV5 software package (coded by A.J. Underwood and M.G. Chapman, University of Sydney, Australia). Prior to analysis, the homogeneity of variance was tested by Cochran’s test, and whenever necessary, data were appropriately transformed. If transformations did not produce homogeneous variances, ANOVA was used on non-transformed data after setting α = 0.01 in order to compensate for the increased likelihood of type I error (Underwood 1997).
Results
The abundance of Antarctic silverfish eggs displayed a highly significant variability at the scale of sites within location, while no significant variability was detected among the locations investigated (Fig. 2; Table 2). Inspection of the graph also reveals that, even though the factor ‘time’ was not investigated formally, a general and notable reduction of the eggs’ abundance occurred between time 1 and 2.
The general pattern of newly hatched larval abundance was similar to that of eggs. Silverfish larvae displayed a significant variability at scale of sites, but not at the scale of locations (Fig. 3; Table 3). The decrease of silverfish larval abundance from time 1 to time 2 was even more pronounced than the decline observed for eggs (Fig. 3).
The visual survey, conducted by the use of a mini video-camera across the platelet ice layer at the silverfish reproductive area in Terra Nova Bay, allowed us to get new in situ insights into the spatial organization of eggs within the platelets. Antarctic silverfish eggs resulted to be arranged in row aggregates adherent to the ice platelets’ surface, mostly at their edges. Furthermore, the video shooting showed that the fish eggs were mainly concentrated in the upper platelet ice level, close to the consolidated ice (Fig. 4). The formal analysis of data performed using ANOVA provided evidence of a clear effect of ‘depth’ (equivalent to ‘distance from the sea ice upper side’) as a main factor explaining the abundance of silverfish eggs under the solid sea ice cover (Table 4). Average egg abundance was highest (around 15 eggs/frame) at −2.5 m, i.e., very close to the underside of solid sea ice and within the platelet ice. Egg abundance decreased dramatically (~5–6 eggs/frame) at quite short distance (15–30 cm down) where the platelet ice was still present, to then stabilize at low values (~0.5–1.5 eggs/frame) beyond ~0.5 m from the underside of solid sea ice, where the platelet ice was not present anymore and the eggs were found to float in the open water (Fig. 3). Such patterns were the same at the three locations investigated (interaction ‘Lo × De’ not significant; Table 4).
Discussion
In the frame of a long-term monitoring program within the PNRA, a two-year survey highlighted the occurrence of P. antarctica eggs in an area of about 260 km2 spanning from the Gerlache Inlet to Cape Washington, with higher concentration at two locations: Gerlache Inlet and Silverfish Bay (Vacchi et al. 2012a).
In the present work, a multi-scale approach was used to analyze the distribution patterns of the Antarctic silverfish eggs and early-hatched larvae in the nursery area identified by Vacchi et al. (2012a), aiming at providing clues to interpret the causal processes underlying the observed patterns. By applying such an approach, we found an uneven distribution at the site scale (i.e., at a scale of kilometers), while no significant variability was found at the scale of locations (i.e., at the scale of tens of kilometers). In this study, also, we found significant densities of eggs and larvae of P. antarctica at Cape Washington, differently from a previous study (Vacchi et al. 2012a), which suggests that, based on environmental conditions that may change from year to year, the relevance of different sites as nursery areas may consequently change.
Having a look at the geographic area where eggs and larvae were found, we can identify some shared traits among the studied locations that could justify the uniform distribution at location scale concomitant with an heterogeneity at the site scale. The most striking feature at location scale certainly is the presence of fast ice covering most of the sea surface in the study area. In sheltered bays adjacent to promontories, the sea ice forms a recurrent and persistent coverage (Massom and Stammerjohn 2010) and the study area is, from this perspective, just characterized by a sequence of bays (e.g., the Gerlache Inlet and Silverfish Bay, where large amount of eggs/larvae have been found) and by the presence of a number of glaciers (e.g., Drygalski Ice Tongue, the Nansen Ice Sheet and the Campbell Glacier Tongue) flowing down from the continent into the ocean. All those features account for the multi-annual fast-ice coverage of the sea surface and for the frequent persistence of fast ice in some locations, where it can form either an annual or perennial cover (Massom and Stammerjohn 2010).
Even though the fast-ice coverage characterizes the whole studied area, the patterns of fast ice formation and breakup are probably highly sensitive to changes in the atmospheric and oceanic conditions. This results in heterogeneous sea ice dynamics that may affect fast-ice features (e.g., thickness) at relatively small scales (Massom and Stammerjohn 2010). A similar small-scale heterogeneity can be hypothesized for the platelet ice, whose amount could be affected by water movement taking place under the fast-ice cover. Therefore, the uneven distribution of eggs/larvae at the scale of kilometers (the site scale), as observed in the present study, might reflect differences in the local hydrodynamic conditions and winds that influence globally the sea ice formation processes and, consequently, the distribution of eggs/larvae underneath the sea ice coverage.
One further factor that could influence the eggs/larvae abundance at the site scale is the occurrence, thickness and extent of the platelet ice under the solid ice. Commonly observed near the ice shelves, at ice depth greater than about one meter, the platelet ice is an important component of the Antarctic land fast ice, consisting of various-sized flat dendritic crystals with a diameter up to 10 cm (McGuinness and Langhorne 2006; Dempsey and Langhorne 2012). Still far from being fully elucidated, the formation of the platelet ice is supposed to be a multistep process influenced by a number of environmental factors such as the heat at the ice/water interface, turbulence, waves, currents and wind (McGuinness and Langhorne 2006). On the whole, the interactions between ice shelf and ocean account for the platelet ice extension, depth, grain boundary and density (Dempsey and Langhorne 2012), thus indirectly influencing the composition, distribution and extent of the cryopelagic community therewith associated (reviewed in Vacchi et al. 2012b).
The occurrence of platelet ice underneath the solid fast ice is documented in the Terra Nova Bay area (Van Woert 1999), where it has been hypothesized that it might play a pivotal role in the early life phases of the Antarctic silverfish (Vacchi et al. 2012a).
The reason why platelet ice is a crucial feature for the Antarctic silverfish nursery still need to be fully elucidated. However, clues to understand the intimate link of the Antarctic silverfish eggs/larvae with this sea ice element could reside in its structural and biologic features. From a purely physical point of view, the platelet ice is a porous layer composed of approximately 20 % ice and 80 % water by volume, it connects the solid and relatively impermeable ice coverage to the liquid sea water below, and it is characterized by intermediate values of ice crystals density, temperature and salinity (Smith et al. 2001). Its tridimensional structure provides a large surface area available for algae, even more apt due to the rate of interstitial water turnover (ranging from 1.6 to 12 days depending of tides) that support the survival of one of the highest accumulation of algae on Earth (Arrigo and Thomas 2004). In a cascade effect fashion, zooplankton, such as copepods (Schnack-Schiel et al. 2004) and other metazoans (Vacchi et al. 2012b), were also found associated to the platelet ice. Moreover, besides its role as a food resource for ice-associated species, the platelet layer dendritic ice crystal maze has potential to act as a refuge for small-sized metazoan from larger predators.
Then, the occurrence of the Antarctic silverfish eggs and larvae in this ice layer appears to be logic. The survival of early life stages is a critical part of the life history of fishes, ultimately impacting on recruitment and overall population dynamics (Leggett and Deblois 1994). The presence of a rich and diversified ice-associated community, including phyto- and mesozooplankton that could be used as a food resource (Giraldo et al. 2011), and the crystal lattice structure, almost inaccessible to predators, makes the platelet layer a suitable ground for eggs and early larvae (Leggett and Deblois 1994). Additionally, to reside in such a stable and protected environment certainly is beneficial for eggs that are left unattended and potentially exposed to high risk of predation.
From this perspective, spawning and hatching of the Antarctic silverfish in the platelet ice might be interpreted as an ecological adaptation of this Antarctic pelagic species.
Unlike most notothenioids, the Antarctic silverfish releases pelagic positively buoyant eggs (Hubold 1984; Faleyeva and Gerasimchuk 1990; Vacchi et al. 2004). Therefore, it was not surprising to find that the abundance of eggs in the platelet ice layer was significantly influenced by the factor ‘depth,’ with most of the eggs located in the upper platelet layer underside the solid ice.
Such a stratification (illustrated in Fig. 5) could result, however, from two scenarios. The eggs could either (1) come across the rapidly changing ice crystal-rich environment that prelude the platelet ice formation and follow the ice platelets in their fate from liquid water to incorporation in under-ice side, or (2) they might reach an almost established platelet ice, driven by currents or because released in the vicinity, and then slide up in the interstices due to their positive buoyancy finally reaching the underside of the solid ice.
The suite of biological adaptations described for the Antarctic silverfish early life stages (Bottaro et al. 2009; Regoli et al. 2005; Evans et al. 2012) is a confirm that the location of eggs and early larvae within the platelet ice is not likely to be fortuitous. Therefore, although challenging (see Vacchi et al. 2012b), the icy platelet ice environment might not be just a suitable and opportune environment for Antarctic silverfish early life stages, but rather the optimal place for them to develop and grow, thus entailing a certain degree of dependence of the Antarctic silverfish early life stages from this particular type of ice, some issues that could be investigated, however, in future studies.
References
Albertson C, Yi-Lin Yan YL, Titus TA, Pisano E, Vacchi M, Yelick PC, Detrich HW, Postlethwait JH (2010) Molecular pedomorphism underlies craniofacial skeletal evolution in Antarctic notothenioid fishes. BMC Evol Biol 10:4. doi:10.1186/1471-2148-10-4
Andrew NL, Mapstone BD (1987) Sampling and the description of spatial pattern in marine ecology. Oceanogr Mar Biol 25:39–90
Arrigo KR, Thomas DN (2004) Large scale importance of sea ice biology in the Southern Ocean. Antarct Sci 16:471–486. doi:10.1017/S0954102004002263
Barange M, Bernal M, Cercole M, Cubillos L, De Moor C, Daskalov G, de Oliveira J, Dickey-Collas M, Hill K, Jacobson L, Køster F, Masse J, Nishida H, Ñiquen M, Oozeki Y, Palomera I, Saccardo S, Santojanni A, Serra R, Somarakis S, Stratoudakis Y, van der Lingen C, Uriarte A, Yatsu A (2009) Current trends in the assessment and management of small pelagic fish stocks. In: Checkley D, Roy C, Oozeki Y, Alheit J (eds) Climate change and small pelagic fish stocks. Cambridge University Press, Cambridge, pp 191–255
Bottaro M, Oliveri D, Ghigliotti L, Pisano E, Ferrando S, Vacchi M (2009) Insights into the life cycle of the Antarctic silverfish Pleuragramma antarcticum: morphology of two developmental stages. Rev Fish Biol Fisher 19:249–259. doi:10.1007/s11160-009-9106-5
Budillon G, Spezie G (2000) Thermohaline structure and variability in the Terra Nova Bay polynya, Ross Sea. Antarct Sci 12:493–508. doi:10.1017/S0954102000000572
Buffoni G, Cappelletti A, Picco P (2002) An investigation of thermohaline circulation in Terra Nova Bay polynya. Antarct Sci 14:83–92. doi:10.1017/S0954102002000615
Chavez FP, Ryan J, Lluch-Cota SE, Niquen M (2003) From anchovies to sardines and back: multidecadal change in the Pacific Ocean. Science 299:217–221. doi:10.1126/science.1075880
Dempsey DE, Langhorne PJ (2012) Geometric properties of platelet ice crystals. Cold Reg Sci Technol 78:1–13. doi:10.1016/j.coldregions.2012.03.002
DeWitt HH (1970) The character of the midwater fish fauna of the Ross Sea, Antarctica. In: Holdgate MW (ed) Antarctic ecology, vol 1. Academic Press, London, pp 305–314
DeWitt HH, Tyler JC (1960) Fishes of the Stanford Antarctic Biological Research Program (1958–1959). Stanford Ichthyol Bull 7:162–199
DeWitt HH, Heemstra PC, Gon O (1990) Nototheniidae. In: Gon O, Heemstra PC (eds) Fishes of the Southern Ocean. JLB Smith Institute of Ichthyology, Grahamstown, pp 279–331
Duhamel G, Hulley PA, Causse R, Koubbi P, Vacchi M, Pruvost P, Vigetta S, Irisson JO, Mormède S, Belchier M, Dettai A, Detrich HW, Gutt J, Jones CD, Kock KH, Lopez Abellan LJ, Van de Putte AP (2014) Biogeographic patterns of fish. In: De Broyer C, Koubbi P (eds) Biogeographic Atlas of the Southern Ocean. Scientific Committee on Antarctic Research, Cambridge, pp 328–362
Eastman JT (1993) Antarctic fish biology: evolution in an unique environment. Academic Press, San Diego
Eastman JT (1997) Phyletic divergence and specialization for pelagic life in the Antarctic Notothenioid fish Pleuragramma antarcticum. Comp Biochem Phys A 118:1095–1101. doi:10.1016/S0300-9629(97)86798-9
Eastman JT (2005) The nature of the diversity of Antarctic fishes. Polar Biol 28:93–107. doi:10.1007/s00300-004-0667-4
Eschmeyer WN (2014) Catalog of Fishes. http://research.calacademy.org/ichthyology/catalog/fishcatmain.asp. Accessed 27 August 2014
Evans CW, Williams DE, Vacchi M, Brimble MA, DeVries AL (2012) Metabolic and behavioural adaptations during early development of the Antarctic silverfish, Pleuragramma antarcticum. Polar Biol 35:891–898. doi:10.1007/s00300-011-1134-7
Faleyeva TI, Gerasimchuk VV (1990) Features of reproduction in the Antarctic sidestripe, Pleuragramma antarcticum (Nototheniidae). J Ichthyol 30:67–79
Ferrando S, Hanchet S, Angiolillo M, Gambardella C, Pisano E, Vacchi M (2010) Insights into the life cycle of the Antarctic Silverfish. Reproduction features of the Ross Sea population. In: Proceedings of international polar year Oslo science conference, 8–12 June 2010, Oslo, Norway
Fuiman LA, Davis RW, Williams TM (2002) Behavior of midwater fishes under Antarctic ice: observations by a predator. Mar Biol 140:815–822. doi:10.1007/s00227-001-0752-y
Gerasimchuck VV (1986) Characteristics of Antarctic silverfish, Pleuragramma antarcticum (Nototheniidae), from Olaf-Pruds Bay (Commonwealth Sea, Eastern Antarctica) with notes on the identification of the species. J Ichthyol 26:10–17
Giraldo C, Cherel Y, Vallet C, Mayzaud P, Tavernier E, Moteki M, Hosie G, Koubbi P (2011) Ontogenic changes in the feeding ecology of the early life stages of the Antarctic silverfish (Pleuragramma antarcticum) documented by stable isotopes and diet analysis in the Dumont d’Urville Sea (East Antarctica). Polar Sci 5:252–263. doi:10.1016/j.polar.2011.04.004
Granata A, Cubeta A, Gugliemo L, Sidoti O, Greco S, Vacchi M, La Mesa M (2002) Ichthyoplankton abundance and distribution in the Ross Sea during 1987–1996. Polar Biol 25:87–202. doi:10.1007/s00300-001-0326-y
Guglielmo L, Granata A, Greco S (1998) Distribution and abundance of postlarval and juvenile Pleuragramma antarcticum (Pisces, Nototheniidae) off Terra Nova Bay (Ross Sea, Antarctica). Polar Biol 19:37–51. doi:10.1007/s003000050214
Guidetti P, Petrillo M, De Benedetto G, Albertelli G (2013) The use of otolith microchemistry to investigate spawning patterns of European anchovy: a case study in the eastern Ligurian Sea (NW Mediterranean). Fish Res 139:1–4
Hubold G (1984) Spatial distribution of Pleuragramma antarcticum (Pisces: Nototheniidae) near the Filchner-and Larsen ice shelves (Weddell sea/Antarctica). Polar Biol 3:231–236. doi:10.1007/BF00292628
Hubold G (1990) Seasonal patterns of ichthyoplankton distribution and abundance in the Southern Weddell Sea. In: Kerry KR, Hempel G (eds) Antarctic ecosystems: ecological change and conservation. Springer, Berlin, pp 149–158
Hubold G, Ekau W (1987) Midwater fish fauna of the Weddell Sea, Antarctica. In: Kullander SO, Fernholm B (eds) Proceedings of the fifth congress of the european ichthyological society. Swedish Museum of Natural History, Stockholm, pp 391–396
Kellermann A (1987) Food and feeding ecology of postlarval and juvenile Pleuragramma antarcticum (Pisces; Notothenioidei) in the seasonal pack ice zone off the Antarctic Peninsula. Polar Biol 7:307–315. doi:10.1007/BF00443949
Kellermann A (1989) The larval fish community in the zone of seasonal ice cover and its seasonal and interannual variability. Arch Fischereiweiss 39:89–109. doi:10.013/epic.12234
Kellermann A (1991) Eggs and larval drift of the Antarctic fish Notothenia coriiceps. Cybium 15:199–210
Kock KH, Kellermann A (1991) Reproduction in Antarctic notothenioid fish. Antarct Sci 3:125–150. doi:10.1017/S0954102091000172
Koubbi P, Hureau JC, Vacchi M, White M (1997) Results of the preliminary survey on the coastal distribution of fish larvae in Adelie Land (Southern Ocean) during January–February 1996. Cybium 21:381–392
Koubbi P, O’Brien C, Loots C, Giraldo C, Smith M, Tavernier E, Vacchi M, Vallet C, Chevallier J, Moteki M (2011) Spatial distribution and inter-annual variations in the size frequency distribution and abundances of Pleuragramma antarcticum larvae in the Dumont d’Urville Sea from 2004 to 2010. Polar Sci 5:225–238. doi:10.1016/j.polar.2011.02.003
Kurtz DD, Bromwich DH (1983) Satellite observed behavior of the Terra Nova Bay polynya. J Geophys Res-Oceans 88:9717–9722. doi:10.1029/JC088iC14p09717
Kurtz DD, Bromwich DH (1985) A recurring, atmospherically forced polynya in Terra Nova Bay. In: Jacobs SS (ed) Oceanology of the Antarctic continental shelf. Antarctic Research Series 43, American Geophysical Union, Washington DC, pp 177–201
La Mesa M, Eastman JT (2012) Antarctic silverfish: life strategies of a key species in the high-Antarctic ecosystem. Fish Fish 13:241–266. doi:10.1111/j.1467-2979.2011.00427.x
La Mesa M, Eastman JT, Vacchi M (2004) The role of notothenioid fish in the food web of the Ross Sea shelf waters: a review. Polar Biol 27:321–338. doi:10.1007/s00300-004-0599-z
Leggett WC, Deblois E (1994) Recruitment in marine fishes: is it regulated by starvation and predation in the egg and larval stages? Ned J Sea Res 32:119–134. doi:10.1016/0077-7579(94)90036-1
Liu Q, Chen DG (1995) Length frequency analysis of Pleuragramma antarcticum, Electrona antarctica, Protomyctophum bolini. Chin J Oceanol Limnol 13:380–384. doi:10.1007/BF02889475
Massom RA, Stammerjohn SE (2010) Antarctic sea ice change and variability–Physical and ecological implications. Polar Sci 4:149–186. doi:10.1016/j.polar.2010.05.001
McGuinness MJ, Langhorne P (2006) A platelet puzzle in Antarctica. Proc KSIAM 2006 Annual Meeting, Nov 24–25, Konkuk University, Seoul, Korea
Moline MA, Karnovsky NJ, Brown Z, Divoky GJ, Frazer TK, Jacoby CA, Torres JJ, Fraser WR (2008) High latitude changes in ice dynamics and their impact on polar marine ecosystems. Ann NY Acad Sci 1134:267–319. doi:10.1196/annals.1439.010
Morales-Nin B, Garcia MA, Lopez O (1998) Distribution of larval and juvenile Nototheniops larseni and Pleuragramma antarcticum off the Antarctic Peninsula in relation to oceanographic conditions. Cybium 22:69–81
O’Driscoll RL, Macaulay GJ, Gauthier S, Pinkerton M, Hanchet S (2011) Distribution, abundance and acoustic properties of Antarctic silverfish (Pleuragramma antarcticum) in the Ross Sea. Deep Sea Res II 58:181–195. doi:10.1016/j.dsr2.2010.05.018
Palomera I, Olivar MP, Salat J, Sabates A, Coll M, Garcia A, Morales-Nin B (2007) Small pelagic fish in the NW Mediterranean Sea: an ecological review. Progr Oceanogr 74:377–396. doi:10.1016/j.pocean.2007.04.012
Regan CT (1916) Larval and postlarval fishes. British Antarctic (“Terra Nova”) Expedition 1910. Nat Hist Report Zool 1:125–156
Regoli F, Nigro M, Benedetti M, Fattorini D, Gorbi S (2005) Antioxidant efficiency in early life stages of the Antarctic silverfish, Pleuragramma antarcticum: responsiveness to pro-oxidant conditions of platelet ice and chemical exposure. Aquat Toxicol 75:43–52. doi:10.1016/j.aquatox.2005.07.003
Schnack-Schiel SB, Dieckmann GS, Kattner G, Thomas DN (2004) Copepods in summer platelet ice in the eastern Weddell Sea, Antarctica. Polar Biol 27:502–506. doi:10.1007/s00300-004-0613-5
Smith IJ, Langhorne PJ, Haskell TG, Joe Trodahl H, Frew R, Ross Vennell M (2001) Platelet ice and the land-fast sea ice of McMurdo Sound, Antarctica. Ann Glaciol 33:21–27. doi:10.3189/172756401781818365
Underwood AJ (1997) Experiments in ecology: Their logical design and interpretations using analysis of variance. Cambridge University Press, Cambridge, p 504
Underwood AJ, Chapman MG, Connell SD (2000) Observations in ecology: you can’t make progress on processes without understanding the patterns. J Exp Mar Biol Ecol 250:97–115. doi:10.1016/S0022-0981(00)00181-7
Vacchi M, La Mesa M, Greco S (1999) Summer distribution and abundance of larval and juvenile fishes in the western Ross Sea. Antarct Sci 11:54–60. doi:10.1017/S0954102099000085
Vacchi M, La Mesa M, Dalù M, Macdonald J (2004) Early life stages in the life cycle of Antarctic silverfish, Pleuragramma antarcticum in Terra Nova Bay, Ross Sea. Antarct Sci 16:299–305. doi:10.1017/s095410200400215
Vacchi M, DeVries AL, Evans CW, Bottaro M, Ghigliotti L, Cutroneo L, Pisano E (2012a) A nursery area for the Antarctic silverfish Pleuragramma antarcticum at Terra Nova Bay (Ross Sea): first estimate of distribution and abundance of eggs and larvae under the seasonal sea-ice. Polar Biol 35:1573–1585. doi:10.1007/s00300-012-1199-y
Vacchi M, Koubbi P, Ghigliotti L, Pisano E (2012b) Sea-ice interactions with polar fish focus on the antarctic silverfish life history. In: Verde C, di Prisco G (eds) Adaptation and evolution in marine environments, from pole to pole series. Springer, Berlin, pp 51–73. doi:10.1007/978-3-642-27352-0-4
Van Woert ML (1999) Wintertime dynamics of the Terra Nova Bay polynya. J Geophys Res 104:1169–7753. doi:10.1029/1999JC900003
Acknowledgments
This work was carried out within the project ‘Vulnerability of polar fishes to climate change: life cycle, habitats and sea-ice relationship of the species P. antarcticum,’ supported by Italian National Programme for Research in Antarctica (PNRA project 2010/A1.11). We wish to thank the entire staff of the Italian Antarctic Base ‘Mario Zucchelli,’ but in particular Davide Riga, Luciano Sartori and Luca De Santis for their invaluable technical/logistical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guidetti, P., Ghigliotti, L. & Vacchi, M. Insights into spatial distribution patterns of early stages of the Antarctic silverfish, Pleuragramma antarctica, in the platelet ice of Terra Nova Bay, Antarctica. Polar Biol 38, 333–342 (2015). https://doi.org/10.1007/s00300-014-1589-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-014-1589-4