Abstract
The Bellingshausen and Amundsen Seas are among the least studied Antarctic areas. Pycnogonids constitute a common and conspicuous component of the Antarctic marine fauna. Antarctic pycnogonids have been widely studied and are usually more abundant than elsewhere. Therefore, they represent a key taxon to understand the zoogeographic and bathymetric distributions of the fauna from these two poorly sampled seas. Furthermore, we aim to compare the diversity and composition of the pycnogonids in these areas with those in other Antarctic zones. Three main surveys were carried out in these regions (Bentart 2003 and 2006 and Biopearl II 2008). In total, 879 pycnogonids belonging to 65 species were recorded in 49 stations. Two new species are described: Heteronymphon krappi n.sp. and Nymphon nakamurai n.sp. Ammothea magniceps and A. hesperidensis are recorded for the second time in the Antarctic. The most abundant family is the Nymphonidae (60.5 %), and Nymphon australe is the most abundant species (25.5 %). The biogeographic analysis revealed 39 species in the Bellingshausen Sea (16 new records) and 19 species in the Amundsen Sea (18 new records). The circumpolar pattern is the most common found. The Bellingshausen Sea seems to be a poor area in terms of abundance and species richness compared to the Amundsen Sea and other Antarctic zones. Faunal similarity was clustered into three main groups: the shallow-water stations, the outer continental shelf stations and the slope stations. The abundance of individuals likely responds to the varying amount of organic matter that reaches each bathymetric zone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antarctica has been isolated for a long time, since the opening of the Drake Passage (23–34 Mya) and the subsequent establishment of the circum-Antarctic Current (Barker and Burrell 1977; Livermore et al. 2005). This isolation has greatly affected its marine fauna, and various processes (recolonization, evolution and speciation) have led to its current composition (Clarke and Crame 1989; Gili et al. 2006). However, these geological and biological processes had different effects on different Antarctic marine fauna: Reptant decapods are nearly absent from Antarctic waters (Thatje and Arntz 2004), few species of fish are found (Eastman and Grande 1989) and many other taxa such as sponges, lophophorata and pycnogonids show species enrichment (Arntz et al. 1997).
Pycnogonids (Chelicerata, Arthropoda) from Antarctic and sub-Antarctic waters have recently been studied more extensively than those from any other oceanic region of a similar size (Mahon et al. 2008; Munilla and Soler-Membrives 2009; Nielsen et al. 2009; Krabbe et al. 2010; Arango et al. 2011; Bamber 2011; Weis et al. 2011; Cano and López-González 2013; Dietz et al. 2013). Some pycnogonid species, such as Ammotheids, tend to be of larger maximal size in colder and deeper waters compared to temperate and tropical waters (Hedgpeth 1969; Griffiths et al. 2011). They are usually more abundant than elsewhere, particularly around Antarctic shelves, as 87 % of the species have been located between 0 and 1,000 m (Munilla 2001). These features make them a common and conspicuous component of the Southern Ocean (SO) benthic samples, in contrast to the typical rarity of reports from elsewhere, probably due to their small size and low abundance. So far, more than 40,000 specimens have been found at approximately 2,100 sampled stations in the Antarctic and sub-Antarctic waters (Munilla and Soler-Membrives 2009). These individuals belong to 31 genera and 262 different species. The SO pycnogonid fauna appears to be more diverse than that at lower latitudes, i.e. 20 % of the total species known are found in this region (Clarke and Johnston 2003). Comparing the percentages of known living species on a global scale, it has been suggested that the pycnogonids (15.5 %) are better represented around Antarctica than are other groups, such as polychaete worms (12 %), amphipods (8–14 %), sponges (6.2 %), echinoderms (4.9 %), cumaceans (4.9 %), fish and gastropod molluscs (<2 %) (Corbera et al. 2009; Munilla 2001). These facts suggest pycnogonids as excellent representatives of the highly diverse and abundant marine invertebrates inhabiting the SO region compared to other parts of the world (Clarke and Johnston 2003).
Studies focused on the biogeographic and biodiversity patterns of austral pycnogonids (Munilla 2001; Munilla and Soler-Membrives 2009; Griffiths et al. 2011) note that the north-west Antarctic area of the Bellingshausen and Amundsen Seas is poorly studied, mostly due to the presence of large rocks on the bottom and the wide and prolonged ice cover, which complicate the survey of the area (San Vicente et al. 2009; Linse et al. 2013). Recent studies of other Antarctic benthic and pelagic fauna also came to a similar conclusion that the Bellingshausen and Amundsen Seas are relatively unknown regions (Linse et al. 2006; Kaiser et al. 2009; Griffiths 2010). As far as it is known, isopods present high species richness in the Amundsen shelf area (Kaiser et al. 2009), whereas the Bellingshausen Sea seems to be poor for molluscs (Troncoso et al. 2007) and moderately rich for fishes (Matallanas and Olaso 2007). Regarding pycnogonids, 23 species have been recorded to date in the Bellingshausen Sea but only one in the Amundsen (Munilla and Soler-Membrives 2009).
The aims of this paper are to (1) contribute to the better understanding of the pycnogonid fauna of these two poorly sampled seas, (2) explain the zoogeographic and bathymetric distributions of these fauna and (3) compare the richness and composition of the pycnogonids in these areas with those in other Antarctic zones.
Materials and methods
The pycnogonids studied herein were collected during two Spanish oceanographic cruises in the Bellingshausen Sea and Western Antarctic Peninsula—Bentart 2003 (from 30 January to 26 February) and Bentart 2006 (from 20 January to 11 February) on board the RV Hespérides—and one British cruise mainly in the Amundsen Sea—Biopearl II 2008 (from February to April) on board the RRS James Clark Ross. The sampling area is shown in Fig. 1, and the exact sampling locations are detailed in Table 1. A total of 69 stations were visited during these three cruises, of which 49 were positive for pycnogonids. The main gears employed were the Agassiz trawl for all cruises, the Macer-GIROQ suprabenthic sledge for the Bentart cruises and the epibenthic Sledge for the Biopearl II cruise. During Bentart cruises, the gears were towed over the bottom for a mean of 8 min at 1.5–2 knots. The deployment protocol for the Biopearl II cruise was standardized to 10 min (at 500 m deep) and 15 min (>1,000 m) trawling at 1 knot with a ratio of 1.5 times cable length to water depth to facilitate comparability between the different sites (for more details of sampling methods see (Troncoso et al. 2007; Corbera et al. 2009; Kaiser et al. 2009)).
When the trawl reached the deck, each sample was sieved through a 500-μm screen, and mega- and macrofauna were preserved in 96 % ethanol. The specimens have been stored at the Universitat Autònoma de Barcelona collection (UABc), where they have been determined to species level, using mainly original descriptions and main monographs listed in Munilla and Soler-Membrives (2009). A list of the species and their authorities are provided in Table 4.
The numbers of specimens and species were counted to determine the abundance (N) and species richness (S) at each station (Online Resource 1). Biodiversity and biogeographic analyses were performed using PRIMER v5 software (Clarke and Warwick 2001). Univariate diversity index of Shannon–Wiener diversity (H′-log2) was calculated for each sampling locality. Faunal similarity among sampling localities with abundances >1 % (i.e., 39 out of 49 stations) was measured with the quantitative Bray-Curtis similarity coefficient (Bray and Curtis 1957) based on fourth-root transformed species abundances, which reduces the effect of the small number of abundant species (Clarke and Warwick 2001). In addition, cluster analysis based on the group-average sorting algorithm was applied to the resemblance data to display the faunal similarities in two dimensions. The species accumulation curves were used to predict total species richness for the areas studied and were calculated by using the species accumulation plot option in PRIMER v5 software (Clarke and Warwick 2001), with 999 permutations.
Results
Taxonomy
-
Class PYCNOGONIDA Latreille, 1810
-
Family NYMPHONIDAE Wilson, 1878
-
Genus Heteronymphon Gordon, 1932
-
Heteronymphon krappi new species (Figs. 2, 3; Table 2).
Table 2 Morphological differences between Heteronymphon krappi n.sp. and other close pycnogonid species Table 3 Morphological differences between Nymphon nakamurai n.sp. and other close pycnogonid species
Material examined
O/V hesperides
Bentart 2006 cruise. Suprabenthic sledge. Station 42: 1,272 m, West Antarctic Peninsula (an ovigerous male, holotype, registration number Bent06-Bell42/17-UABc). Station 34: 608–620 m, Bellingshausen Sea (a female with ovules, paratype, registration number Bent06-Bell34/84-UABc).
Description of holotype
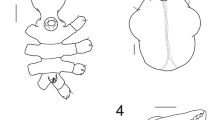
Body length 4.1 mm (proboscis and trunk) (Figs. 2a, b, 3a). Body smooth, without apophyses, setae or spines. Proboscis almost cylindrical slightly inclined downward, glabrous, long and thin (0.75 and 0.15 mm in length and maximum width, respectively) with a very feeble constriction at 1/4 of the rounder tip, and 3 or 4 pairs of lateral setae (Fig. 2d). Trunk 4.3 times as long as proboscis, very slender and fully segmented, with lateral processes about 4 times as long as wide, separated by intervals six times their diameters. Ocular tubercle very low, located at the cephalic front, with four transparent papillae and without pigmented eyes. Abdomen very short, inclined about 45º upward and its tip not reaching distal margin of fourth lateral process.
Heteronymphon krappi n. sp. Dorsal (a) and lateral (b) view of the body. Detail of the chelifore (c). Detail of the proboscis (d) and palp (e). Detail of the strigilis (f) showing a pair of compound spines (g). Second leg (h), detailing the distal part of the leg (i) with the propodus and terminal claw
Chelifores with scape slightly curved in dorsal and lateral view, slightly more than proboscis length, with small and scarce spines (Figs. 2c, 3c). Chelae with size similar to half of the scape, finger length is equal to the palm and each with 6–9 similar teeth; spines might be more abundant on the palm region of the chela
Palps 5-articled, slender, 2.3 times as long as proboscis (Fig. 2e). Articles 2, 4 and 5 subequal in length. Article 3 the longest, 4 times as long as second article. Article 5 more setose than others and swollen apically.
Ovigers conventional with ten articles; its insertion is between the tip of cephalon and the first pair of lateral processes, 2/3 behind the ocular tubercle level. Article 5 the longest, 3 times as long as article 6. Strigilis recurved, with some ectal normal spines and 24 endal denticulate spines in formula 7:6:5:6, with three or four pairs of teeth each (Fig. 2f, g). Article 10 with a small terminal claw without serrations. A couple of egg masses with 20 eggs each are attached to the left oviger.
Legs very thin, with sparse and small spines, more abundant in the second tibiae—the longest article— tarsus and propodus; leg lengths 3–4 times as long as the trunk (Fig. 2h, i). Second coxa length 4–5 times as long as the first one. Long segments straight, with some spines longer than the rest. Propodus straight, without heel, but with 20 endal and similar spines along the entire sole (Fig. 2i). Claw large, about half propodal length, without auxiliary claws (Fig. 2i).
The female is slighlty shorter than the male (trunk length: 2.8 mm). Oviger formula 5:5:5:5 compound spines, each with four pair of teeth. Moreover, this female has ovules inside its second coxae and femora, and it has neither eyes nor ocular tubercle.
Measurements of holotype (in mm)
Length of trunk (tip of cephalic segment to tip of fourth lateral process): 3.2. Width of trunk across second lateral process: 1.1. Length of proboscis: 0.75. Greatest diameter of proboscis: 0.15. Length of abdomen: 0.17. Length of chelifore: 1.3. Length of scape: 0.9. Length of chelae and palm: 0.4. Length of palp: 1.7; length of palp articles: 1–0.1, 2–0.2, 3–0.8, 4–0.3, 5–0.3. Length of third leg: 9.7; length of leg articles: coxa 1–0.3, coxa 2–1.1, coxa 3–0.3, femur-1.7, tibia 1–2.3, tibia 2–2.7, tarsus—0.5, propodus—0.5, claw—0.3. Length of oviger: 5.05; articles of oviger: 1–0.1, 2–0.3, 3–0.2, 4–1.2, 5–1.8, 6–0.6, 7–0.3, 8–0.2, 9–0.2, 10–0.15.
Etymology
The name of this species is dedicated to our colleague Dr. Franz Krapp in recognition of his comprehensive work on taxonomy of pycnogonids.
Remarks
This new species has some similarities to several known ones, especially with H. profundum Turpaeva, 1956—N. bioculatum Turpaeva, 1956 has been synonymised to H. profundum by herself (Turpaeva and Yampolsky 1992)—and N. horikoshii Nakamura, 1985: Their fourth palpal segments are subequal or longer than fifths segments, and tibia 1 is shorter than tibia 2. H. exiguum Hodgson, 1927 is the other Antarctic species, but the fourth palpal article is much shorter than the fifth, and the claw of the oviger is absent (Nakamura 1985). The comparison of the main differences between these species is shown in Table 2. In brief, major characters that distinguish this new species from the others are their small size, the distance between lateral processes, the scarce finger teeth number, the insertion of the oviger and the number of compound spines of the strigilis.
-
Class PYCNOGONIDA Latreille, 1810
-
Family NYMPHONIDAE Wilson, 1878
-
Genus Nymphon Fabricius, 1794
-
Nymphon nakamurai new species (Fig. 4; Table 3).
Table 4 List of the specimens per station of the species found in each cruise
Material examined
O/V Hesperides. Bentart 2006 cruise. Suprabenthic sledge. Station 29: 3280 m, near to Peter I Island in the Bellingshausen Sea. One sub-adult, holotype, registration number Bent06-Bell29/95-UABc.
Description of holotype
Body length 3.3 mm (proboscis and trunk) (Fig. 4a, b). Trunk and lateral processes glabrous and without apophyses. The appearance is that of a young male recently moulted due to the slightly transparent body and the complete presence of the oviger. Proboscis almost cylindrical and truncate apically, with some lateral setae on its distal half, almost three times as long as wide and with similar length to scape (Fig. 4e). Trunk 3.1 times as long as proboscis, 2.5 times as long as wide and fully segmented, with lateral processes separated by intervals 2–3 times their diameter and as long as wide or a bit longer. Ocular tubercle low, transparent, located on the basal zone of neck, without eyes but with two apical horns (Fig. 4f). Anterior part of cephalon heart-shaped followed of a triangular neck, giving to ensemble a biconcave lateral profile. Abdomen short and bifid, inclined about 45° upward, reaching the end of the fourth lateral process.
Nymphon nakamurai n. sp. Dorsal (a) and lateral (b) view of the body. Detail of the chelifore (c) and chela (d). Detail of the proboscis (e), ocular tubercle (f) and palp (g). Detail of the strigilis (h) showing the distal segment and terminal claw (i). Second leg (j), detailing the distal part of the leg (k) with the propodus and terminal claw
Chelifores with scape glabrous, except for 3–4 distal setae, and slightly curved in dorsal and lateral view (Fig. 4c, d). Chelae with size similar to half of the scape, palm slightly longer than fingers with some distal and exterior setae, fingers with 6–7 similar teeth each (Fig. 4d).
Palps 5-articled, slender, 1.9 times as long as proboscis. Article 2 ≥ 3 > 4 < 5, article 4 0.7 times as long as article 5 (Fig. 4g). The two last articles more setose than the others and article 3 with some setae at the distal part only.
Ovigers conventional with ten articles; ventral insertion at level of the first pair of lateral processes. Article 4 with a slight bulge in its middle. Article 5 the longest, 1.4 times as long as article 4. Strigilis recurved, with some ectal normal spines and 20 endal denticulate spines in formula 7: 5: 4: 4, having two or three pairs of teeth each (Fig. 4h, i). Article 10 with a small terminal claw with four serrations.
Legs very thin, with sparse and small spines, more abundant on second tibiae—the longest article—tarsus and propodus (Fig. 4j, k); leg length 4 up to 5 times as long as the trunk (leg 2 vs leg 4, respectively). Second coxa length almost 3 times as long as the first one. Propodus straight, about 1.1 times as long as tarsus, without heel, but with 8 endal and similar spines along the entire sole (Fig. 4k). Main claw about the half propodus length; auxiliary claws between 1/4 and 1/5 of the main claw length.
This specimen still has no cement gland or sexual pores.
Measurements of holotype (in mm)
Length of trunk (tip of cephalic segment to tip of fourth lateral processes): 2.5. Width of trunk across second lateral processes: 1.0. Length of proboscis: 0.8. Greatest diameter of proboscis: 0.3. Length of abdomen: 0.3. Length of chelifore: 1.2. Length of scape: 0.8. Length of chelae and palm: 0.4. Length of palp: 1.5; articles of palp: 1–0.1, 2–0.5, 3–0.4, 4–0.2, 5–0.3. Length of fourth leg: 12.4; articles of leg: coxa 1–0.3, coxa 2–0.8, coxa 3–0.3, femur—1.4, tibia 1–3.0, tibia 2–4.3, tarsus—0.7, propodus—0.8, main claw—0.8, auxiliary claw: 0.2. Length of oviger: 3.0; articles of oviger: 1–0.2, 2–0.2, 3–0.2, 4–0.5, 5–0.7, 6–0.4, 7–0.3, 8–0.2, 9–0.2, 10–0.1.
Etymology
This species is named in honour of Dr. Koichiro Nakamura who dedicated a whole life to the study of pycnogonids, mainly in Japanese waters.
Remarks
This specimen is similar to N. paucidens Gordon 1932 and N. subtile Gordon, 1932 (differences are shown in Table 3), but rather distant from the hamatum species (Child 1995a), since they all lack auxiliary claws. The combination of characters that define the new species and separate it from the others are the following: the truncated end of the cylindrical proboscis with some lateral setae, the two horns without eyes of the ocular tubercle, the similar length of tarsus and propodus and the auxiliary claws proportion to the main claw length (0.20–0.25).
Biodiversity and faunal composition
The total number of individuals captured was 879, belonging to 65 species, 16 genera and 9 families (Table 4). The most species-rich families are the Nymphonidae and Ammotheidae, with 16 species each. The most species-rich genus is Nymphon (S = 13), followed by Ammothea and Colossendeis with 10 species each. Ammothea magniceps and A. hesperidensis were recorded near Low Island around 82–97 m depth, constituting the second records for each species in Antarctic waters. All specimens found from both species agree with the holotypes.
Likewise, Nymphonidae and Ammotheidae families are also the most abundant, although the former (60.5 %) is much more abundant than the latter (11.2 %). The abundances of the species recorded during each cruise are shown in Table 4. There are only 4 species whose abundance exceeds 5 % of the total individuals, with the most abundant being Nymphon australe (23.4 %), followed by N. longicoxa (14.3 %). Most of the species (41 of 65) were found at 1 or 2 stations, 19 species were present at 3–9 stations and five species (N. australe, N. longicoxa, Austropallene cornigera, Austrodecus glaciale and Pallenopsis pilosa) were present at 10 stations or more. There are three species with a mean number of individuals per station (N/nSt) ≥ 20: N. australe, N. biarticulatum and P. macronyx.
The average species richness per station (S/St) was similar for the three cruises, oscillating between 3.30 and 4.55, but the average abundance per station (N/St) was markedly higher in the Biopearl II cruise (mainly in the Amundsen Sea, 23.8) when compared to the Bentart cruises (Bellingshausen Sea and West Antarctic Peninsula, 15.1–17.8) (Table 4). Stations 21 (open sea, West Antarctic Peninsula) and 47 (Low Island, Shetland I) showed the highest values for species richness (14 and 12 species, respectively), and station 54 in the Amundsen Sea showed the highest abundance, with 138 specimens (Online Resource 1).
Regarding the Shannon–Wiener diversity index (Online Resource 1), three main groups can be detected, with a mean value of 1.2 and a maximum value of 3.5 found at station 21 (106 m depth). Nine of the twelve shallow stations (depths above 200 m) showed relatively high diversities, with indices H′ greater than 2. Most of the stations with depths ranging from 200 to 700 m (21 of 23) present intermediate diversity values (1 ≤ H′ ≤ 2). All 14 stations deeper than 700 m are characterized by the lowest diversity (H′ ≤ 1).
Based on pycnogonid assemblages, three main groups of stations with 20 % similarity are grouped by the quantitative Bray-Curtis similarity clustering of the 39 sampling sites selected, based on the abundance >1 % (see Fig. 5). Group A includes 10 stations located in very shallow waters (depth range 82–215 m), seven stations located near the South Shetland Islands and West Antarctic Peninsula, and another three stations at Peter I Island. Group B contains 9 stations at depths ranging from the continental shelf (>200 m) up to the shelf break at approximately 700 m and a single station (St. 42) at 1,272 m depth, most of them found in open areas of the Bellingshausen Sea or in the Amundsen Sea. Group C includes 11 stations, 4 belonging to the outer continental shelf (400–700 m), station 57 at approximately 1,000 m depth, and 6 stations grouped in a sub-cluster with 60 % similarity with the slope at greater depths (938–1,457 m). All of these stations are located in open areas of the Bellingshausen Sea or in the Amundsen Sea. The three very deep stations (Sts. 17, 29 and 30 at depths greater than 1,700 m) selected for the similarity analysis are located as outgroups, each separated from the others, though they are all located in open parts of the Bellingshausen Sea.
The comparison of species richness by area (Fig. 6) shows that increased sampling leads to increased species richness. The Bellingshausen area (both islands and open sea) showed the lowest species richness per station sampled compared to the other zones. The Amundsen Sea showed an intermediate rate of species accumulation with increased sampling. The third grouping, including the West Antarctic Peninsula and the Shetland Islands, showed a relatively high degree of species accumulation.
Discussion
To date, the sampling of the Amundsen and Bellingshausen Seas has been very scarce (Griffiths et al. 2011; Linse et al. 2013), making the present study of major importance understanding the Antarctic marine benthic biodiversity.
Biodiversity of the Amundsen and Bellingshausen Seas
Previous reports documented 23 species of pycnogonids in the Bellingshausen Sea and a single species in the Amundsen Sea (Colossendeis brevirostris) (Bouvier 1913; Giltay 1935; Fry and Hedgpeth 1969; Pushkin 1993; Müller 1993; Child 1995b; Munilla and Soler-Membrives 2009). Our study (Table 5) increases the number of species in the Bellingshausen Sea to 39 (including 16 new records) and to 19 in the Amundsen Sea (including 18 new records), with 11 species being common to the two areas. Finally, A. magniceps and A. hesperidensis were recorded for the second time in the Antarctic: the first was previously recorded in the Weddell Sea (Weis et al. 2011) as well as in New Zealand and Australia (Child 1998). The latter was only recorded before in Livingston Island (Munilla 2000). The new species N. nakamurai is the only Bellingshausen Sea endemic species and C. brevirostris the only Amundsen Sea endemic species.
Rarefaction curves (Fig. 6) provide a useful way to compare regional species richness between different areas. The results shown here are consistent with studies encompassing several pycnogonid data (Griffiths et al. 2011; Munilla and Soler-Membrives 2009). These results confirm that increasing sampling leads to increases in species richness (Griffiths et al. 2011). Taking into account the low sample size, the Bellingshausen Sea has the shallowest curve, indicating the paucity of species in this area. Among the zones studied, the Antarctic Peninsula area is the richest in species. Within this grouping, the South Shetland Islands (SSI) has the steepest slope and appears to be even richer than the West Antarctic Peninsula (WAP). These results are consistent with previous data that predict an asymptotic curve levelling off at around 35–50 species for WAP and >60 for the SSI (Griffiths et al. 2011). The figure also reveals that both the Bellingshausen and Amundsen seas are poorly sampled for pycnogonids. Therefore, it is probable that the number of pycnogonid species recorded from each of these areas will increase with further sampling. Thus, the circumpolar pattern may also become clearer.
Bathymetric patterns
The known bathymetric distribution of seven species has increased thanks to these cruises (Table 5): Achelia serratipalpis, Cilunculus acanthus, Nymphon inferum, N. microgracilipes, N. paucidens, Pantopipetta lata and Pentanymphon minutum.
Depth is the most important environmental factor accounting for the differences within and among some benthic taxa communities, such as Isopoda (Brandt et al. 2007) and Pycnogonida (San Vicente et al. 1997). Regarding bathymetric patterns, diversity indices are based on the grouping encountered by the bathymetric cluster analysis (Fig. 5). Concerning the cluster results, it must be acknowledged that the Amundsen Sea sample size is not large enough to accurately determine depth ranges. Considering that shelf break in these areas is found at approximately 600–700 m depth (Kaiser et al. 2009), a better explanation for grouping is as follows: (1) a shallow continental shelf group, composed of stations with depths shallower than 200 m (A group); most of these stations (8 out of 10) have Shannon-Wiener values H′ between 2 and 3.5, and only stations 5 and 6 present lower values, due to high abundances of N. australe and A. cornigera; (2) an outer continental shelf group (B and C groups without C1), composed of stations found between 200 and 700 m depth with H′ ranging from 1 to 2.5, with the exception of the deep stations 42 and 57 (1,272 m, H′ = 0.77 and 1,000, H′ = 0.68, respectively) and (3) a slope group, composed of those stations found at depths greater than 900 m (sub-cluster C1), with a similarity over 60 %. Slope stations present H′ values lower than 1. This bathymetric grouping also occurs generally throughout the Antarctic (Griffiths et al. 2011). The shallow continental shelf group (A group) is dominated by two of the three main families of Antarctic pycnogonids, the Ammotheidae and Colossendeidae, and 96 and 85 % of the sampled individuals of these families, respectively, belong to this group. Although the faunal composition of the continental shelf varies among different areas, the slope seems to be more homogeneous among the Antarctic zones, which are species-poor (H′ usually <1) and frequently dominated by a single genus or even a single species, in this case, the presence of the genus Nymphon (Soler-Membrives et al. 2009). In fact, the other main Antarctic pycnogonid family, the Nymphonidae—and its main genus Nymphon—is found at all depths according to its eurybathy (1–6,135 m depth), but it is also the main family found at deep sea sites, not only in the Southern Ocean waters but also worldwide (Arango et al. 2011).
Comparison to other Antarctic zones: ecological observations
To date, the geographic distribution pattern that best fits with Antarctic pycnogonids as well as for many other Antarctic fauna is the single functional unit or circumpolar model (Griffiths et al. 2009). Both seas analysed in this study fit with this pattern, and 25 from the 46 species listed in Table 5 follow this distribution. In fact, approximately 29 % of Antarctic pycnogonids are described as circumpolar (Munilla and Soler-Membrives 2009). The higher values found at the seas analysed here may be due to the still-limited nature of the sampling in these two areas.
Although differences in faunal composition observed in the clusters (see above) may be based on depth and there may not be a clear grouping by area, differences observed when analysing overall abundances and species richness align with geographic location. The comparative analysis among different Antarctic zones (Table 6) shows that the areas richest in terms of abundance are the Amundsen Sea, the Weddell Sea and the SW Scotia Sea (Shetland Is. + Drake Passage + Bransfield Strait) whose N/Sts range between 29.4 and 27.4, almost twice the global average (16.2). The last two zones have also been reported to be abundant in other studies (Griffiths et al. 2011). The Amundsen Sea is an optimal area for phytoplankton to bloom within coastal polynyas (Arrigo et al. 2012; Mills et al. 2012). A previous study showed that these Amundsen coastal ecosystems were the most productive of 37 identified coastal polynya systems in the Antarctic (Arrigo and van Dijken 2003). Therefore, the high phytoplankton productivity within the polynyas of the Amundsen Sea might be the reason for the high levels of benthic faunal abundances reported in the Amundsen Sea (Linse et al. 2013).
The less abundant areas are the Ross and Bellingshausen Seas (5 and 12.3, respectively). Within the Bellingshausen Sea, islands (Table 6) show similar species richness (S/Sts ≈ 1.3–1.8) to the open sea area, but abundance is much lower in the latter (N/Sts of 21 and 6 in the islands and open sea, respectively). Nutrient levels of the Bellingshausen Sea are low in comparison to most other parts of Antarctic waters, and corresponding to the paucity of nutrients, the primary production and phytoplankton biomass in the central part of the Bellingshausen Sea, which is reported to be poor (Ameneiro et al. 2012). Furthermore, low levels of dissolved iron are limited to phytoplankton production. These biogeographic differences among the Antarctic zones are fully consistent with the previous reports (Munilla and Soler-Membrives 2009) that showed a subdivision within the Antarctic into two branches, one including the Scotia Arc, the Weddell Sea and the East Zone and the other including the Bellingshausen and Ross Seas. It may also be that the Bellingshausen and Ross Seas showed lower abundances because the ice plate covers their continental shelf during most of the year, preventing photosynthesis. Other taxa, such as molluscs (Troncoso et al. 2007), cumaceans (Corbera et al. 2009) and hydroids (Peña Cantero 2012), also present lower abundances in the Bellingshausen Sea when compared to other Antarctic zones. These results agree with the species richness per station (mean S/St). The richest areas are the SW Scotia Sea (Shetland Islands, Drake Passage and Bransfield Strait) and the West Antarctic Peninsula. The SW Scotia Sea has previously been identified as the richest zone in the Antarctic, and this is related to the hypothesis of benthic insular refuge (Munilla and Soler-Membrives 2009) in which the numerous archipelagos of this area retain many fauna dragged by the circum-Antarctic Current. Moreover, species from islands (South Shetland Islands and Peter I Island) are clustered separately from those from the open Bellingshausen Sea and the Amundsen Sea. Therefore, species composition from islands seems to be different from that in open sea locations.
Conclusions
This is a novel report on the pycnogonid fauna from two relatively unknown Antarctic areas, the Bellingshausen and Amundsen Seas. Based on the study from three cruises (Bentart 2003 and 2006 and Biopearl II), two new species of pycnogonid were described (Heteronymphon krappi n.sp. and Nymphon nakamurai n.sp.), A. magniceps and A. hesperidensis were recorded for the second time in the Antarctic and the known bathymetric ranges of seven species were increased. The most species-rich families are the Ammotheidae and Nymphonidae, and N. australe is the most abundant species (25.5 %), which is consistent with the results from other Antarctic studies (Child 1995a; Munilla and Soler-Membrives 2009). The biogeographic analysis provided 39 species in the Bellingshausen Sea (16 new records) and 19 species in the Amundsen Sea (18 new records). The Bellingshausen Sea, especially the central part, seems to be poor in terms of abundance compared to the Amundsen Sea and other Antarctic zones, probably due to its low superficial primary production. Two main conclusions might be taken from the cluster similarity among sampling localities: first, the shallow group (stations less than 200 m depth, with moderately high diversity levels and characterized by the dominance of the Ammotheidae and Colossendeidae families) is different from the continental shelf and slope stations. Second, the slope stations (>900 m, grouped in a sub-cluster with an outstanding similarity and characterized by low levels of diversity and the dominance of the genus Nymphon) are a subset within the continental shelf stations.
References
Ameneiro J, Mouriño-Carballido B, Parapar J, Vázquez E (2012) Abundance and distribution of invertebrate larvae in the Bellingshausen Sea (West Antarctica). Polar Biol 35:1359–1373
Arango CP, Soler-Membrives A, Miller KJ (2011) Genetic differentiation in the circum—Antarctic sea spider Nymphon australe (Pycnogonida; Nymphonidae). Deep Sea Res II 58:212–219
Arntz WE, Gutt J, Klages M (1997) Antarctic marine biodiversity: an overview. In: Battaglia B, Valencia J, Walton DW (eds) Antarctic marine communities: species, structure and survival. Cambridge University Press, Massachussetts, pp 3–14
Arrigo KR, van Dijken GL (2003) Phytoplankton dynamics within 37 Antarctic coastal polynya systems. J Geophys Res Oceans 108:3271
Arrigo KR, Lowry KE, van Dijken GL (2012) Annual changes in sea ice and phytoplankton in polynyas of the Amundsen Sea, Antarctica. Deep Sea Res II 71–76:5–15
Bamber R (2011) The sea-spiders (Arthropoda: Pycnogonida) of Admiralty Bay, King George Island. Pol Polar Res 32:27–38
Barker PF, Burrell J (1977) The opening of Drake Passage. Mar Geol 25:15–34
Bouvier EL (1913) Pycnogonides du “Pourquoi-Pas?”. Deuxième expédition antarctique française (1908–1910) 6:1–169
Brandt A et al (2007) The biodiversity of the deep Southern Ocean benthos. Philos Trans R Soc B B Sci 362:39–66
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of Southern Wisconsin. Ecol Monogr 27:325–349
Cano E, López-González P (2013) Two new species of Ammothea (Pycnogonida, Ammotheidae) from Antarctic waters. Helgol Mar Res 67:337–347
Child CA (1995a) Antarctic and Subantarctic Pycnogonida III. The family Nymphonidae. Biology of Antarctic Seas XXIII. Antarct Res Ser 69:1–68
Child CA (1995b) Antarctic and Subantarctic Pycnogonida IV. The families Colossendeidae and Rhynchothoraxidae. Biology of Antarctic Seas XXIII. Antarct Res Ser 69:69–111
Child CA (1998) The Marine Fauna of New Zealand: pycnogonida (Sea Spiders). NIWA Biodivers Mem 109:1–71
Clarke A, Crame JA (1989) The origin of the Southern Ocean marine fauna. Geological Society, London, Special Publications 47:253–268
Clarke A, Johnston N (2003) Antarctic Marine Benthic Diversity. Oceanogr Mar Biol Annu Rev 41:47–114
Clarke KR, Warwick RM (2001) A further biodiversity index applicable to species lists: variation in taxonomic distinctness. Mar Ecol Prog Ser 216:265–278
Corbera J, San Vicente C, Sorbe JC (2009) Cumaceans (Crustacea) from the Bellingshausen Sea and off the western Antarctic Peninsula: a deep-water link with fauna of the surrounding oceans. Polar Biol 32:611–622
Dietz L, Krapp F, Hendrickx M, Arango C, Krabbe K, Spaak J, Leese F (2013) Evidence from morphological and genetic data confirms that Colossendeis tenera Hilton, 1943 (Arthropoda: Pycnogonida), does not belong to the Colossendeis megalonyx Hoek, 1881 complex. Org Divers Evol 13:151–162
Eastman JT, Grande L (1989) Evolution of the Antarctic fish fauna with emphasis on the recent notothenioids. Geol Soc Lond Spec Publ 47:241–252
Fry WG, Hedgpeth JW (1969) Pycnogonida, 1: colossendeidae, Pycnogonidae, Endeidae, Ammotheidae. Fauna of the Ross Sea, 7. NZ Oceanogr Inst Mem 198:1–139
Gili JM et al (2006) A unique assemblage of epibenthic sessile suspension feeders with archaic features in the high-Antarctic. Deep Sea Res II 53:1029–1052
Giltay L (1935) Pycnogonides. In: Résultats du voyage de la Belgica en 1897–1899 (Expédition antarctique belge). Rapports scientifiques. Bruxelles, pp 1–16
Gordon I (1932) Re-description of some type-specimens of pycnogonids of the genus Nymphon. Ann Mag Nat History (10) 9:97–120
Griffiths HJ (2010) Antarctic marine biodiversity—what do we know about the distribution of life in the Southern Ocean? PLoS ONE 5:e11683
Griffiths HJ, Barnes DKA, Linse K (2009) Towards a generalized biogeography of the Southern Ocean benthos. J Biogeogr 36:162–177
Griffiths HJ, Arango CP, Munilla T, McInnes SJ (2011) Biodiversity and biogeography of Southern Ocean pycnogonids. Ecography 34:616–627
Hedgpeth JW (1969) Pycnogonida. In: Distribution of selected groups of marine invertebrates in waters south of 35°S latitude. Antarctic Map Folio Series, Folio 11:26–28
Kaiser S, Barnes DA, Sands C, Brandt A (2009) Biodiversity of an unknown Antarctic Sea: assessing isopod richness and abundance in the first benthic survey of the Amundsen continental shelf. Mar Biodivers 39:27–43
Krabbe K, Leese F, Mayer C, Tollrian R, Held C (2010) Cryptic mitochondrial lineages in the widespread pycnogonid Colossendeis megalonyx Hoek, 1881 from Antarctic and Subantarctic waters. Polar Biol 33:281–292
Linse K, Griffiths HJ, Barnes DKA, Clarke A (2006) Biodiversity and biogeography of Antarctic and sub-Antarctic mollusca. Deep Sea Res II 53:985–1008
Linse K et al (2013) The macro- and megabenthic fauna on the continental shelf of the eastern Amundsen Sea, Antarctica. Cont Shelf Res 68:80–90
Livermore R, Nankivell A, Eagles G, Morris P (2005) Paleogene opening of Drake Passage. Earth Planet Sci Lett 236:459–470
Mahon AR, Arango CP, Halanych KM (2008) Genetic diversity of Nymphon (Arthropoda: Pycnogonida: Nymphonidae) along the Antarctic Peninsula with a focus on Nymphon australe Hodgson 1902. Mar Biol 155:315–323
Matallanas J, Olaso I (2007) Fishes of the Bellingshausen Sea and Peter I Island. Polar Biol 30:333–341
Mills MM, Alderkamp AC, Thuróczy CE, van Dijken GL, Laan P, de Baar HJW, Arrigo KR (2012) Phytoplankton biomass and pigment responses to Fe amendments in the Pine Island and Amundsen polynyas. Deep Sea Res II 71–76:61–76
Müller HG (1993) World catalogue and bibliography of the recent Pycnogonida. Wissenschaftlicher Verlag HG, Wetzlar
Munilla T (2000) A new species of Ammothea (Pycnogonida) and other pycnogonids from Livingston Island and surrounding waters (South Shetland Islands, Antarctica). Antarct Sci 13:144–149
Munilla T (2001) Synopsis of the pycnogonids from Antarctic and Subantarctic waters. Polar Biol 24:941–945
Munilla T, Soler-Membrives A (2009) Check-list of the pycnogonids from Antarctic and sub-Antarctic waters: zoogeographic implications. Antarct Sci 21:99–111
Nakamura K (1985) A new species of pycnogonid, Heteronymphon horikoshii, from the waters adjacent to Honshu, Japan. Bull Biogeogr Soc Japan 40:31–34
Nielsen J, Lavery S, Lörz A-N (2009) Synopsis of a new collection of sea spiders (Arthropoda: Pycnogonida) from the Ross Sea, Antarctica. Polar Biol 32:1147–1155
Peña Cantero Á (2012) Filling biodiversity gaps: benthic hydroids from the Bellingshausen Sea (Antarctica). Polar Biol 35:851–865
Pushkin AF (1993) The Pycnogonida fauna of the South Ocean. Biological results of the Soviet Antarctic Expeditions. Explorations of the fauna of the seas XX (XXX). Biol Res Sov Antarct Exped 8:1–398
San Vicente C, Ramos A, Jimeno A, Sorbe JC (1997) Suprabenthic assemblages from South Shetland Islands and Bransfield Strait (Antarctica): preliminary observations on faunistical composition, bathymetric and near-bottom distribution. Polar Biol 18:415–422
San Vicente C, Munilla T, Corbera J, Sorbe J, Ramos A (2009) Suprabenthic fauna from the Bellingshausen Sea and western Antarctic Peninsula: spatial distribution and community structure. Sci Mar 73:357–368
Soler-Membrives A, Turpaeva E, Munilla T (2009) Pycnogonids of the Eastern Weddell Sea (Antarctica), with remarks on their bathymetric distribution. Polar Biol 32:1389–1397
Thatje S, Arntz W (2004) Antarctic reptant decapods: more than a myth? Polar Biol 27:195–201
Troncoso JS, Aldea C, Arnaud P, Ramos A, García F (2007) Quantitative analysis of soft-bottom molluscs in the Bellingshausen Sea and around Peter I Island. Polar Res 26:126–134
Turpaeva EP, Yampolsky AD (1992) Variability of the eurybathic pycnogonid, Heteronymphon profundum (Pycnogonida, Nymphonidae) from the north-western Pacific Ocean. Zool Zh 71:32–38
Weis A, Friedrich S, Melzer RR (2011) Antarctic Pycnogonida housed at the Bavarian State Collection of Zoology. Zoosyst Evol 87:297–317
Acknowledgments
The BENTART cruises were performed under the auspices of two Spanish Ministry of Science and Technology (MCYT) Antarctic programs (REN2001-1074/ANT and CGL2004-01856). We express our gratitude to the head of campaign Ana Ramos, to the officers and crew of the RV Hespérides and to our colleagues from the BENTART cruises in 2003 and 2006 for their help in this paper. Likewise, we are grateful to Dr. Barnes (BAS) who kindly provided us samples from the British cruise, to Drs. K. Linse and P. Enderlein for organizing and running the BIOPEARL II cruise and to the officers and crew of RRS James Clark Ross, whose patience, accuracy and timing have made this work possible. We also acknowledge the British Antarctic Survey and the Natural Environment Research Council for funding the British expedition. We thank the reviewers for their valuable comments which helped to considerably improve the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Munilla, T., Soler-Membrives, A. Pycnogonida from the Bellingshausen and Amundsen seas: taxonomy and biodiversity. Polar Biol 38, 413–430 (2015). https://doi.org/10.1007/s00300-014-1585-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-014-1585-8