Abstract
Although scientific knowledge about the biodiversity of Antarctic benthic hydrozoans has considerably increased in recent years, little is known about their spatial distribution and underpinning factors. Trying to contribute to filling this gap, benthic hydroid spatial distribution in the Bellingshausen Sea (Southern Ocean) was studied. Samples were collected at 32 stations at depths between 86 and 3,304 m during Spanish Antarctic expeditions in 2003 and 2006. Sediments and bottom water properties were analyzed using an USNEL-type box corer and a Neil Brown Instrument System Mark III CTD, respectively. Forty species were reported (Acryptolaria sp., Stegopoma plicatile, Staurotheca dichotoma having the highest percentages of occurrence), representing ca. 19 % of the species richness of the known benthic hydroid fauna of the Southern Ocean. Three well-defined assemblages (shallow, deep and transitional) were established based on significant differences in species occurrence. Benthic hydroid spatial distribution in the Bellingshausen Sea seems to be controlled mainly by depth and substrate (most hydrozoan species are epibiotic), by species dispersal abilities and by species resilience to changing hydrodynamic conditions. The level of species richness found in the present study, compared with other Antarctic areas, gives support to arguments stated by authors against the idea that the Bellingshausen Sea is “a benthos desert” controlled by oligotrophic conditions and intense iceberg traffic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Bellingshausen Sea constitutes the third largest Antarctic sea. It also has significant zoogeographical importance for the dispersal of species along both sides of the Antarctic Peninsula, through waters around the Antarctic continent and along the Scotia Arc (Saiz-Salinas et al. 2008). Yet the Bellingshausen Sea has been widely recognized as one of the lesser studied areas of the Southern Ocean (Clarke and Johnston 2003).
With the aim of improving the limited scientific knowledge on benthos inhabiting its shelf and deep-sea communities, a sampling program of sea bottoms of the Bellingshausen Sea (including Peter I Island) was carried out during the Bentart 2003 and Bentart 2006 Spanish Antarctic expeditions with BIO Hespérides in 2003 and 2006.
As for hydrozoans, only three papers had dealt with benthic hydroids collected in the Bellingshausen Sea proper before the Bentart surveys (Hartlaub 1904; Broch 1948; Blanco and Bellusci de Miralles 1972).
Two recent papers (Peña Cantero 2010, 2012) reviewed data from those three articles and provided results from faunistic studies of benthic hydroids collected during the Bentart surveys. A total of 27 species was found in the Bellingshausen Sea (including two new species and 21 new records for the area) and 23 species (including a new species and 14 new records) in samples obtained from Peter I Island.
Based on all distribution data collected during previous surveys as well as in the literature, the present study aims to understand the biogeographical and ecological factors that control spatial distribution of benthic hydroids in the Bellingshausen Sea. Hydroid records were analyzed with regards to environmental variables and biocoenosis data investigated during the Bentart surveys and supported by several studies for other zoological groups, such as crustaceans (García Raso et al. 2005, 2008; San Vicente et al. 2009), fishes (Matallanas and Olaso 2007; Eakin et al. 2008), sponges (Ríos and Cristobo 2007), mollusks (Troncoso et al. 2007; Troncoso and Aldea 2008; Aldea et al. 2008, 2009), ascidians (Varela and Ramos-Esplá 2008) and echinoderms (O’Loughlin et al. 2009; Moya et al. 2012) as well as macrobenthos general studies (Saiz-Salinas et al. 2008).
Materials and methods
Study area
The Bellingshausen Sea is considered to be the part of the Southern Ocean located between Thurston Island to the west and Marguerite Bay to the east (from 70° to 100°W and from the coast to 68°S), as adopted by other authors (e.g., Fairbridge 1966; Turner and Owens 1995; Grotov et al. 1998). It is one of the less investigated areas of the Southern Ocean, mainly because of its remoteness and ice prevalence during most of the year (Fairbridge 1966; Turner and Owens 1995; Grotov et al. 1998; Clarke and Johnston 2003).
Sedimentological and geomorphological characteristics are consequences of glacier dynamics, their effects on the ice layer and the activity of icebergs, which have plowed deep grooves on the bottom (Starmans et al. 1999; Gutt 2000; O´Cofaigh et al. 2005).
Oceanographic features are the result of the combined effects of shallow and deep water masses (Klinck et al. 2004), with water temperatures varying from −1.8 to 0.3 °C (Stambler 2003).
Field sampling
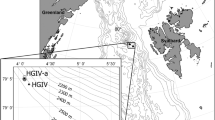
Sampling was carried out during BENTART expeditions (Spanish Antarctic Research Program) with the BIO Hespérides from January to March 2003 and from January to February 2006. Samples were collected at 32 stations, at depths between 86 and 3,304 m (Fig. 1; Table 1, ESM), using an Agassiz trawl with horizontal and vertical openings of 2.01 and 1.12 m, respectively, and a 10-mm mesh size (Ramos 1995; Arnaud et al. 1998). A USNEL-type box corer with a maximum breakthrough of 60 cm and an effective sampling area of 0.25 m2 (Saiz-Salinas et al. 2008) was used for infaunal organisms and sediments. Suprabenthic samples were collected with a modified Macer-GIRO Q sledge (Carter and Hunter 1994). This sledge was equipped with three superimposed nets (0.5-mm mesh size). Hydrographic casts were made with a Neil Brown Instrument System Mark III CTD. Hydroids were fixed in 70 % ethanol. For faunistic results, see Peña Cantero (2010, 2012).
Data analyses
A majority of species in the samples form stalked or bushy colonies. However, many colonies came on board fragmented. This makes abundance studies very inaccurate since it is too difficult, or even impossible, to estimate the exact number of colonies collected. Accordingly, no quantitative analysis was performed in the present study.
Hydroid species were classified according to their frequencies across samples, a surrogate for evaluating their importance in the community. Based on the percentage frequency of occurrence across all stations, four categories were recognized: ubiquitous species (>30 % of stations), very common species (between 30 and 20 %), common species (between 20 and 10 %), and rare or accidental species (<10 %) (Mora 1980; Manjón-Cabeza and García Raso 1994; Manjón-Cabeza and Ramos 2003; Moya et al. 2012). To investigate the structure of hydroid assemblages, similarities between samples were computed by a hierarchical cluster analysis using the UPGMA agglomerative algorithm (Sneath and Sokal 1973; RMACOQUI ver. 1.0 software Olivero et al. 2011, 2013). It was based on the similarity matrix of the Baroni-Urbani and Jaccard coefficients, calculated from presence/absence data (Jaccard 1901; Baroni-Urbani and Buser 1976; Real and Vargas 1996; Real 1999). Robustness of each cluster was estimated with a test of biological significance performed on boundaries between hydroid assemblages (McCoy et al. 1986). Strong and weak boundaries were defined between assemblages following McCoy et al. (1986), in both cases for a p < 0.05 (Olivero et al. 1998). Stations were plotted using a detrended correspondence analysis and a canonical correspondence analysis (CCA) computed from the presence/absence matrix and based on the eigenvalues of χ 2 distances between all data points (Ter Braak and Prentice 1988; Hennebert and Lees 1991; Legendre and Legendre 1998), using the PAST-paleontological statistics computer program, version 1.181 (Hammer et al. 2001). The CCA was performed using the environmental variables that best matched the hydroid assemblages identified in the cluster analysis (those stations without environmental data were not included in this analysis). They were used to define ordination axes on which hydroid data (with both stations and specimens) were plotted. Environmental variables were plotted as well as correlations with ordination axes.
The ArcGIS v10.2 program and GEBCO Digital Atlas v2.12 were used for mapping and digital support.
Results
Hydroids were present in 18 of the 32 stations (Fig. 1). In total, 85 records, representing 40 species within 11 families and 2 orders, were assembled (Table 1, ESM). Some taxa (Eudendrium sp. 1, Eudendrium sp. 2, Eudendrium sp. 3, Anthoathecate sp. and Acryptolaria sp.) could not be identified to species (see Peña Cantero 2010, 2012).
Of the 40 species recorded, three (Acryptolaria sp., Stegopoma plicatile, Staurotheca dichotoma) were classified as ubiquitous (>30 %), five (Stegella lobata, Symplectoscyphus cumberlandicus, Halecium frigidum, Antarctoscyphus spiralis, Symplectoscyphus glacialis) as very common (between 30 and 20 %) and eight (Symplectoscyphus curvatus, Schizotricha vervoorti, Billardia subrufa, Halecium pallens, Filellum antarcticum, Filellum magnificum, Lafoea dumosa, Halecium delicatulum) as common (between 20 and 10 %). The rest were taken to be rare or accidental (<10 %) (cf. Table 1, ESM).
Species richness by station is found in Table 1, ESM. Peter I stations contained 57.5 % of the total number of species, whereas the Bellingshausen stations accounted for 67.5 % (MB34 being the richest station).
Results from cluster and correspondence analyses (Figs. 2, 3) show significant differences (Jaccard and Baroni index p < 0.05) between three well-defined assemblages, called S (shallow), D (deep) and SH, based on species occurrence (Figs. 1b, 4). The assemblages were separated by strong boundaries in all cases. In addition, within the S assemblage there was a clear partitioning between deeper (S1) and shallower stations (S2) (Figs. 2c, 3, 4b).
a Canonical correspondence analysis (CCA) (eigenvalues λ 1 = 0.84; p < 0.05 and λ2 = 0.54; p = 0.5 with 10,000-replicate permutation test). b Correspondence analysis with groups identified as defined by the hierarchical cluster analysis (eigenvalues shown in the text). D: Depth (m); OD: organic deposit (%); G: gravel (%); S: sand (%); M: mud (%)
Composition and occurrence percentage of hydroid assemblages as defined by hierarchical cluster analysis. a Three main assemblages. b Only shallow-water assemblages. Abbreviations are referred to in the text. Colors represent different substrates for the species: green (star) ascidians; blue (plus) bryozoans; pink (square) dead organisms; black other hydroids. (Color figure online)
Species composition of hydroid assemblages is structured as follows (B: assemblage from the Baroni index; J: assemblage from the Jaccard index; MB: Bellingshausen Sea stations; PI: Peter I stations):
-
SH: MB3. Deep-water station with mixed bottom, distinguished by only one species, Symplectoscyphus hesperides, which is absent from any of the other stations.
-
B4; J5 (except MB26 and MB12): PI27, PI28, MB30 (D). Assemblage formed by some of the deepest stations, with sandy muds, characterized by low species richness values and high occurrence of A. spiralis.
-
B3: MB26. Deep-water station with sandy bottom, defined by four species absent from the rest (Bouillonia denhartogi, Antarctoscyphus elongatus, Symplectoscyphus exochus, Symplectoscyphus plectilis) and a single shared species, A. spiralis.
-
J4: MB12. Deepest, mono-specific station (S. curvatus), with sandy muds.
-
B1; J1, J2 (S1): MB4, MB14, MB11, MB13, MB37, MB34. Assemblage dominated by S. plicatile, which was present in 100 % of the stations. The stations are characterized by mixed bottoms with a relatively high presence of gravel (apart from MB34) located at shallower depths.
-
B2; J3: PI5, PI8, PI6, PI7, MB9, MB10 (S2). Assemblage found on sandy muds at shallower depths and mainly characterized by S. lobata, S. cumberlandicus (83.33 %) and the presence of S. glacialis (66.67 %).
Results from the CCA (Fig. 3a) show that the sediment granulometry (mud, sand and gravel percentages), organic deposit percentage and depth are the five variables that best explain the structure of the hydroid assemblages. The two first axes (eigenvalues λ 1 = 0.84, p < 0.05; λ 2 = 0.54, p = 0.5 with 10,000-replicate permutation test) show a very good match, except for the organic deposit percentage, which does not seem to have a great influence on the assemblages. Depth appears to be the main factor controlling division of the hydroid assemblages into the deep-sea (D), continental slope (S1) and shallowest stations (S2) (Figs. 1b, 3).
Species were plotted in the same graphic with the aim of depicting their environmental preferences. The main distinction can be made between species exclusive to the deep basin (S. hesperides, S. exochus, S. plectilis, A. spiralis, A. elongatus, B. denhartogi, Sertularella sanmatiasensis) and the rest from the continental slope or from the shallowest stations. The only species present in both environments is S. curvatus, although it is absent at the shallowest stations.
Discussion
Our study summarizes information on the benthic hydroid fauna from the Bellingshausen Sea, as reported by Peña Cantero (2010, 2012, with Peter I samples included). Forty species were reported, with 3 of them new to science and 14 others constituting new records for the area. They represent about 19 % of the species richness of the 209 known Antarctic benthic hydroids. This important level of species richness, compared with other Antarctic areas, lends support to arguments stated by some authors (cf. Moya et al. 2012) against the idea that the Bellingshausen Sea is “a benthos desert” (Saiz-Salinas et al. 2008; San Vicente et al. 2009) controlled by oligotrophic conditions and intense iceberg traffic (Peck et al. 1999; Gutt 2000).
Such species richness is unexpected considering the substrate dependence of hydroids (Calder 1991; Gili et al. 2008; Ronowicz et al. 2008), the type of bottoms present in the area studied (mostly muddy and sandy bottoms) and the scarcity of dense and rich communities of large filter feeders in the Bellingshausen Sea (Ramil et al. 2006a, b) compared to other Antarctic areas such as the Weddell and Ross seas and closer areas (e.g., Ramos 1987, 1995; Luján and Ramos-Esplá 1996a, b; Saiz-Salinas et al. 1997; Ramil and Ramos 1997; Manjón-Cabeza et al. 2001; Ramos and Moya 2003, Manjón-Cabeza and Ramos 2003; Ramos and Moya 2003).
In addition to depth, hydrozoan assemblages of the Bellingshausen Sea seem to be determined by substrate type (see Peña Cantero 2010 for a discussion about the substrata on which the species live), species dispersal abilities and species resilience to changing hydrodynamic conditions (Calder 1991; Gili et al. 2008; Ronowicz et al. 2008).
Two well-structured hydroid assemblages were recognized in the study area. The first one (D) was found in deep-water stations (<−1N500 m) of the slope with bottoms of sand and sandy mud. It was dominated by A. spiralis, which had not been found before at such depths (cf. Peña Cantero 2012). This species is usually collected in shallower areas, from 6 to 720 m depth, as is also the case for the other species of the assemblage. They are circumpolar eurybathic species, capable of being widely transported by currents. In the Bellingshausen Sea, however, they are only present at the deepest stations.
The second assemblage (S) occurs on bottoms at intermediate depths and encompasses two minor groups of species, S1 and S2.
S1 contains species with a particular preference for mixed bottoms with an abundance of gravel at the edge of the Continental Shelf, perhaps because of the hydrodynamic conditions. The dominant species was S. plicatile. The assemblage is also characterized by the presence of an endemic species, Symplectoscyphus bellingshauseni, not shared with any other assemblage.
Stegopoma plicatile is a species widely distributed all around the Antarctic continent, but also outside Antarctic waters. It is an epibiotic species that typically grows on other hydroids such as species of the genera Eudendrium and Halecium.
In general, this assemblage is an association of a small number of species that are epibiotic in habit. This increases their substrate availability and enhances their survival chances on soft bottoms having a paucity of filter feeders, as is characteristic of the Bellingshausen Sea.
S2 embraces shallower stations located on muddy bottoms off Peter I Island and on sandy muds on the Antarctic Continental Shelf. It comprises the richest species assemblage, despite the a priori not suitable type of bottom, with a more balanced dominance shared by three species, S. dichotoma, S. lobata and S. cumberlandicus. Most species are epibiotic, occurring on bryozoans (López-Fé 2005) and large filter feeders such as desmosponges, hexactinellids and ascidians, like Cnemidocarpa verrucosa, which is very abundant at these stations, and particularly at Peter I Island (Ramos and Moya 2003; Primo and Vazquez 2009).
Conclusion
Hydroid assemblages from the Bellingshausen Sea show a structure similar to that found for other zoological groups, such as echinoids, fishes or mollusks (Matallanas and Olaso 2007; Aldea et al. 2008; Troncoso and Aldea 2008; San Vicente et al. 2009; Moya et al. 2012).
As in other taxocoenosis, depth is the factor best explaining both the structure of the hydroid assemblages and the species dominance.
Other abiotic factors, such as sediment features, also contribute to determining these associations, which are dependent on biotic parameters, too, particularly substrate dependency, enhancing their chance of survival on soft bottoms with a paucity of filter feeders (desmosponges, hexactinellids and ascidians), as is characteristic of the Bellingshausen Sea.
References
Aldea C, Troncoso JS (2008) Systematics and distribution of shelled mollusks (Gastropoda, Bivalvia and Scaphopoda) from the South Shetland Island to the Bellingshausen Sea, West Antarctica. Iberus 26:43–117
Aldea C, Olabarria C, Toncoso JS (2009) Spatial patterns of benthic diversity in molluscs from West Antarctica. Antarct Sci 21:341–353
Arnaud PM, López CM, Olaso I, Ramil F, Ramos-Esplá AA, Ramos A (1998) Semi-quantitative study of macrobenthic fauna in the region of the South Shetland islands and the Antarctic Peninsula. Polar Biol 19:160–166
Baroni-Urbani C, Buser MW (1976) Similarity of binary data. Syst Zool 25:251–259
Blanco OM, Bellusci de Miralles DA (1972) Hidrozoos de la isla Pedro I. Contrib Inst Antárt Argent 145:1–41
Broch H (1948) Antarctic hydroids. Sci Res Nor Antarct Exped 1927–1928 28:1–23
Calder DR (1991) Associations between hydroid species assemblages and substrate types in the mangal at Twin Cays, Belize. Can J Zool 69:2067–2074
Carter SJB, Hunter JR (1994) The physical oceanographic effects of jarosite dumping at sea. C.S.I.R.O. Division of Oceanography Report, OMR-66/42
Clarke A, Johnston NM (2003) Antarctic marine benthic diversity. Oceanogr Mar Biol 41:47–114
Eakin RR, Eastman JT, Matallanas J (2008) New species of Pogonophryne (Pisces, Artedidraconidae) from the Bellingshausen Sea, Antarctica. Polar Biol 31:1175–1179
Fairbridge RW (1966) The encyclopaedia of oceanography. Reinhold, New York
García Raso JE, Manjón-Cabeza ME, Ramos A, Olaso I (2005) New record of Lithodidae (Crustacea Decapoda, Anomura) from the Antarctic (Bellingshausen Sea). Polar Biol 28:642–646
García Raso JE, García Muñoz JE, Manjón-Cabeza ME (2008) First record of Munidopsis albatrossae (Crustacea: Decapoda: Galatheidae) from Antarctic waters. Polar Biol 31:1281–1285
Gili JM, Orejas C, Robson E (2008) Hydrozoan biology: the view from a workshop. J Mar Biol Assoc UK 88:1515–1517
Grotov AS, Nechaev DA, Panteleev GG (1998) Large-scale circulation in the Bellingshausen and Amundsen seas as a variational inverse of climatological data. J Geophys Res 103:13011–13022
Gutt J (2000) Some “driving forces” structuring communities of the sublittoral Antarctic macrobenthos. Antarct Sci 72:297–373
Hammer O, Harper DAT, Ryan PD (2001) PAST version 1.39: paleoontological statistical software package for education and data analysis. Paleontol Eletronica 4:9
Hartlaub C (1904) Hydroiden. Result voyage S.Y. Belgica 1897–1899 (Zool) 7:1–19, pls 1–4
Hennebert M, Lees A (1991) Environmental gradients in carbonate sediments and rocks detected by correspondence analysis: examples from the Recent of Norway and the Dinantian of southwest England. Sedimentology 38:623–642
Jaccard P (1901) Étude comparative de la distribution florale dans une portion des Alpes et des Jura. Mém Soc Vaud Sci Nat 37:547–579
Klinck JM, Hofmann EE, Beardsley RC, Salihoglu B, Howard S (2004) Water mass properties and circulation on the west Antarctic Peninsula continental shelf in austral fall and winter 2001. Deep Sea Res II 51:1925–1946
Legendre P, Legendre L (1998) Numerical ecology, 2nd edn. Elsevier, Amsterdam
López-Fé CM (2005) Cheilostomate Bryozoa of the Bellingshausen Sea (Western Antarctica): a preliminary report of the results of the ‘Bentart 2003’ Spanish expedition. In: Moyano HI, Cancino JM, Wyse J, Patrick N (eds) Bryozoan studies 2004. Taylor and Francis Group, London, pp 173–179
Luján l, Ramos-Esplá AA (1996a) Afinidad por el substrato en diferentes familias de ascidias recolectadas en las campañas españolas BENTART-94 y BENTART-95. VI Simp Esp Est Antárt Madrid
Luján I, Ramos-Esplá AA (1996b) Revisión de Synoicum adareanarum Herdman (Polyclinidae, Ascidiacea), ejemplares recolectados en la campaña BENTART-94. VI Simp Esp Est Antárt Madrid
Manjón-Cabeza ME, García Raso JE (1994) Structure and evolution of a decapod crustacean community from the coastal detritic bottoms of Barbate (Cádiz, Southern Spain). J Nat Hist 32:1619–1630
Manjón-Cabeza ME, Ramos A (2003) Ophiuroid community structure of the South Shetlands Islands and Antarctic Peninsula region. Polar Biol 26:691–699
Manjón-Cabeza ME, Lirio Y, Ramos A (2001) Distribution of asteroid genera (Echinodermata) off the South Shetland Islands and the Antarctic Peninsula. Bol Inst Esp Oceanogr 17:1–13
Matallanas J, Olaso I (2007) Fishes of the Bellingshausen Sea and Peter I Island. Polar Biol 30:333–341
McCoy ED, Bell SS, Walters K (1986) Identifying biotic boundaries along environmental gradients. Ecology 68:749–759
Mora J (1980) Poblaciones bénticas de la Ría de Arousa. Dissertation, Universidad de Santiago de Compostela
Moya F, Saucède T, Manjón-Cabeza ME (2012) Environmental control on the structure of echinoid assemblages in the Bellingshausen Sea (Antarctica). Polar Biol 35:1343–1357
O´Cofaigh C, Dowdeswell JA (2005) Flow dynamics and till genesis associated with a marine-based Antarctic palaeo-ice stream. Quat Sci Rev 24:709–740
O’Loughlin PM, Manjón-Cabeza ME, Moya Ruiz F (2009) Antarctic holothuroids from the Bellingshausen Sea, with descriptions of new species (Echinodermata: Holothuroidea). Zootaxa 2016:1–16
Olivero J, Real R, Vargas J (1998) Distribution of breeding, wintering, and resident waterbirds in Europe: biotic regions and macroclimate. Ornis Fenn 75:153–175
Olivero J, Real R, Marquez A (2011) Fuzzy chorotypes as a conceptual tool to improve insight into biogeographic patterns. Syst Biol 60:645–660
Olivero J, Marquez A, Real R (2013) Integrating fuzzy logic and statistics to improve the reliable delimitation of biogeographic regions and transition zones. Syst Biol 62:1–21
Peck LS, Brockington S, Vanhove S, Beghyn M (1999) Community recovery following catastrophic iceberg impacts in a soft-sediment shallow-water site at Signy Island, Antarctica. Mar Ecol Prog Ser 186:1–8
Peña Cantero AL (2010) Benthic hydroids (Cnidaria: Hydrozoa) from Peter I Island (Southern Ocean, Antarctica). Polar Biol 33:761–773
Peña Cantero AL (2012) Filling biodiversity gaps: benthic hydroids from the Bellingshausen Sea (Antarctica). Polar Biol 35:851–865
Primo C, Vazquez E (2009) Antarctic ascidians: an isolated and homogeneous fauna. Polar Res 28:403–414
Ramil F, Ramos A (1997) Estudio de la fauna bentónica asociada a los edificios volcánicos de la cuenca central del Estrecho de Brandsfield. In: Vericad JR, Cacho J (eds) Informe sobre las actividades científicas de España en la Antártida durante la Campaña 1996–1997. CICYT, Madrid, pp 21–30
Ramil F, Ramos-Esplá AA, Ramos A, Lopez Fé CM, Gambi MA, Mutschke E, Moya F, Rios P (2006a) Resultados preliminares de la Campaña Bentart 2006 Epifauna macrobentónica del Mar de Bellingshausen y Península Antártica. XIV SIEBM Simp Ibérico Est Biol Mar Barcelona
Ramil F, Arnaud PM, Castro JM, Gambi MC, López-Fé CM, Moya F, Mutschke E, Olaso I, Manjón M, Parapar J, Peña A, Ramos-Esplá AA, Ríos P, Ramos A (2006b) La epifauna de la Antártida del Oeste: Una visión global basada en las campañas Bentart. VII Simp Esp Est Polares Granada
Ramos A (1987) Informe de Resultados “Antártida 8611”. Invertebrados. Inf Inst Esp Oceanogr MAPA, SGMP, 23 pp
Ramos A (1995) Informe de la Campaña BENTART95. Inf Inst Esp Oceanogr MAPA, SPM, 23 pp
Ramos A, Moya F (2003) Informe de la Campaña BENTART-2003 Inf Inst Esp Oceanogr Málaga, 189 pp
Real R (1999) Tables of significant values of Jaccard’s index similarity. Misc Zool 220:29–40
Real R, Vargas JM (1996) The probabilistic bases of Jaccard’s index of similarity. Syst Biol 45:380–385
Ríos P, Cristobo J (2007) A new species of Phorbas (Porifera: Poecilosclerida) from the Bellingshausen Sea, Antarctica. J Mar Biol Assoc UK 87:1485–1490
Ronowicz M, Wlodarska-Kowalczuk M, Kuklinski P (2008) Factors influencing hydroids (Cnidaria: Hydrozoa) biodiversity and distribution in Arctic kelp forest. J Mar Biol Assoc UK 88:1567–1575
Saiz-Salinas JI, Ramos A, García FJ, Troncoso JS, San Martín G, Sanz C, Palacín C (1997) Quantitative analysis of macrobenthic soft-bottom assemblages in South Shetland waters. Polar Biol 17:393–400
Saiz-Salinas JI, García FJ, Manjón-Cabeza ME, Parapar-Vegas J, Peña-Cantero A, Saucède T, Troncoso JS, Ramos A (2008) Community structure and spatial distribution of benthic fauna in the Bellingshausen Sea (West Antarctica). Polar Biol 31:735–743
San Vicente C, Munilla T, Corbera J, Sorbe J-C, Ramos A (2009) Suprabenthic fauna from the Bellingshausen Sea and western Antarctic Peninsula: spatial distribution and community structure. Sci Mar 73:357–368
Sneath PHA, Sokal RR (1973) Numerical taxonomy. The principles and practices of numerical classification. Freeman, San Francisco
Stambler N (2003) Primary production, light absorption and quantum yields of phytoplankton from the Bellingshausen and Amundsen Seas (Antarctica). Polar Biol 26:438–451
Starmans A, Gutt J, Arntz WE (1999) Mega-epibenthic communities in Arctic and Antarctic shelf areas. Mar Biol 135:269–280
Ter Braak CJF, Prentice CI (1988) A theory of gradient analysis. Adv Ecol Res 18:271–317
Troncoso JS, Aldea C (2008) Macrobenthic mollusc assemblages and diversity in West Antarctica from the South Shetland Islands to the Bellingshausen Sea. Polar Biol 31:1253–1265
Troncoso JS, Aldea C, García FJ, Arnaud PM, Ramos A (2007) Quantitative analysis of soft bottom Molluscs in Bellingshausen Sea and around Peter I Island. Polar Res 26:126–134
Turner DR, Owens NJP (1995) A biogeochemical study in the Bellingshausen Sea: overview of the STERNA 1992 expedition. Deep Sea Res II 42:907–932
Varela MM, Ramos-Esplá A (2008) Didemnum bentarti (Chordata: Tunicata) a new species from the Bellingshausen Sea, Antarctica. Polar Biol 31:209–213
Acknowledgments
The ‘BENTART-03’ cruise was included within the Project REN2003-01881/ANT supported by the Spanish MCYT funds. The ‘BENTART-06’ cruise was funded by the Antarctic Program CGL2004-21066-E of the Spanish Government. The faunal studies were supported by the Spanish MEC, MCYT and MCI and the European Regional Development Fund (ERDF) funds coming from the projects: REN2001-1074/ANT, REN2003—01881/ANT, GLC2004-01856/ANT, CGL2004-04684/ANT and CTM2009-11128ANT. We would like to express our thanks to the crew and UTM technicians of the RV ‘Hespérides’ for their help in collecting samples, to Dr. Calder for his proposals and help with the English language, and Sanjay Giany, native English teacher, for the revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
300_2014_1557_MOESM1_ESM.xls
Taxa occurrence and environmental variables per station registered during BENTART 2003-2006 cruise. O%: Occurrence; S(%): Species richness; Lat: Latitude (°); Lon: Longitude (°); D: Depth (m); OD: Organic deposit (%); G: Gravel (%); S: Sand (%); M: Mud (%); R: Redox; T: Temperature (ºC); Sl: Salinity (ups); O2: Oxygen (mmol/L) (XLS 42 kb)
Rights and permissions
About this article
Cite this article
Peña Cantero, Á.L., Manjón-Cabeza, M.E. Hydroid assemblages from the Bellingshausen Sea (Antarctica): environmental factors behind their spatial distribution. Polar Biol 37, 1733–1740 (2014). https://doi.org/10.1007/s00300-014-1557-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-014-1557-z