Abstract
Movement patterns of highly mobile animals can reveal life history strategies and ecological relationships. We hypothesized that wolves (Canis lupus) would display similar movement patterns as their prey, barren-ground caribou (Rangifer tarandus groenlandicus), and that movements of the two species would co-vary with season. We tested for interspecific movement dynamics using animal locations from wolves and caribou monitored concurrently from mid-October to June, across the Northwest Territories and Nunavut, Canada. We used a correlated random walk as a null model to test for pattern in movements and the bearing procedure to detect whether movements were consistently directional. There was a statistical difference between the movements of caribou and wolves (F 1,9 = 13.232, P = 0.005), when compared to a correlated random walk, and a significant interaction effect between season and species (F 1,9 = 6.815, P = 0.028). During winter, the movements of caribou were strongly correlated with the 80°–90° (\(\overline{X}\) r = 0.859, SE = 0.065) and 270°–280° (\(\overline{X}\) r = 0.875, SE = 0.059) bearing classes suggesting an east–west movement gradient. Wolf movements during winter showed large variation in direction, but were generally east to west. Peak mean correlation for caribou movements during spring was distinct at 40°–50° (\(\overline{X}\) r = 0.978, SE = 0.006) revealing movement to the north-east calving grounds. During spring, wolf movements correlated with the 80°–90° (\(\overline{X}\) r = 0.861, SE = 0.043) and 270°–280° (\(\overline{X}\) r = 0.850, SE = 0.064) bearing class. Directionality of movement suggested that during winter, caribou and wolves had a similar distribution at the large spatial scales we tested. During spring migration, however, caribou and wolves employed asynchronous movement strategies. Our findings demonstrate the utility of the correlated random walk and bearing procedure for quantifying the movement patterns of co-occurring species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spatiotemporal variation in the distribution of organisms is one of the primary mechanisms underlying evolutionary and ecological processes. The simple act of movement often has been quoted as the ‘glue’ that relates population dynamics to ecological processes (Turchin 1998; Cagnacci et al. 2010). Studies of movement dynamics of wide-ranging mammals have increased with the availability of new tracking technologies such as satellite or GPS collars (Hebblewhite and Haydon 2010). When connected via line segments, a time series of frequent location data can approximate movement paths. A number of techniques—correlated random walk (Kareiva and Shigesada 1983; Turchin 1998), fractal dimension (With 1994; Nams and Bourgeois 2004), first-passage time analysis (Fauchald and Tverra 2003; Bailleul et al. 2010), and Lévy flight (Viswanathan et al. 1999; Schreier and Grove 2010)—are available for quantifying the shape and scale of these paths.
Movement paths can be compared among individuals within a population and among species to reveal patterns or strategies for locating and using resources, interspecific interactions, or the influence of human activities on the distribution of animals (Whittington et al. 2004; Bailey and Thompson 2006; Brooks and Harris 2008). For example, movement paths of large herbivores have been observed to change between large-scale excursions and fine-scale movements related to foraging (Morales and Ellner 2002; Johnson et al. 2002a). Such movement mechanics likely apply across a range of spatiotemporal scales. As illustrated by Fryxell et al. (2008), herbivores will demonstrate variation in movement according to internal conditions and external stimuli, and these responses will vary according to the period of exposure.
Considering predation as an external stimulus for movement response, researchers have reported a relationship between the distribution and movements of herbivores and areas of high predation risk (Johnson et al. 2002a; Fortin et al. 2005; Hebblewhite and Merrill 2007; Briand et al. 2009; Gervazi et al. 2013). These works have demonstrated that predation risk is seasonally and temporally variable and that herbivores can modify their behaviour in response to this variability. Furthermore, such variation in risk can have direct implications for individual fitness and population productivity leading to ecological and evolutionary outcomes (Creel et al. 2007; Whittington et al. 2011). Unfortunately, the majority of studies designed to understand the spatial interactions of co-occurring species have focused on the recorded movement of one species and the inferred distribution of the second. Rarely are the movements of two or more species monitored and compared concurrently (but see Creel et al. 2005; Laundré 2010). Such comparisons of the distribution of predator and prey are necessary for testing an extensive body of theory that provides general explanation for the behaviour and outcomes of predator–prey interactions (Sih 1984; Brown et al. 1999; Mitchell 2009).
The wolf (Canis lupus), although extensively studied throughout much of North America and Europe (Messier 1985; Hayes and Harestad 2000; Cuicci et al. 2003; McPhee et al. 2012; Sand et al. 2012), has seldom been examined at the northern extents of its range where it resides along and above the treeline (Walton et al. 2001). The movements of these tundra wolves differ from those found in forested habitats in that they do not maintain a defendable, stable home range (Walton et al. 2001; Musiani 2003). Due to the migratory nature of their primary prey, the barren-ground caribou (Rangifer tarandus groenlandicus), wolves are assumed to move with these herds during most of the year and thus maintain relatively large seasonal ranges (Cluff et al. 2002; Musiani 2003; Mattson et al. 2009). During spring and early summer, however, reproducing wolves are constrained to den sites near treeline where they must support altricial pups while caribou migrate farther north to calving grounds (Heard and Williams 1992; Walton et al. 2001). Alternative prey is few for wolves during these seasons, with muskox (Ovibos moschatus), Arctic hare (Lepus arcticus), and Arctic ground squirrel (Urocitellus parryii) being at relatively low densities near den sites (Fournier and Gunn 1998; Frame et al. 2004).
The movements and hunting behaviours of tundra wolves during winter are not well understood. Wolves may closely track their prey within and among seasons (Bergman et al. 2006). Alternatively, wolves may employ a range of behavioural search strategies for caribou and only demonstrate concurrent movements at some spatial scales or portions of caribou range (Williamson Ehlers 2012). This relationship is likely complicated by avoidance responses of caribou to direct and indirect predation risk (Johnson et al. 2002b; Kittle et al. 2008; Briand et al. 2009; Pinard et al. 2011; Whittington et al. 2011).
In this study, we examined the movements of wolves in the Canadian central Arctic relative to barren-ground caribou. We assumed that movement paths would serve as a measure of hunting behaviour by wolves at the scale of the seasonal range. This scale of analysis represented co-varying seasonal movements and distribution not the patch choice and avoidance decisions of caribou or wolves (Laundré 2010). We analysed movement of wolves and caribou during the winter and spring. For both species, winter is a time of relatively sedentary movements focused on hunting and foraging, while spring involves large-scale migration to distant calving/denning areas (Gunn et al. 2001; Walton et al. 2001; Cluff et al. 2002).
We began by testing the relationship between the movement paths of individual caribou and wolves and a correlated random walk (CRW). Given the south to north migration to calving and denning habitats during spring, we used the bearing procedure to test movement paths for consistent directionality within and between seasons (Rosenberg 2000). We hypothesized that caribou movement may be a primary driving force behind wolf search strategies, and thus, movement should be similar in both pattern and directionality. If wolf movement mimics that of the only large prey, we would conclude that wolves were engaging in behaviours that allowed them to associate closely in space and time with the distribution of caribou. If wolf movement differed from that of the caribou, wolves may be employing an alternative search strategy that is not premised on continual reference to large groups of wintering or migrating caribou.
Materials and methods
Study area
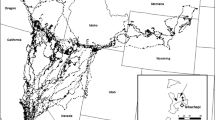
From 1997 to 2000, caribou from the Bathurst herd and co-occurring wolves were captured and monitored, north-east of Yellowknife, Northwest Territories, Canada (see Gunn et al. 2001; Walton et al. 2001; Barrier and Johnson 2012; Fig. 1). The winter distribution of caribou constitutes the largest seasonal range at approximately 256,000 km2. At the time of the study, the Bathurst herd consisted of approximately 349,000 individuals (±94,900 SE; Adamczewski et al. 2009). However, the herd was likely in decline during that period as 186,005 (±15,990 SE; Gunn et al. 2005) animals were estimated in 2003.
Study area for movements of barren-ground caribou of the Bathurst herd and a co-occurring wolf population, Northwest Territories and Nunavut Territory, Canada. Seasonal movement locations and inferred paths of example caribou and a wolf are presented. The annual range of the Bathurst herd is delineated by a 95 % minimum convex polygon and is centred at approximately 64°17′36.49″N 110°43′42.51″W
The study area encompasses low arctic tundra in the northern region, and forest tundra and northern boreal forest in the southern region. Dominant shrub and tree species include Salix spp., Vaccinium spp. and Picea mariana, P. glauca, and Pinus banksiana. The topography is gently sloped with frequent rock outcrops and glacial–fluvial landscape features such as eskers (see Walton et al. 2001). Winter temperatures often fall below −30 °C, and the region receives a yearly average of 151 cm of snowfall (Environment Canada 2006).
Wolf and caribou movement paths
Wolves were located in early June at den sites and fitted with Argos-certified satellite collars (Telonics ST-10 and ST-14 models). Selection of wolves for collaring was dependent on terrain; also, capture crews attempted to collar at least 1 breeding adult in each pack sampled (see Walton et al. 2001). Collars were scheduled to generate approximately 1 location every 4 days. Monitoring for this study ended in the summer of 1999.
Female caribou were captured and collared during the late fall and winter of 1996–1998 (Gunn et al. 2001). As with wolves, capture crews employed a quasi-random encounter-based sampling protocol. Caribou were collared with Argos-certified satellite collars (Telonics ST-10 model). Collars were scheduled to generate approximately 1 location every 5 days.
To detect changes in movement paths due to seasonal behavioural patterns, seasons were segregated by date based on previous studies of migratory movements of the monitored wolves (Walton et al. 2001; Musiani 2003) and annual movements of barren-ground caribou (Gunn et al. 2001). We defined the winter season as occurring between October 16 and March 15, and the spring season as March 16–June 1. The spring season began earlier than defined by Gunn et al. (2001) for caribou, but accommodated the northward movement of wolves in late March and allowed for sufficient sample size of animal movements for that relatively shorter time period. We included wolf or caribou locations of class (LC) 1 or better (<1,500 m error radius; Argos 2008) and LC A; although not associated with an accuracy estimate, LC A locations have been shown to be similar in accuracy to LC 1 locations (Vincent et al. 2002). We generated movement paths for 6 wolves from individual packs, and 5 caribou monitored over the winter and spring of 1998/1999. Each of the animals had between 30 and 38 reliable locations during winter and 16–20 during the spring.
Correlated random walk
We used the CRW to quantify the inferred movement paths of caribou and wolves. A CRW represents a theoretical movement strategy where each successive move occurs in a random direction that is correlated with the most recent movement. For many applications in ecology, the observed movement is compared to the expected net displacement of a movement path generated from a CRW (Bergman et al. 2000). The animal demonstrates a more tortuous movement path when the observed movement is less than that predicted by a CRW. Likewise, if the movement path exceeds the displacement expected from a CRW, the animal is assumed to be pursuing a more linear movement strategy (Kareiva and Shigesada 1983). When proposed as a model of animal movement, the CRW can serve as a null test against which more complex scale-specific decision-making processes can be assessed. Also, a CRW can serve as a standardized measure of movement paths that can be compared among individuals, populations, or species (Turchin 1996; Mårell et al. 2002; Nams and Bourgeois 2004).
The CRW analysis assumes independence between length of movement steps and turning angle between each step (Kareiva and Shigesada 1983; Turchin 1998). There is no accepted measure of independence for this analysis, and other tests can be excessively conservative (McNay et al. 1994). Considering the long interval between successive animal locations, minimum of 4 days for wolves and 5 days for caribou, we assumed independence. To test the hypothesis of a CRW for collared caribou and wolves, we compared the squared net displacement of the actual movement path to that of a CRW. The net displacement of a movement path can be less or greater than that expected for a CRW. Such deviations can be measured using the CRWDiff, test statistic: the scaled difference between the expected and observed squared distance travelled, averaged over a range of steps travelled (see Appendix 1 Nams 2006a). The CRWDiff can be a negative or positive value with 0 representing a true CRW (Nams 2006b). Negative CRWDiff values indicate that the movement path has a shorter net displacement and thus less directionality than a CRW or a path with a positive CRWDiff value (Nams 2006b). Relative to some techniques for quantifying animal movement (e.g. Johnson et al. 2006), the calculation of the CRWDiff is robust to few animal locations and resulting path segments (Nams 2006b) and produces an easily interpretable measure of deviation from the null hypothesis. Error estimates and a P value for a test for significance are provided by the net distance function (Fractal 5 v.5.05, Nams 2006a).
Direction analysis
We used the bearing procedure to quantify the direction of movement for each animal (Rosenberg 2000). The procedure used a Mantel test to generate a correlation between the directionality of pairs of successive movements and a predetermined compass bearing. The largest positive correlation suggested the dominant direction of movement for caribou and wolves. As with the CRW, the bearing procedure was robust to low sample sizes and thus provided an interpretable measure of spatial direction for the animals we monitored in this study (Rosenberg 2000). We specified the bearing procedure be performed on 18 set bearings each differing by 10 degrees. Directionality was oriented across a 180° arc of movement from west (270°–280°) to north (0°) to east (80°–90°). Because caribou were at the southern extent of their distribution during winter, a strong correlation between paired locations and 0° (fixed bearing of north–south) suggested a northerly direction of movement. Animals were pooled by season, and the correlation measures, r, were averaged for each bearing class. Calculations were performed using PASSaGE v.1.1 (Rosenberg 2001).
Statistical comparison of movements
We used repeated measures ANOVA to test for mean differences in CRWDiff statistics. Where sphericity was violated (Mauchly’s test), we used the Greenhouse–Geisser or Huynh–Feldt corrected degrees of freedom. Season served as a within subject effect, and species was tested as a between-subjects effect. Due to a relatively small sample of animal locations, we used a Pearson’s correlation to test for a relationship between the number of locations for each path and the corresponding CRWDiff measure. We considered results with P < 0.05 as statistically significant.
Results
Correlated random walk
During winter and spring, both caribou and wolves demonstrated extensive movements across the central Arctic study area (Fig. 1). However, the majority of caribou and wolf paths in both seasons did not differ significantly from a CRW (Fig. 2). Movement paths that differed from CRWs occurred more frequently in the spring. In general, caribou produced CRWDiff values that were negative, especially in the spring season, while wolves’ CRWDiff were almost always positive (Fig. 2). This trend of negative and positive values suggested that the squared net displacement of caribou and wolves was less and greater, respectively, than expected from individuals demonstrating a CRW. Average CRWDiff values differed statistically between caribou and wolves (F 1,9 = 13.232, P = 0.005). Movement paths did not differ significantly between seasons for either caribou or wolves (F 1,9 = 3.638, P = 0.089), but there was a significant interaction effect between season and species (F 1,9 = 6.815, P = 0.028; Fig. 3). There was no significant relationship between number of animal locations and CRWDiff values (r = 0.348, P = 0.112).
Mean (±1 SE) deviation from a correlated random walk (CRWDiff) for individual caribou and wolves monitored in the Northwest Territories and Nunavut Territory, Canada, during 1998/1999 for the winter (Opened circle) and spring (Filled circle) seasons. Zero indicates a true CRW. Plots marked with asterisk indicate the movement path differed significantly from a CRW
Direction analysis
The directionality analysis revealed differences in correlation of turning angles between the winter and spring seasons and species (Fig. 4). Caribou movement during the winter was strongly correlated with the 80°–90° and 270°–280° bearing classes (respectively, mean (\(\overline{X}\)) r = 0.859, SE = 0.065 and \(\overline{X}\) r = 0.875, SE = 0.059; Fig. 4). This suggested an east–west movement gradient. Peak mean correlation for caribou during spring was distinct at 40°–50° with little variation around the mean (\(\overline{X}\) r = 0.978, SE = 0.006), suggesting focused movement to the north-east. During spring, wolves demonstrated peak mean correlation values at 80°–90° (\(\overline{X}\) r = 0.861, SE = 0.043) and 270°–280° (\(\overline{X}\) r = 0.850, SE = 0.064), suggesting movement focused in an east–west manner (Fig. 4). Wolf directionality in the winter showed large variation, yet slight peaks in correlation were evident at 80°–90° and 270°–300°, similar to movements during spring and that of caribou in the winter (Fig. 4).
Mean (±1 SE) correlation of movement path with bearing class for wolves (Filled circle) and barren-ground caribou (Opened circle) monitored during winter (top) and spring (bottom) in the Northwest Territories and Nunavut Territory, Canada. Degree notation follows typical compass bearings, where west is 270°–280°, north is 0°, and east is 80°–90°. Lines connecting means were retained for clarity in following trends by species
Discussion
A CRW is a valid yet simple strategy for animals seeking resources in a heterogeneous environment. However, a CRW may be strongly dependent on external and internal stimuli that vary across a range of observational or behavioural scales—from the individual foraging decision to movement among seasonal ranges (Fryxell et al. 2008). For example, Brooks and Harris (2008) found intrapopulation differences in the CRW demonstrated by zebra (Equus burchelli antiquorum). Alternatively, Mårell et al. (2002) did not test for a CRWDiff, but reported that for many observation scales, the movement of semi-domesticated reindeer (R. t. tarandus) exceeded the net squared displacement expected from a CRW. Frost et al. (2009) reported that a CRW was the least accurate of a set of models designed to represent the movements of deer (Odocoileus virginianus and O. hemionus).
Applying a CRW to the movements of woodland caribou (R. t. caribou), Bergman et al. (2000) reported similar results to our findings. They suggested that a CRW may be the most efficient strategy for locating forage, primarily dominated by terrestrial lichens, during winter. The population of barren-ground caribou in this study was likely in decline, but still at a relatively high density (~349,000); thus, there was the potential to overgraze lichens if foraging was concentrated in any one place for an extended period of time (Arseneault et al. 1997). Also, the multi-year winter range of the Bathurst caribou herd was large (~246,000 km2) and dynamic, being influenced by wildfire. These factors suggest a patchy distribution of terrestrial lichen (Barrier and Johnson 2012). Adopting a random movement and search strategy may help ensure that caribou disperse widely across their winter range, increasing the likelihood of finding forest stands not recently burned or grazed heavily in past years.
The pattern of caribou movements were inconsistent with our expectation for the spring. During that season, female caribou have a relatively short period of time to reach distant (400–600 km) calving grounds where synchronous births reduce the risk of predation and may maximize the nutritional gain from emerging plants (Dauphiné and McClure 1974; Post et al. 2003). Thus, we expected spring movements to be less tortuous (positive CRWDiff) and in the direction of the northern calving ground. The values for spring were on average negative, although not significantly for the majority of caribou, suggesting shorter more tortuous paths.
We suspect that the contradiction between our hypothesis, positive CRWDiff, and observed data is a function of the ‘spring’ season incorporating multiple interseasonal behaviours. The scale of observation is important when testing whether a movement path is consistent with a CRW (Mårell et al. 2002; Nams 2006b). Migration to the calving ground occurred over a large area and a relatively short time period. Our definition of spring movement inadvertently included locations of caribou that were associated with non-migratory behaviour. Russell et al. (1993) identified 15 periods in the annual life cycle of the migratory Porcupine caribou herd. They defined both a ‘spring’ and ‘spring migration’ season for the period that we considered as spring migration. More recently, Gunn and Poole (2010) redefined the spring migration period for caribou (not wolves) as beginning in mid-April, 4 weeks after the date that we adopted. One caribou we tracked was likely barren and did not proceed to the calving ground (Fig. 1; caribou 127; Gunn et al. 2008). As we might expect, this animal had a relatively large negative CRWDiff value. Although counterintuitive relative to our hypothesis, these findings illustrate the power of the CRWDiff statistic to elucidate multi-scale behaviours and identify seasonal patterns of movement for migratory animals (Nams 2006b).
The movement paths of wolves generally approximated a CRW during winter. Caribou adopted a CRW during this season; thus, it is reasonable to assume their predators did as well. A broad-scale association between the distribution and movements of wolves and caribou on the Bathurst herd’s annual range also was noted by Walton et al. (2001). Where wolves were not tracking groups of caribou directly, they may have increased their likelihood of intercepting prey and prey sign, including prey scent, by adopting a search strategy that mimicked caribou movements.
The CRWDiff test statistic for wolf movements during spring was on average slightly more positive than winter movements. This resulted from the actual path being longer than a CRW model would predict (Nams 2006b) and indicated more straight-line movement. Zollner and Lima (1999) reported that some element of nearly straight movements is an effective search strategy for resources, such as groups of migrating caribou. The spring is an energetically demanding season with the pregnant females preparing for parturition and rearing of pups at dens that are on average 200 km to the south of the caribou’s calving range (Heard and Williams 1992). Increased movement, if associated with hunting, may be a final effort to secure food resources while caribou are still accessible. Alternatively, wolves may employ increased straight-line movement to quickly gain access to denning habitat prior to parturition.
In general, caribou and wolf movements during winter were directed in an east–west direction. This possibly allowed fuller use of the winter range that was oriented in a similar direction along the treeline (Fig. 1). With the onset of spring, most caribou focused their movements in a north-east direction. This corresponded with the known migration to calving grounds located to the north of the winter range (Gunn et al. 2001). Although we hypothesized that wolves would follow migrating caribou, we did not record a similar direction of movement. Wolves can travel >250 km to dens north of treeline (Walton et al. 2001), but they did not follow caribou to the more northerly calving grounds. Reproductive wolves were constrained to more southerly areas with a combination of suitable soils for digging dens and a greater temporal likelihood of encountering caribou moving both north and later south (Heard and Williams 1992; McLoughlin et al. 2004).
Aerial surveys have confirmed that collared caribou represent the distribution of the Bathurst caribou herd on the winter range (Mattson et al. 2009). For this work, however, we monitored only a very small fraction of the herd. Thus, it is difficult to identify general patterns of movement for caribou that apply across this or other populations of herbivores. We monitored wolves from 6 packs likely representing a much larger percentage of that population, although we have no data to suggest total number of packs on the winter range during the study period. Regardless of sample size and the generality of the findings, our application of the CRW and bearing procedures provided insight on the usefulness of these techniques for other populations where it is not possible to collect frequent animal locations and construct detailed movement paths.
The CRW and bearing analyses provide some insights on movement pattern and by inference animal distribution, but they do not reveal the mechanisms for such patterns. We would gain a deeper understanding of caribou–wolf interactions if we combined the description of movement direction and displacement with parameters influencing behaviour. As examples of those process-related parameters, Johnson et al. (2002a) reported that predation risk altered the movements of woodland caribou across spatial scale and Kunkel and Pletscher (2001) found that environmental features such as snow depth and hiding/stalking cover influenced predation strategy. Where fine-scale data are available, such environmental responses could be investigated using empirically based state-space models (Patterson et al. 2008).
References
Adamczewski J, Boulanger J, Croft B, Cluff D, Elkin B, Nishi J, Kelly A, D’Hont A, Nicolson C (2009) Decline in the Bathurst caribou herd 2006–2009: A technical evaluation of field data and modeling. Government of Northwest Territories manuscript report, Yellowknife, NT
Argos (2008) Argos user’s manual. CLS/Service Argos, Toulouse
Arseneault D, Villeneuve N, Boismenu C, Leblanc Y, Deshaye J (1997) Estimating lichen biomass and caribou grazing on the wintering grounds of northern Québec: an application of fire history and landsat data. J Appl Ecol 34:65–78
Bailey H, Thompson P (2006) Quantitative analysis of bottlenose dolphin movement patterns and their relationship with foraging. J Anim Ecol 75:456–465
Bailleul F, Lesage V, Hammill MO (2010) Spherical first passage time: a tool to investigate area-restricted search in three-dimensional movements. Ecol Model 221:1665–1673
Barrier T, Johnson CJ (2012) The influence of fire history on selection of foraging sites by barren-ground caribou. Ecoscience 19:177–188
Bergman CM, Schaefer JA, Luttich SN (2000) Caribou movement as a correlated random walk. Oecologia 123:364–374
Bergman EJ, Garrott RA, Creel S, Borkowski JJ, Jaffe R, Watson FGR (2006) Assessment of prey vulnerability through analysis of wolf movements and kill sites. Ecol Appl 16:273–284
Briand Y, Ouellet J-P, Dussault C, St-Laurent M-H (2009) Fine-Scale habitat selection by female forest-dwelling caribou in managed boreal forest: empirical evidence of a seasonal shift between foraging opportunities and antipredator strategies. Ecoscience 16:330–340
Brooks CJ, Harris S (2008) Directed movement and orientation across a large natural landscape by zebras, Equus burchelli antiquorum. Anim Behav 76:277–285
Brown JS, Laundré JW, Gurung M (1999) The ecology of fear: optimal foraging, game theory, and trophic interactions. J Mammal 80:385–399
Cagnacci F, Boitani L, Powell RA, Boyce MS (2010) Animal ecology meets GPS-based radiotelemetry: a perfect storm of opportunities and challenges. Philos T Roy Soc B 365:2157–2162
Cluff HD, Walton LR, Paquet PC (2002) Movements and habitat use of wolves denning in the central Arctic, Northwest Territories and Nunavut, Canada. Final report to the West Kitikmeot/Slave Study Society, Yellowknife
Creel S, Winnie J, Maxwell B, Hamlin K, Creel M (2005) Elk alter habitat selection as an antipredator response to wolves. Ecology 86:3387–3397
Creel S, Christianson D, Liley S, Winnie JA (2007) Predation risk affects reproductive physiology and demography of elk. Science 315:960
Cuicci P, Masi M, Boitani L (2003) Winter habitat and travel route selection by wolves in the northern Apennines, Italy. Ecography 26:223–235
Dauphiné TC, McClure RL (1974) Synchronous mating in barren-ground caribou. J Wildlife Manage 38:54–66
Environment Canada (2006) Canadian climate normals and averages 1971–2010. http://www.climate.weatheroffice.gc.ca/climate_normals/
Fauchald P, Tverra T (2003) Using first-passage time in the analysis of area-restricted search and habitat selection. Ecology 84:282–288
Fortin D, Beyer HL, Boyce MS, Smith DW, Duchesne T, Mao JS (2005) Wolves influence elk movements: behaviour shapes a trophic cascade in Yellowstone National Park. Ecology 86:1320–1330
Fournier B, Gunn A (1998) Muskox numbers and distribution in the Northwest Territories, 1997. GNWT filet report no. 121. Yellowknife, NT
Frame PF, Hik DS, Cluff HD, Paquet P (2004) Long foraging movement of a denning tundra wolf. Arctic 57:196–203
Frost CJ, Hygnstrom SE, Tyre AJ, Eskridge KM, Baasch DM, Boner JR, Clements GM, Gilsdorf JM, Kinsell TC, Vercauteren KC (2009) Probabilistic movement model with emigration simulates movements of deer in Nebraska, 1990–2006. Ecol Model 220:2481–2490
Fryxell JM, Hazell M, Börger L, Dalziel BD, Haydon DT, Morales JM, McIntosh T, Rosatte RC (2008) Multiple movement modes by large herbivores at multiple spatiotemporal scales. P Natl Acad Sci USA 105:19114–19119
Gervazi V, Sand H, Zimmerman B, Mattison J, Wabakken P, Linnell JDC (2013) Decomposing risk: landscape structure and wolf behavior generate different predation patterns in two sympatric ungulates. Ecol Appl dx.doi.org/10.1890/12-1615.1
Gunn A, Poole KG (2010) Environmental trends across the range of the Bathurst caribou herd and timing of the arrival of cows on their calving ground 1996–2009. Unpublished Report for Government of the Northwest Territories, Yellowknife
Gunn A, Dragon J, Boulanger J (2001) Seasonal movements of satellite-collared caribou from the Bathurst herd. Final Report to the West Kitikmeot/Slave Study Society, Yellowknife, Canada
Gunn A, Nishi J, Boulanger J, Williams J (2005) An estimate of breeding females of the Bathurst herd of barren-ground caribou, June 2003. Government of Northwest Territories manuscript report no. 164, Yellowknife, NT
Gunn A, Poole KG, Wierzchowski J (2008) A geostatistical analysis for the patterns of caribou occupancy on the Bathurst calving grounds 1996–2007. Unpublished report for Indian and Northern Affairs Canada, Yellowknife
Hayes RD, Harestad AS (2000) Demography of a recovering wolf population in the Yukon. Can J Zool 78:36–48
Heard DC, Williams TM (1992) Distribution of wolf dens on migratory caribou ranges in the Northwest Territories, Canada. Can J Zool 70:1504–1510
Hebblewhite M, Haydon DT (2010) Building the bridge between animal movement and population dynamics. Philos T Roy Soc B 365:2303–2312
Hebblewhite M, Merrill EH (2007) Multiscale wolf predation risk for elk: does migration reduce risk. Oecologica 152:377–387
Johnson CJ, Parker KL, Heard DC, Gillingham MP (2002a) Movement parameters of ungulates and scale-specific responses to the environment. J Anim Ecol 71:225–235
Johnson CJ, Parker KL, Heard DC, Gillingham MP (2002b) A multi-scale behavioral approach to understanding the movements of woodland caribou. Ecol Appl 12:1840–1860
Johnson CJ, Parker KL, Heard DC, Gillingham MP (2006) Unrealistic animal movement rates as behavioural bouts: a reply. J Anim Ecol 75:303–308
Kareiva PM, Shigesada N (1983) Analyzing insect movement as a correlated random walk. Oecologia 56:164–170
Kittle AM, Fryxell JM, Desy GE, Hamr J (2008) The scale-dependent impact of wolf predation risk on resource selection by three sympatric ungulates. Oecologia 157:163–175
Kunkel K, Pletscher DH (2001) Winter hunting patterns of wolves in and near Glacier National Park, Montana. J Wildlife Manage 65:520–530
Laundré JW (2010) Behavioral response races, predator-prey shell games, ecology of fear, and patch use of pumas and their ungulate prey. Ecology 91:2995–3007
Mårell A, Ball JP, Hofgaard A (2002) Foraging and movement paths of female reindeer: insights from fractal analysis, correlated random walks, and Levy flights. Can J Zool 80:854–865
Mattson IJK, Johnson CJ, Cluff HD (2009) Winter survey of Bathurst Caribou and associated wolf distribution and abundance. GNWT manuscript report no. 185. Yellowknife, NT
McLoughlin PD, Walton LR, Cluff HD, Paquet PC, Ramsay MA (2004) Hierarchical habitat selection by tundra wolves. J Mammal 85:576–580
McNay RS, Morgan JA, Bunnell FL (1994) Characterizing independence of observations in movements of Columbian black-tailed deer. J Wildlife Manage 58:422–429
McPhee HM, Webb NF, Merrill EH (2012) Hierarchical predation: wolf (Canis lupus) selection along hunt paths at kill sites. Can J Zool 90:555–563
Messier F (1985) Social organization, spatial distribution, and population density of wolves in relation to moose density. Can J Zool 63:1068–1077
Mitchell WA (2009) Multi-behavioral strategies in a predator–prey game: an evolutionary algorithm analysis. Oikos 118:1073–1083
Morales JM, Ellner SP (2002) Scaling up animal movements in heterogeneous landscapes: the importance of behaviour. Ecology 83:2240–2247
Musiani M (2003) Conservation biology and management of wolves and wolf-human conflicts in Western North America. Ph.D. Dissertation, University of Calgary, Calgary
Nams VO (2006a) Fractal 5 user’s guide. Available online at http://www.nsac.ns.ca/envsci/staff/vnams/Fractal.htm
Nams VO (2006b) Detecting oriented movement of animals. Anim Behav 72:1197–1203
Nams VO, Bourgeois M (2004) Fractal analysis measures habitat use at different spatial scales: an example with American marten. Can J Zool 82:1738–1747
Patterson TA, Thomas L, Wilcox C, Ovaskainen O, Matthiopoulos J (2008) State–space models of individual animal movement. Trends Ecol Evol 23:87–94
Pinard V, Dussault C, Ouellet J-P, Fortin D, Courtois R (2011) Calving rate, calf survival rate, and habitat selection of forest-dwelling caribou in a highly managed landscape. J Wildlife Manage 76:189–199
Post E, Bøving PS, Pedersen C, MacArthur MA (2003) Synchrony between caribou calving and plant phenology in depredated and non-depredated populations. Can J Zool 81:1709–1714
Rosenberg MS (2000) The bearing correlogram: a new method of analyzing directional spatial autocorrelation. Geogr Anal 32:267–278
Rosenberg MS (2001) Passage. Pattern analysis, spatial statistics and geographic exegesis version 1.1. Department of Biology, Arizona State University, Tempe, AZ. http://www.passagesoftware.net/download1.php
Russell DE, Martell AM, Nixon WA (1993) Range ecology of the porcupine Caribou Herd in Canada. Rangifer special issue, 8
Sand H, Vucetich JA, Zimmermann B, Wabakken P, Wikenros C, Pedersen HC, Peterson RO, Liberg O (2012) Assessing the influence of prey-predator ratio, prey age structure and pack size on wolf kill rates. Oikos 121:1454–1463
Schreier AL, Grove M (2010) Ranging patterns of hamadryas baboons: random walk analyses. Anim Behav 80:75–87
Sih A (1984) The behavioral response race between predator and prey. Am Nat 123:143–150
Turchin P (1996) Fractal analyses of animal movement: a critique. Ecology 77:2086–2090
Turchin P (1998) Quantitative analysis of movement: measuring and modeling population redistribution in animals and plants. Sinauer Associates Inc., Sunderland
Vincent C, Mcconnell B, Ridoux V, Fedak MA (2002) Assessment of Argos location error from satellite tags deployed on captive gray seals. Mar Mammal Sci 18:156–166
Viswanathan GM, Buldyrev SV, Havlin S, da Luz MGE, Raposo EP, Stanley HE (1999) Optimizing the success of random searches. Nature 401:911–914
Walton LR, Cluff HD, Paquet PC, Ramsay MA (2001) Movement patterns of barren-ground wolves in the central Canadian Arctic. J Mammal 82:867–876
Whittington J, St. Clair CC, Mercer G (2004) Path tortuosity and the permeability of roads and trails to wolf movement. Ecol Soc 9:4. http://www.ecologyandsociety.org/vol9/iss1/art4/
Whittington J, Hebblewhite M, DeCesare NJ, Neufeld L, Bradley M, Wilmshurst J, Musiani M (2011) Caribou encounters with wolves increase near roads and trails: a time-to-event approach. J Appl Ecol 48:1535–1542
Williamson Ehlers E (2012) Impacts of industrial developments on the distribution and movement ecology of wolves (Canis lupus) and woodland caribou (Rangifer tarandus caribou) in the South Peace region of British Columbia. MSc Thesis, University of Northern British Columbia, Prince George, BC
With KA (1994) Using fractal analysis to assess how species perceive landscape structure. Landscape Ecol 9:25–36
Zollner PA, Lima SL (1999) Search strategies for landscape-level interpatch movements. Ecology 80:1019–1030
Acknowledgments
We acknowledge support of the Natural Sciences and Engineering Research Council, University of Northern British Columbia, and the Government of the Northwest Territories. Collaring of caribou and wolves was funded by the West Kitikmeot Slave Study Society. We thank K. Poole, C. Demars, and one anonymous reviewer for constructive comments that improved the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hansen, I.J., Johnson, C.J. & Cluff, H.D. Synchronicity of movement paths of barren-ground caribou and tundra wolves. Polar Biol 36, 1363–1371 (2013). https://doi.org/10.1007/s00300-013-1356-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-013-1356-y