Abstract
The Antarctic krill, Euphausia superba, and the Northern krill, Meganyctiphanes norvegica, are closely related species but occupy significantly different trophic and climatic environments. E. superba holds a key position as a phytoplankton grazer in the Southern Ocean. The omnivorous M. norvegica is an important member of plankton communities in the Northeast Atlantic. Both species expressed high proteolytic activities which were dominated by serine proteinases. In the stomachs of Antarctic krill, activities of total proteinase, trypsin, and chymotrypsin were significantly higher than in Northern krill. In the midgut glands, however, total proteinase and trypsin activities were similar in both species, but chymotrypsin activity was significantly higher in Antarctic krill. Moreover, Antarctic krill expressed four trypsin isoforms while only one isoform appeared in Northern krill. Chymotrypsin was present in either species as one single isoform. Antarctic krill adapted to the low and patchy distribution of food by elevated enzyme activities and the expression of trypsin isoforms with slightly different catalytic properties. Presumably, these enzymes facilitate in concerted action the efficient utilization of proteins from phytoplankton, the major food. Northern krill, in contrast, seems not to be equipped to face food limitation. It expresses a “simple” or “basic” set of digestive enzymes for utilizing abundant and easily digestible prey.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Euphausiids are important members of many marine pelagic ecosystems all over the world (Mauchline and Fisher 1969; Mauchline 1980). Some species may even be considered outstanding due to their abundance and ecological relevance. Among them are the Antarctic krill, Euphausia superba, and the Northern krill, Meganyctiphanes norvegica.
Euphausia superba is endemic in the Southern ocean and holds a key position in Antarctic marine food webs. It adapted to an environment with low temperature around 0°C and patchy and seasonally changing food availability (Meyer 2012). While predominantly feeding on phytoplankton, Antarctic krill forms an important link between primary producers and higher trophic levels (e.g., Smetacek et al. 1990; Falk-Petersen et al. 2000). M. norvegica inhabits the North Atlantic Ocean and adjacent seas. The area of distribution extends from the east coast of Canada to Iceland and the Barents Sea. To the south and the east, M. norvegica is abundant in the Mediterranean Sea and has its southern distributional limits around the Canary Islands. According to this wide latitudinal distribution, Northern krill faces a wide range of temperatures from 4 to 16°C and is exposed to different trophic conditions (Einarsson 1945; Mauchline 1960; Matthews et al. 1999). The Northern krill is considered omnivorous feeding on zoo- and phytoplankton but also on detritus (Lass et al. 2001).
In both species, alimentary proteins form the major source for amino acids and nitrogen. Accordingly, the utilization of proteins from the food is an essential metabolic requirement. Proteolysis is also one of the most ancient metabolic processes (Neurath 1984). Several classes of proteinases (serine-, cysteine-, and metallo-proteinases) and isoforms of various enzymes have evolved in crustaceans. The expression of certain proteinase classes in crustaceans seems to be related to taxa (Teschke and Saborowski 2005; Navarrete del Toro et al. 2006). Euphausiids express high levels of serine proteinases which are dominated by trypsin-like enzymes. Trypsin-like activity but also carboxypeptidase A-, carboxypeptidase B-, and aminopeptidase activities were measured in Antarctic krill by Chen et al. (1978), Kimoto et al. (1981, 1983), Nishimura et al. (1983), Kimoto and Murakami (1984) and Osnes and Mohr (1985a, b). Mayzaud et al. (1987) separated six different enzymes which hydrolyze the synthetic substrate N-benzoyl-l-arginine p-nitroanilide (BANA). Turkiewicz et al. (1991) as well as Bucht and Karlstam (1991) isolated highly active serine proteinases and enzymes with wide substrate specificity. In contrast to Antarctic krill, detailed investigations on digestive proteinases in Northern krill are rare (Spicer and Saborowski 2010). The postmortem proteolysis of M. norvegica was studied by Saether et al. (1987) and, similar to Antarctic krill, the authors found high levels of peptide hydrolase. Autoproteolysis was mainly due to digestive enzymes in the midgut gland.
Because of the close taxonomical relationship between both species, a comparative study on adaptations to different habitats appears rewarding. Therefore, this work is focused on the activities and expression pattern of digestive endopeptidases and how they may be related to the climatic and, particularly, trophic environment of either species.
Materials and methods
Origin of samples
Northern krill, M. norvegica, were caught with an Isaacs-Kidd Midwater Trawl in the central part of the Gullmarnfjord, Sweden (58°19.9′N, 11°33.8′E) in spring 2000. The net was deployed during daytime at a depth of 80–90 m (van den Thillart et al. 1999). After capture, krill were transported to Kristineberg Marine Research Station where they were shock-frozen at −80°C.
Antarctic krill, E. superba, were captured off the South Shetland Islands in February/March 2000 during an expedition of the Russian vessel R/V Yuzhmorgeologiya (Stübing and Hagen 2003). Antarctic krill were briefly rinsed with distilled water, blotted dry, and then frozen and stored at −80°C until analysis at the marine Station Helgoland.
Dissection of organs
Organs were dissected from frozen animals under a binocular microscope. In order to prevent thawing, the krill were placed on a cooling block (−80°C). The stomach and the midgut gland were rapidly excised, relieved of adhering tissue, transferred into pre-weighed reaction tubes, and immediately placed on ice.
Extracts
Crude extracts for enzyme assay were prepared from individual stomachs and midgut glands of either species. After weighing, 1 ml of ice-cold demineralised water (aqua dem.) was added to the reaction cup which contained the tissue. Subsequently, homogenization was performed on ice with a Branson Sonifier cell disrupter (Model B15, equipped with a microtip) by applying three ultrasonic bursts of 5 s at 30% of maximum energy. The homogenates were centrifuged for 10 min at 15,000×g (Heraeus, Biofuge A). Thereafter, the supernatants were transferred into new tubes and assayed for enzyme activity.
Enzyme assays
Total proteolytic activity was determined with the substrate azocasein Na-salt (Serva, 14391) at 0°C and at 30°C. Reaction cups (1.5 ml) were equipped with 200 μl phosphate buffer (0.05 mol l−1 NaH2PO4, 0.05 mol l−1 Na2HPO4, 0.15 mol l−1 NaCl, pH 6.8) and 20 μl of sample and were pre-incubated in a thermomixer (Eppendorf, 5436) at the respective temperature (0°C and 30°C) for 5 min. The reaction was started by the addition of 100 μl of azocasein solution (1% w/v in phosphate buffer). After 30 min of incubation, the reaction was terminated by the addition of 500 μl of trichloroacetic acid (TCA, 20% w/v in aqua dem.) and cooling on ice. Controls were run in parallel in which the sample was added after termination with TCA. Samples were run in triplicate and controls were run in duplicate. Finally, the reaction cups were centrifuged at 15,000×g (10 min, 4°C), and the absorption of the supernatant was measured at 366 nm against air. The activity was expressed as ΔA 366 min−1 g −1fw .
Trypsin activity (peptidase) was determined in routine with the substrate N α-benzoyl-l-arginine 4-nitroanilide hydrochloride (l-BAPA, Merck 1.10754) according to Erlanger et al. (1961) with the following modifications: 600 μl of buffer (0.05 mol l−1 Tris–HCl, pH 7.5) and 20 μl of sample were pre-incubated for 5 min at 30°C in a temperature-controlled cuvette holder. The reaction was started by the addition of 20 μl of l-BAPA (32 mmol l−1 in dimethyl sulfoxide, DMSO) and continuously monitored at 405 nm for another 5 min. The substrate concentration in the cuvette was 1 mmol l−1. The activity was expressed as U g −1fw (=μmol min−1 g −1fw ) using the extinction coefficient ε405 = 10.2 l mmol−1 cm−1 (Geiger and Fritz 1988).
Trypsin activity (esterase) was determined with N α-p-tosyl-l-arginine methyl ester (TAME, Sigma T-4626) after Rick (1974). TAME was dissolved in aqua dem. and applied to the assay at a final concentration of 1 mmol l−1. The cuvette containing the reaction mixture was incubated at 25°C in temperature-controlled cuvette holder. The change in absorbance at 247 nm was recorded for 5 min. The activity was expressed as U g −1fw (=μmol min−1 g −1fw ) using the extinction coefficient ε405 = 0.54 l mmol−1 cm−1 (Rick 1974).
Chymotrypsin activity was measured with the substrate N-succinyl-ala-ala-pro-phe p-nitroanilide (SAAPPNA, Sigma S-7388). The assay conditions were the same as described above for tryptic peptidase activity. The activity of chymotrypsin was also expressed as U g −1fw (ε405 = 10.2 l mmol−1 cm−1, Geiger 1988).
Inhibitor assays
Enzyme extracts from the midgut gland were incubated with the serine proteinases inhibitor 4-(2-aminoethyl)enzenesulfonyl-fluorid hydrochloride (AEBSF, Merck 124839) and with the cysteine proteinase inhibitor trans-epoxy-succinyl-l-leucylamido-(4-guanidino)-butane (E-64, Sigma E3132). The inhibitors were dissolved in aqua dem. at a concentration of 1 mmol l−1. One hundred microliters of the extract and the same amount of inhibitor solution were incubated for 60 min at room temperature in a thermomixer (Eppendorf 5436). Thereafter, the extract/inhibitor solution was used for the assay of azocasein digestion as described above. Controls were run with aqua dem. (the solvent) instead of inhibitor solution. The residual activity was calculated and expressed as percent of the uninhibited activity.
Chromatography
Extracts for liquid chromatography were prepared from individual animals or from batches of up to 120 mg of tissue (5–10 organs). Depending on the amount of tissue, samples were homogenized in 1 or 2 ml of imidazole buffer (0.01 mol l−1, pH 6.8) by ultrasonication as described above. After centrifugation (15,000×g, 10 min, 4°C), the supernatant was desalted over Sephadex G-25 PD10 columns (AP Biotech, 52-1308-00).
Chromatographic separation of enzymes was performed with a FPLC system (AP Biotech) using the anionic exchange column UNO Q-1R (BioRad, 720-0011). The sample was applied onto the column over a 2-ml sample loop. Elution of proteins was achieved with increasing concentration of NaCl (0–1 mol l−1) in imidazole buffer (0.01 mol l−1, pH 6.8) at a flow rate of 1 ml min−1. During each run, 70 fractions of 0.5 ml each were separated into pre-cooled reaction tubes. During the run, the absorption was monitored at 280 nm (Uvicord II) as a measure for the relative protein concentration.
The molecular weights of isolated proteins were determined by gel filtration over Superdex 200 prep grade (High Load 16/60, Pharmacia, 17-1069-01). The buffer used was imidazole (0.01 mol l−1, pH 6.8) at a flow rate of 1 ml min−1. Calibration was done with Ribonuclease A (13.7 kDa), Chymotrypsinogen A (25 kDa), Ovalbumin (43 kDa), Albumin (67 kDa), Aldolase (158 kDa), Catalase (232 kDa), and Ferritin (440 kDa), (Pharmacia 17-0441-01 and 17-0442-01). Fractions of 1 ml were collected.
Activity screening of FPLC fractions
In order to obtain activity profiles after chromatographic separation, the collected fractions were screened for total protease, trypsin, and chymotrypsin. Total protease was measured as described above in 1.5 ml reaction cups. However, 50 μl of sample was used instead of 20 μl. Trypsin-like activity was measured in microplates: first, 50 μl of sample was transferred from the fraction-collector tubes into the wells of a 96-well microplate. Subsequently, 250 μl of buffer (0.05 mol l−1 Tris–HCl, pH 7.5) which was supplemented with the substrate l-BAPA (final concentration 1 mmol l−1) was quickly added to the samples in the microplate. The plate was incubated at room temperature for up to 1 h. The optical density of the plate was read at 405 nm after 15, 30, 45, and 60 min. Chymotrypsin activity was measured in the same way but with SAAPPNA as substrate (final concentration 1 mmol l−1).
Effect of calcium
Calcium significantly affects the activity of vertebrate serine proteases. In order to investigate the effect of calcium on krill trypsin and chymotrypsin, the activities in crude extracts of either species were determined without Ca2+ and with Ca2+. The extracts were prepared and desalted as described above. The concentration of CaCl2 in the reaction buffer was 1 mmol l−1.
Thermal stability
In order to investigate the thermal stability, isolated enzymes were incubated for 20 min at temperatures between 25 and 55°C in intervals of 5°C. Between 40 and 50°C, the temperature intervals were 2.5°C. After incubation, enzyme activities were measured at 30°C. The assays were run as described above.
Statistics
Data were expressed as means and standard deviation (SD). Differences among means were analyzed by ANOVA followed by a Tukey’s multicomparison test. Differences are reported as statistically significant when P < 0.05. The significance levels in graphs are indicated by asterisks: P < 0.05 (*), P < 0.01 (**), P < 0.001 (***).
Results
Enzyme activities and enzyme classes in digestive organs
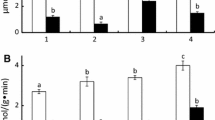
Total proteinase activity was measured in individual stomachs and midgut glands at 0 and at 30°C (Fig. 1). In both species, weight-specific activities were always higher in the stomachs than in the midgut glands. In the stomach, total proteinase activity was significantly higher in E. superba than in M. norvegica (Fig. 1a). It amounted on average to 4 ΔA 366 min−1 g −1fw in M. norvegica and 10.8 ΔA 366 min−1 g −1fw in E. superba. In contrast, activities in the midgut gland did not differ significantly between species (3.8 vs. 5.8 ΔA 366 min−1 g −1fw ). At 30°C, activities in both species were 10–20 times higher than at 0°C (Fig. 1b). However, a similar ratio of activities remained between species and between organs. The increase in activity with temperature corresponds to Q 10 -values between 2.0 and 2.7.
The effects of inhibitors were determined at 30°C (Fig. 2). The inhibitory effect of AEBSF was significantly higher than that of E-64 (P < 0.001). In M. norvegica, AEBSF reduced total proteolytic activity in midgut gland extracts to 54% and E-64 to 86% of initial activity. In E. superba extracts, residual activity remained at 46% after AEBSF treatment and at 93% after E-64 treatment.
Trypsin activities (l-BAPA) amounted on average to 2.9 U g −1fw in the stomachs of M. norvegica and to 10.3 U g −1fw in the stomachs of E. superba (Fig. 3a). The difference between both species statistically significant (P < 0.001). In the midgut gland, however, activities did not differ significantly between both species. They amounted to 5.6 U g −1fw in M. norvegica and 6.8 U g −1fw in E. superba.
Chymotrypsin activities were significantly higher in E. superba than in M. norvegica in both digestive organs (Fig. 3b). In the stomach of M. norvegica, chymotrypsin activities amounted to 49.5 U g −1FW and in E. superba to 125 U g −1fw . In the midgut glands, the activities were 28.5 and 52.3 U g −1fw , respectively.
Separation of trypsin and chymotrypsin isoforms
Anionic exchange chromatography of midgut gland extract of M. norvegica showed a good separation of proteins. Only a little amount of protein did not bind to the column and eluted in the fractions 6–8 before the concentration of NaCl increased (Fig. 4). At 0.9 mol l−1 of NaCl, almost all proteins eluted from the column. Several protein peaks were detected between fractions 17 and 33 (5–35% NaCl) and fractions 45 and 60 (60–90% NaCl).
FPLC elution profiles of midgut gland extracts from Northern krill (M. norvegica) and Antarctic krill (E. superba). Shaded areas in the graphs denote enzyme activities of total proteinase, trypsin, and chymotrypsin as indicated. The trypsin isoforms of E. superba are denoted with number I, II, III, and IV in the graph e
Three major peaks of total proteinase were detected in fractions 39–44, 47–52, and 55–58 (Fig. 4a). Minor peaks appeared in fractions 25, 27, 30, 33, and 37. No proteolytic activity was detected prior to fraction 17 assuming that proteases entirely bound to the column.
Trypsin (determined with l-BAPA as substrate) eluted in one single and well-defined peak between fractions 48–51 (Fig. 4b). Some low activity was detected in fraction 43. The same pattern was obtained when TAME was used as substrate. The fractions of activity were pooled for further analysis. The enzyme was denoted as M.n.TryI.
Chymotrypsin eluted also as a single peak between fractions 38–44 (Fig. 4c). The chymotrypsin peak was wider than the trypsin peak. The fractions of activity were pooled and the enzyme was denoted as M.n.ChyI.
The chromatographic profiles of midgut glands from E. superba differed from the profiles of Northern krill. The share of non-bound proteins was higher and the proteins showed a different elution pattern (Fig. 4d). Major protein peaks appeared at fractions 22, 25, 34, 36, 42, 44, 47, and 51.
Total proteinase activity was detected in four major peaks in fractions 33–36, 39–41, 49–53, and 55–58 (Fig. 4d). Some minor activity was detected between fractions 18–31 and between 60 and 65.
Trypsin activity (l-BAPA) appeared in three sharp and distinct peaks between fractions 34–36, 52–55, and 58–61 (Fig. 4e). Minor activity was detected at fraction 39–41. Trypsin activity with TAME as substrate was detected in the two latter peaks. Compared to those, however, only little activity was present around fraction 34. In contrast, a major peak appeared between fraction 39 and 41 which was not present when the trypsin assay was run with l-BAPA. Fractions of activity were pooled for further analysis, and the enzymes were denoted as E.s.TryI, E.s.TryII, E.s.TryIII, and E.s.TryIV.
Chymotrypsin eluted as a single peak between fraction 41 and 46 (Fig. 4f). No activity was detected aside this peak. The enzyme was denoted as E.s.ChyI.
Molecular weight
The molecular weights (MW) were determined under native conditions by gel filtration. The MW of all trypsin and chymotrypsin isoforms showed uniform results of 31–34 kDa (Table 1). No distinct differences in MW were evident between species.
Effect of calcium
The presence of calcium had no significant effect on the activities of chymotrypsin or trypsin of M. norvegica. The activity of chymotrypsin decreased slightly to 98% of the activity with unsupplemented buffer. The activity of trypsin rose slightly to 102% when measured with both, BAPA and TAME as substrate (results not shown).
Thermal stability
The trypsin and chymotrypsin from M. norvegica remained fully active up to 35°C when pre-incubated for 20 min (Fig. 5a). Activities began to decrease at 40°C, and the most rapid loss of activity appeared between 42 and 46°C. At 50°C both enzymes showed less than 10% of the initial activities.
The stability profiles of E. superba enzymes showed similar patterns as those of M. norvegica (Fig. 5b). Again, denaturation started at 40°C. E.s.TryII maintained 50% of activity after incubation at 42°C and of E.s.TryI after incubation at 46°C. Chymotrypsin (E.s.ChyI) showed the same course of denaturation as the trypsins did. Half maximum activity remained at 44°C.
Discussion
This work confirms the high proteolytic activities and the presence of several trypsin isoforms in Antarctic krill. However, it also shows that Northern krill maintains, at least in the midgut gland, a similar level of activity as the Antarctic krill. Accordingly, the major differences between both species appear to be the expression of different trypsin isoforms and the elevated proteolytic activities in the stomachs. These features, however, can help to understand the physiological adaptation of either species to its particular environment.
Northern krill feed on phytoplankton and zooplankton (Beyer 1992; Båmstedt and Karlson 1998; Onsrud and Kaartvedt 1998; Kaartvedt et al. 2002). Even dead organic matter such as detritus was found in the stomachs (Lass et al. 2001). Usually, M. norvegica is not limited by food. Only in particular environments, such as the oligotrophic Ligurian Sea, it may be exposed to food limitation (Fabiano 1984; Saborowski and Buchholz 2002). Compared to Antarctic krill, the proteinases expressed by Northern krill seem to represent a set of enzyme which may be denoted “simple” or “basic”, suitable for animals with abundant food supply. Moreover, Northern krill is distributed in waters with moderate temperatures of about 4–16°C. Therefore, rate limiting conditions due to low temperatures affect Northern krill to a lesser extent than Antarctic krill, which live at around 0°C. Again, a “simple” set of enzymes seems sufficient to successfully cope with these conditions. Finally, Northern krill is an omnivorous feeder utilizing micro- and mesozooplankton which provide both, sufficient energy to fuel metabolic demands and, particularly, sufficient nitrogen for the synthesis of proteins and nucleic acids. Moreover, zooplankton species possess endogenous digestive enzymes which may contribute to the digestive process and, thus, accelerate digestion and improve the utilization of nutrients in krill (Dabrowski and Glogowski 1977; Le Ruyet et al. 1993).
Antarctic krill, in contrast, inhabits environments where food is often limiting. Although krill is capable of feeding on smaller zooplankton (Huntley et al. 1994; Atkinson and Snÿder 1997; Cripps and Atkinson 2000), phytoplankton is the major food of E. superba. The thoracopods form a highly specialized filter basket (Hamner et al. 1983; Kils 1983) which enables krill to exploit efficiently pelagic microalgae (Quetin and Ross 1985) as well as ice algae (Smetacek et al. 1990; Meyer 2012). Phytoplankton contains less protein than zooplankton (e.g., Mayzaud and Martin 1975; Brown et al. 1997). Therefore, the utilization of protein from phytoplankton has to be optimized. This may be facilitated by increasing the digestive enzyme activities and/or by increasing the gut retention times (Mayzaud et al. 1998).
These suggestions are in agreement with observations on decapods. Kumlu and Jones (1997) compared trypsin-like activity in various decapod larvae and found high levels in herbivorous, low levels in carnivorous and intermediate levels in omnivorous species. High trypsin activity was suggested to facilitate protein utilization from “less digestible” algae, whereas in carnivorous larvae, comparatively low activity was considered sufficient to utilize large and “easily digestible” prey. Similarly, LeVay et al. (2001) concluded that herbivorous decapod larvae adapted to low energy values of food with high enzyme activities, rapid food turnover, and low assimilation efficiency. In contrast, carnivorous larvae show lower levels of enzyme activities but compensate it by extending the gut retention time thereby increasing assimilation efficiency. Similar results were obtained in feeding experiments with Penaeus japonicus. Trypsin activity was sixfold higher in larvae which were fed with the diatom Chaetoceros gracilis than in larvae fed with nauplii of the brine shrimp Artemia sp. (Rodriguez et al. 1994)
The midgut gland and the stomach form a functional unit in terms of both anatomy and physiology. Digestive enzymes are synthesized and secreted in the midgut gland and accumulate in the stomach (Saborowski and Buchholz 1999). Both species, E. superba and M. norvegica, showed similar levels of total proteinase as well as trypsin activity in their midgut glands. However, in the stomachs, activities were significantly higher in E. superba. On one hand, these high activities may be due to higher enzyme accumulation as a result of elevated synthesis rates. But on the other hand, the proteinases in the midgut gland of Antarctic krill are thoroughly regulated by a set of specific inhibitors (Ellingsen and Mohr 1987). Upon release from the cells, the regulatory effects of the inhibitors collapse and the enzymes develop full activity. In any case, the ability to provide elevated activity in the stomach prior to food uptake enables Antarctic krill to digest food immediately after ingestion. This feature represents an efficient adaptation to the patchy occurrence of phytoplankton in the Southern ocean.
Expression of different trypsin isoforms appears beneficial when the isoforms differ slightly in specificity or in their kinetic properties. In E. superba, one trypsin isoforms showed predominantly peptidase activity (E.s.TryI), two showed peptidase and esterase activities (E.s.TryIII and E.s.TryIV), and one isoform showed predominantly esterase activity (E.s.TryII). It is likely that the isoenzymes act synergetically and, thus, hydrolyze proteins more efficiently than a single enzyme with defined specificity could do. Besides trypsin, high activities of chymotrypsin were present in both species. Chymotrypsin hydrolyzes peptide bonds after tyrosine, tryptophan, phenylalanine. Thus, it complements the hydrolytic action of trypsin which cleaves at the amino acids arginine and lysine. Accordingly, most efficient protein digestion seems to be provided by the concerted action of a set of endopeptidases, each with slightly different specificities.
In both species of krill, most proteolytic activity was contributed by serine proteinases. Their share amounted to about 50% of total proteolytic activity as shown by inhibitor assays. In contrast, cysteine proteinases, which may prevail in caridean shrimps (Teschke and Saborowski 2005) contributed only a minor share. Other endopeptidases which were not inhibited by AEBSF or E64 may belong to the classes of metallo- or aspartic proteinases. These, however, were not considered in the present work. Nevertheless, the serine proteinase trypsin is regarded as the most important and most frequent endopeptidase in invertebrates and, particularly, in crustaceans (Dall and Moriarty 1983). This study has shown that beside trypsin, also chymotrypsin plays a significant role in the digestion of proteins in the studied euphausiids. Different to vertebrate trypsin, none of the krill enzymes were affected by calcium. This observation is in agreement with the results by Osnes and Mohr (1985b), and this feature seems to separate crustacean serine proteinases from those of many vertebrates.
There was no obvious adaptation of E. superba proteolytic enzymes in terms of thermal properties, stability or activities at low temperatures. Apparently, the proteolytic enzymes of E. superba do not compensate low temperatures by elevated activities. At 0°C, the activity of E. superba was not elevated in comparison to M. norvegica. Moreover, the enzyme activities of either species increased with temperature at the same rate which resulted in similar ratios of activities between both species at 0 and at 30°C. Probably, adaptations to low temperature are more distinct in other physiological systems. These might be membrane transfer processes in the midgut gland since the transfer of nutrients through the membrane into the cell appears to be one of the crucial and probably limiting steps in nutrition.
In conclusion, both krill species expressed serine proteinases, trypsin, and chymotrypsin, which constitute a significant share of digestive endopeptidases. M. norvegica shows more simple proteolytic characteristics than E. superba which allows living in an environment without significant food limitation. E. superba, in contrast, exhibit more complex digestive properties which comprise elevated activities in stomach and the expression of isoenzymes with slightly different substrate specificities. Both features enable rapid digestion of incidental food and, thus, support viability in the extreme Antarctic environment.
References
Atkinson A, Snÿder R (1997) Krill-copepod interactions at South Georgia, Antarctica, I. Omnivory by Euphausia superba. Mar Ecol Prog Ser 160:63–76
Båmstedt U, Karlson K (1998) Euphausiid predation on copepods in coastal waters of the Northeast Atlantic. Mar Ecol Prog Ser 172:149–168
Beyer F (1992) Meganyctiphanes norvegica (M. Sars) (Euphausiacea): a voracious predator on Calanus, other copepods, and Ctenophores, in Oslofjorden, southern Norway. Sarsia 77:189–206
Brown MR, Jeffrey SW, Volkman JK, Dunstan GA (1997) Nutritional properties of microalgae for mariculture. Aquaculture 151:315–331
Bucht A, Karlstam B (1991) Isolation and immunological characterization of three highly purified serine proteinases from Antarctic krill (Euphausia superba). Polar Biol 11:495–500
Chen C-S, Yan T-R, Chen H-Y (1978) Purification and properties of trypsin-like enzymes and a carboxypeptidase A from Euphausia superba. J Food Biochem 2:349–366
Cripps GC, Atkinson A (2000) Fatty acid composition as an indicator of carnivory in Antarctic krill, Euphausia superba. Can J Fish Aquat Sci 57(Suppl 3):31–37
Dabrowski K, Glogowski J (1977) Studies on the role of exogenous proteolytic enzymes in digestion processes in fish. Hydrobiologia 54:129–134
Dall W, Moriarty DJW (1983) Functional aspects of nutrition and digestion. In: Mantel LH (ed) The biology of crustacea, vol 5 internal anatomy and physiological regulation. Academic Press, New York, pp 215–261
Einarsson H (1945) Euphausiacea. I. Northern Atlantic species. Dana Rep Carlberg Found 27:1–184
Ellingsen TE, Mohr V (1987) Biochemistry of the autolysis process in Antarctic krill post mortem. Autoproteolysis. Biochem J 246:295–305
Erlanger BF, Kokowsky N, Cohen W (1961) The preparation and properties of two new chromogenic substrates for trypsin. Arch Biochem Biophys 95:271–278
Fabiano M (1984) Production of the Ligurian coastal waters. 2. Primary production. Mem Biol Mar Oceanogr 14:43–58
Falk-Petersen S, Hagen W, Kattner G, Clarke A, Sargent J (2000) Lipids, trophic relationships, and biodiversity in Arctic and Antarctic krill. Can J Fish Aquat Sci 57(Suppl 3):178–191
Geiger R (1988) Chymotrypsin. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 5, 3rd edn. Enzymes 3: peptidases, proteinases and their inhibitors. VCH Verlagsgesellschaft, Weinheim, pp 99–118
Geiger R, Fritz H (1988) Trypsin. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 5, 3rd edn. Enzymes 3: peptidases, proteinases and their inhibitors. VCH Verlagsgesellschaft, Weinheim, pp 119–129
Hamner WM, Hamner PP, Strand SW, Gilmer RW (1983) Behaviour of Antarctic krill, Euphausia superba: chemoreception, feeding, schooling, and moulting. Science 220:433–435
Huntley ME, Nordhausen W, Lopez MDG (1994) Elemental composition, metabolic activity and growth of Antarctic krill Euphausia superba during winter. Mar Ecol Prog Ser 107:23–40
Kaartvedt S, Larsen T, Hjelmseth K, Onsrud MSR (2002) Is the omnivorous krill Meganyctiphanes norvegica primarily a selectively feeding carnivore? Mar Ecol Prog Ser 228:193–204
Kils U (1983) Swimming and feeding of Antarctic krill, Euphausia superba—some outstanding energetics and dynamics, some unique morphological details. Ber Polarf Sonderh 4:130–155
Kimoto K, Murakami K (1984) Purification and characterization of aminopeptidase from Euphausia superba. Agric Biol Chem 48:1819–1823
Kimoto K, Than VV, Murakami K (1981) Acid proteinases from Antarctic krill, Euphausia superba. Partial purification and some properties. J Food Sci 46:1881–1884
Kimoto K, Kusama S, Murakami K (1983) Purification and characterization of serine proteinases from Euphausia superba. Agric Biol Chem 47:529–534
Kumlu M, Jones DA (1997) Digestive protease activity in planktonic crustaceans feeding at different trophic levels. J Mar Biol Assoc UK 77:159–165
Lass S, Tarling GA, Virtue P, Matthews JBL, Mayzaud P, Buchholz F (2001) On the food of northern krill Meganyctiphanes norvegica in relation to its vertical distribution. Mar Ecol Prog Ser 214:177–200
Le Ruyet JP, Alexandre JC, Thébaud L, Mugnier C (1993) Marine fish larvae feeding: formulated diets or live prey? J World Aquac Soc 24:211–224
Le Vay L, Jones DA, Puello-Cruz AC, Sangha RS, Ngamphongsai C (2001) Digestion in relation to feeding strategies exhibited by crustacean larvae. Comp Biochem Physiol 128A:623–630
Matthews JBL, Buchholz F, Saborowski R, Tarling GA, Dallot S, Labat JP (1999) On the physical oceanography of the Kattegat and the Clyde Sea area, 1996–98, as background to ecophysiological studies on the planktonic crustacean, Meganyctiphanes norvegica (Euphausiacea). Helgol Mar Res 53:70–84
Mauchline J (1960) The biology of euphausiid crustacean Meganyctiphanes norvegica Sars. Proc R Soc Edinb B (Biol) 67:141–179
Mauchline J (1980) The biology of euphausiids. Adv Mar Biol 18:373–623
Mauchline J, Fisher LR (1969) The biology of euphausiids. Adv Mar Biol 7:1–454
Mayzaud P, Martin J-L M (1975) Some aspects of the biochemical and mineral composition of marine plankton. J Exp Mar Biol Ecol 17:297–310
Mayzaud P, Van Wormhoudt A, Roche-Mayzaud O (1987) Spatial changes in the concentrations and the activities of amylase and trypsin in Euphausia superba. A comparison between activity measurement and immunoquantitation. Polar Biol 8:73–80
Mayzaud P, Tirelli V, Bernard JM, Roche-Mayzaud O (1998) The influence of food quality on the nutritional acclimation of the copepod Acartia clausi. J Mar Sys 15:483–493
Meyer B (2012) The overwintering of Antarctic krill, Euphausia superba, from an ecophysiological perspective. Polar Biol 35:15–37
Navarrete del Toro MA, García-Carreño FL, Díaz-López M, Celis-Guerrero L, Saborowski R (2006) Aspartic proteinases in the digestive tract of marine decapod crustaceans. J Exp Zool 305A:645–654
Neurath H (1984) Evolution of proteolytic enzymes. Science 224:350–357
Nishimura K, Kavamura Y, Matoba T, Yonezawa D (1983) Classification of proteases in Antarctic krill. Agric Biol Chem 47:2577–2583
Onsrud MSR, Kaartvedt S (1998) Die vertical migration of the krill Meganyctiphanes norvegica in relation to physical environment, food and predators. Mar Ecol Prog Ser 171:209–219
Osnes KK, Mohr V (1985a) Peptide hydrolases of Antarctic krill, Euphausia superba. Comp Biochem Physiol 82B:599–606
Osnes KK, Mohr V (1985b) On the purification and characterization of three anionic, serine-type peptide hydrolases from Antarctic krill, Euphausia superba. Comp Biochem Physiol 82B:607–619
Quetin LB, Ross RM (1985) Feeding by Antarctic krill, Euphausia superba: does size matter? In: Siegfried WR, Condy PR, Laws RM (eds) Antarctic nutrient cycles and food webs. Springer, Berlin, pp 372–377
Rick W (1974) Trypsin: measurement with Nα-p-toluenesulfonyl-l-arginine methyl ester as substrate. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 2, 2nd edn. Academic Press, New York, pp 1021–1024
Rodriguez A, Le Vay L, Mourente G, Jones DA (1994) Biochemical composition and digestive enzyme activity in larvae and postlarvae of Penaeus japonicus during herbivorous and carnivorous feeding. Mar Biol 118:45–51
Saborowski R, Buchholz F (1999) A laboratory study on digestive processes in the Antarctic krill, Euphausia superba, with special regard to chitinolytic enzymes. Polar Biol 21:295–304
Saborowski R, Buchholz F (2002) Metabolic properties of Northern krill, Meganyctiphanes norvegica, from different climatic zones: enzyme characteristics and activities. Mar Biol 140:557–565
Saether O, Ellingsen TE, Mohr V (1987) Proteolysis post mortem in North Atlantic krill. Comp Biochem Physiol 88B:165–176
Smetacek V, Scharek R, Nöthig EM (1990) Seasonal and regional variation in the pelagial and its relationship in the life history cycle of krill. In: Kerry KR, Hempel G (eds) Antarctic ecosystems. Ecological change and conservation. Springer, Berlin, pp 103–114
Spicer J, Saborowski R (2010) Physiology and metabolism of Northern krill (Meganyctiphanes norvegica Sars). In: Tarling G (ed) Advances in marine biology. Vol 57. The biology of northern krill. Elsevier, Amsterdam, pp 91–126
Stübing D, Hagen W (2003) Fatty acid biomarker ratios—suitable trophic indicators in Antarctic euphausiids. Polar Biol 26:774–782
Teschke M, Saborowski R (2005) Cysteine proteinases substitute for serine proteinases in the midgut glands of Crangon crangon and Crangon allmani (Decapoda: Caridea). J Exp Mar Biol Ecol 316:213–229
Turkiewicz M, Galas E, Kalinowska H (1991) Collagenolytic serine proteinase from Euphausia superba Dana (Antarctic krill). Comp Biochem Physiol 99B:359–371
van den Thillart G, George RY, Strömberg J-O (1999) Hypoxia sensitivity and respiration of the euphausiid crustacean Meganyctiphanes norvegica from Gullmarn Fjord, Sweden. Sarsia 85:105–109
Acknowledgments
Many thanks are due to Dr. Dorothee Stübing who supplied Antarctic krill. The help of Gerrit Sahling in the laboratory is greatly acknowledged and the staff of the Marine Stations at Kristineberg (Sweden) and the Biologische Anstalt Helgoland (Germany) greatly supported this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saborowski, R. Related antipodes: a comparative study on digestive endopeptidases from Northern krill and Antarctic krill (Euphausiacea). Polar Biol 35, 1003–1012 (2012). https://doi.org/10.1007/s00300-011-1147-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-011-1147-2