Abstract
The use of indirect dietary markers, including stable isotopes, has immensely improved our knowledge of seabird trophic ecology throughout their annual cycle. Important aspects include differences in trophic niche between adults and chicks at the intra- and inter-specific levels and tissue-dependent differentiation in chicks. Using stable isotopic niche as a proxy for trophic ecology, we investigated how three closely related albatross species co-exist in the sub-Antarctic Prince Edward Islands. The effects of age, sex, tissue, and species on the isotopic niche were observed for Grey-headed Thalassarche chrysostoma, Sooty Phoebetria fusca, and Light-mantled Phoebetria palpebrata Albatrosses breeding on Marion Island. At the end of chick-rearing, carbon and nitrogen stable isotope values differed according to age, tissue, and species but not the sex of either adults or chicks. A complex pattern was revealed as the three species exhibited contrasting results. For example, values for δ13C or δ15N of chick blood could be depleted, enriched or similar relative to that of adults, depending on species. Stable isotope differences between blood and feathers likely reflect differences in their amino acid composition, while adult/chick differences will relate to their different physiological needs and diet. The results indicate that co-existence of the three species on the island is facilitated through resource partitioning among species in terms of foraging areas and in the trophic levels at which adults feed for themselves and their chicks. This work brings new insights into the effect of intrinsic factors on the foraging ecology of marine top predators.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Knowledge of the foraging ecology of seabirds throughout their annual cycle has increased tremendously since the development of bio-logging and the use of dietary or biochemical tracers (Burger and Shaffer 2008; Ramos and González-Solís 2012). Biochemical tracers, such as stable isotopes, follow the concept of ‘you are what you eat (plus [minus] a few per mil)’ first voiced by DeNiro and Epstein (1976). This means that nutrients pass from prey to predators in a predictable manner and that values in predators may be explained using both prey data and species- and tissue-specific discrimination factors. In marine ecosystems, the most frequently used elements in stable isotope analyses are carbon and nitrogen (Michener and Kaufman 2007). The carbon stable isotope ratio 13C/12C (δ13C) provides information on the food sources underpinning the food web because there is only a small enrichment from prey to predators (Caut et al. 2009). This enrichment results from preferential loss of 12C during respiration, preferential uptake of 13C enriched compounds during digestion and/or assimilation, or metabolic fractionation during synthesis of tissues (Michener and Kaufman 2007). The nitrogen stable isotope ratio 15N/14N (δ15N) gives an indication of the trophic level occupied by the predator, with a stepwise increase of ~ 4‰ between prey and marine predator from the same ecosystem (Caut et al. 2009) due to preferential excretion of 15N-depleted nitrogen usually in the form of urea and ammonia (Michener and Kaufman 2007). The precise discrimination factors can, however, vary according to species, tissue, diet or else nutritional stress (reviewed in Caut et al. 2009). As in all dietary methods, limitations in the use of stable isotopes in food web studies exist, such as the near impossibility of identifying prey species except in very simple food webs, and the impossibility of measuring and sexing prey consumed (reviewed in Barrett et al. 2007 and Karnovsky et al. 2012). Hence, it is advantageous of combining dietary methods to get the most complete picture as possible (e.g. Connan et al. 2014).
An important aspect of dietary tracers is that the integration time will depend on the tissue analysed as the signal is influenced by the metabolic turnover rate of the tissue. Tracers can, for example, provide information from a few days prior to sampling (blood plasma), up to several months with adipose tissue, and ultimately the whole lifetime an individuals in the case of bones and teeth (Dalerum and Angerbjörn 2005). In stable isotope analyses, the two tissues that are most commonly used in seabird studies to infer foraging areas and trophic levels are feathers and blood. In flying birds (as opposed to diving birds such as penguins), feathers store the isotopic information at the time of their growth (Mizutani et al. 1992), while whole blood provides isotopic information from the last few weeks (Hobson and Clark 1992).
In the last two decades, carbon and nitrogen stable isotopes have been used extensively to delineate trophic links in marine food webs including those in the Southern Ocean (e.g. Corbisier et al. 2004; Quillfeldt et al. 2005; Michener and Lajtha 2007; Cherel et al. 2010; Ceia et al. 2015). Clear latitudinal variations have been observed in carbon stable isotope ratios of the particulate organic matter (François et al. 1993; Trull and Armand 2001; Quillfeldt et al. 2010), and the use of these isoscapes in the southern Indian Ocean has recently been validated to infer migration patterns of seabirds (Jaeger et al. 2010b). Numerous studies conducted on marine top predators in the Southern Ocean have used stable isotopes to reveal the migration patterns of a wide range of seabird and fur seal species (e.g. Kernaléguen et al. 2012; Jaeger et al. 2013), the individual specialization of albatrosses (Jaeger et al. 2010a), and temporal shifts in the environment through analyses of feathers and teeth (Hilton et al. 2006; Hanson et al. 2009; Quillfeldt et al. 2010).
The development of biochemical tracers has permitted the inclusion of intra-specific factors such as sex and age in dietary studies as these may influence the trophic ecology of seabird species at particular times of their annual cycle (Quillfeldt et al. 2008a; Whitehead et al. 2017; Xavier et al. 2017). Indeed, male and female breeders face differing physiological needs, including the cost of egg production for female birds (Hayward and Gillooly 2011), and differences in parental care in some species (Burke et al. 2015). In the Southern Ocean, sexual differences have been investigated in a number of studies for the moulting period (e.g. Phillips et al. 2009), but studies during chick-rearing remain rare (but see Quillfeldt et al. 2008b; Ceia et al. 2012). Sexual segregation may occur at the adult level and potentially also at the chick level. In the dimorphic Wandering Albatross Diomedea exulans; regular weighing of chicks showed that male chicks received more food than female chicks (Weimerskirch et al. 2000) but whether the food quality differs is unknown.
In our study of three albatross species, we investigated: (1) whether adults of both sexes exhibited similar isotopic niches during both chick-rearing and moulting, (2) whether differences in isotopic niche existed between adults and chicks, (3) whether male and female chicks exhibited similar isotopic niches, and (4) to what extent tissue type affected the stable isotope values in chicks. We hypothesized that sex will affect stable isotope values in adults due to the differing physiological needs of breeding males and females. Similarly, we expected to find differences between conspecific adults and chicks due to their different physiological needs. However, since the three species exhibit very little sexual dimorphism, we did not expect any diet differences between male and female chicks. Finally, we anticipated differences between the tissues of chicks produced concurrently due to their different compositions. Ultimately, this work complements the study by Connan et al. (2014) in which spatial and trophic segregations were highlighted using stomach content, lipid and stable isotope analyses. The present work clarifies how the three closely related species share their resources when competition for food is most likely, that is during the breeding season.
Materials and methods
Study site and sampling
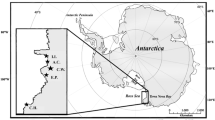
Marion Island forms part of the Prince Edward Islands (46°55′S, 037°54′E) and is located in the sub-Antarctic zone between the sub-Antarctic front to the north and the Antarctic polar front to the south (Ansorge and Lutjeharms 2002; Fig. 1). The island is a breeding ground for three species of medium-sized albatrosses: Grey-headed Thalassarche chrysostoma, Sooty Phoebetria fusca and Light-mantled Albatrosses Phoebetria palpebrata. The endangered Grey-headed Albatross is a colonial species with c.a. 6709 pairs breeding in the southern area of Marion Island in 2013 (~ 7% world population; BirdLife International 2016). The Sooty Albatross is also listed as endangered in the IUCN red list and Marion Island is home to 14% of the world’s breeding population (1950 pairs in 2014–2015; Schoombie et al. 2016). Only 1% of the world’s breeding population of the near-threatened Light-mantled Albatross is found on Marion Island (~ 246 pairs in 2014) with a decreasing trend since 2006 (Schoombie et al. 2016). At the island, Sooty Albatrosses exclusively nest on coastal cliffs while Light-mantled Albatrosses nest on both coastal and inland cliffs. The three species are summer breeders, returning to colonies in August–September, laying a single egg in October. Hatching occurs in December, and chicks fledge in May–June (Berruti 1979, pers. obs.).
Geographic situation of the Prince Edward Islands with respect to the frontal systems of the Southern Indian Ocean (APF: Antarctic Polar Front; SAF: sub-Antarctic Front; STF: sub-Tropical Front; adapted from Durgadoo et al. 2010). Sampling locations of Grey-headed Albatrosses (white star) and fuliginous albatrosses (black stars) are indicated
Fieldwork was conducted on the island in April and May 2009, at the end of chick-rearing for all three species. Blood and feathers were collected from adults and all feathered chicks from the east and south of the island (Fig. 1). All well-feathered chicks were preferred to downy chicks to reduce as much as possible the influence of intense growth on stable isotope values (Sears et al. 2009). Four feathers were plucked from the back of each bird’s neck, placed in a ziploc bag and stored at − 20 °C as soon as possible until lab processing. Blood was collected from the tarsal vein using a slightly heparinised sterile syringe with a 24 or 25G needle. Given the remoteness of some of the sampling sites, it was necessary to store blood samples immediately in 70% ethanol. Preservation in ethanol 70% does not significantly alter the carbon or nitrogen stable isotope values of blood samples (Hobson et al. 1997).
Morphometric measurements, including culmen length, tube length, bill depth pre-tube and at gonys, tarsus and wing lengths were taken from adults using Vernier callipers (± 0.1 mm) or a ruler (± 1 mm), as required. Birds were weighed using a Pesola spring balance (± 25 g). Species identification for Sooty and Light-mantled Albatross chicks were based on colour and extent of their eye-ring (Connan et al. 2011), and confirmed by molecular analysis for borderline cases. Overall, 45 Grey-headed (24 adults, 21 chicks), 37 Sooty (15 adults, 22 chicks), and 27 Light-mantled (8 adults, 19 chicks) Albatrosses were sampled.
Molecular sexing
Fieldwork was conducted at the end of the chick-rearing period, but outside of the monitoring colonies which are already in use for demographic studies; therefore no prior information was available on the sex of sampled adults. Behaviour (observed copulatory position or pre-laying attendance; Phillips et al. 2004) or culmen morphometrics (birds with depth at the gonys > 29 mm were considered males: Nel et al. 2000) have been used to sex Grey-headed Albatrosses. Even though adult males of the three species tend to be heavier and to exhibit larger features than adult females (Berruti 1979; Jouventin and Weimerskirch 1984), sexual dimorphism is not sufficiently pronounced to be reliable for accurate sexing; molecular analysis was therefore used to confirm sex. Total genomic DNA was extracted from blood samples using the DNeasy Blood and tissue Kit (Qiagen), according to the manufacturer’s protocol. Birds were sexed by polymerase chain reaction (PCR) amplification of partial fragments of two chromo-helicase-DNA-binding genes (CHD) present on the sex chromosomes (Fridolfsson and Ellegren 1999); the CHD-Z gene is present in both sexes, whilst the CHD-W gene is present in females only. The sex linked CHD was amplified using a PCR amplification and the two 2550F and 2718R primers (Fridolfsson and Ellegren 1999). Amplification reactions were performed in 20 µL final volumes, including 3 mM MgCl2, 2 µL 10 × NH4 Buffer, 160 µM each deoxyribonucleotide triphosphate (dNTP), 0.4 µM each primer, 0.5 U Bioline BioTAQ™ DNA polymerase, and 5 µL of template. Thermocycling conditions for amplification included an initial denaturation step of 2 min at 94 °C, followed by a touch-down cycle lowering the annealing temperature in 1 °C decrements, from 50 to 42 °C. Thirty additional cycles were then run at 42 °C. Cycles comprised a denaturation step at 94 °C for 30 s, 30 s at annealing temperature, and extension at 72 °C for 1 min. A final extensive phase at 72 °C lasted 5 min. PCR products were bound with SYBR® Green I and checked via electrophoresis on a 1.8% agarose gel with TBE Buffer and subsequent visualization under UV radiation.
Stable isotope analyses
Whole blood samples were dried at 50 °C for 24–48 h and finely ground to a homogenous powder. Individual feathers were cleaned in a 2:1 chloroform:methanol solution placed in an ultrasonic bath for 2 min, rinsed in successive baths of methanol and deionised water, and then dried (50 °C, 24 h). Each whole body feather was homogenized by finely cutting with scissors. Relative isotope abundances of carbon and nitrogen were determined by combusting samples in a Flash 2000 organic elemental analyzer and passing the gases to a Delta V Plus isotope ratio mass spectrometer (IRMS) via a Conflo IV gas control unit (all three items were Thermo Scientific, Bremen, Germany; Stable Light Isotope Unit, University of Cape Town, South Africa). Carbon and nitrogen stable isotope results are presented in the usual δ notation relative to Vienna Pee Dee Belemnite and atmospheric N2 standards, respectively: \(\delta^{13} {\text{C or }}\delta^{15} {\text{N }}\left( \permille \right) \, = \, \left[ {\left( {{{R_{\text{sample}} } \mathord{\left/ {\vphantom {{R_{\text{sample}} } {R_{\text{standard}} }}} \right. \kern-0pt} {R_{\text{standard}} }}} \right) \, - \, 1} \right] \, \times 10^{3}\).
Here, Rsample and Rstandard are the ratios of 13C/12C (for δ13C) or 15N/14N (for δ15N) for the samples and the references (Vienna Pee Dee Belemnite, atmospheric N2), respectively. Replicate measurements of internal laboratory standards (Merck gel δ13C = − 20.05‰, δ15N = 7.50‰; seal bone δ13C = − 11.97‰, δ15N = 15.84‰; valine δ13C = − 26.80‰, δ15N = 12.14‰; calibrated against reference materials from the International Atomic Energy Agency [IAEA, Vienna, Austria]) indicated measurement errors < 0.17 and < 0.12‰ for carbon and nitrogen stable isotope measurements, respectively. All stable isotope analyses were conducted in the Stable Light Isotope Unit, University of Cape Town, South Africa.
Data analysis
Possible within species differences between the sexes in adult morphometric measurements and in stable isotope values were tested using parametric t-tests or non-parametric Mann–Whitney tests, depending on the outcome of tests for normality (Shapiro–Wilk test) and homogeneity of variance (Levene’s test). A stepwise discriminant analysis was used on bill measurements to investigate which of these were the most suitable to distinguish males from females. A similar parametric/non-parametric approach was used to investigate differences between adults and chicks of the same species. Differences between tissues were investigated for chicks only because blood and feathers in adults correspond to two different periods, i.e. chick-rearing and moulting, respectively. Blood and feather stable isotope data for chicks were compared using a t test for paired samples or a Wilcoxon test if the data did not conform to the assumptions of normality or homogeneity of variance.
Comparisons among adults of the three species of albatrosses have previously been investigated (Connan et al. 2014). We therefore focussed on chick data for the comparison among species. Isotopic richness (total convex hull areas) was estimated separately for blood and feather stable isotope values by first scaling the data between 0 and 1 as advised by Cucherousset and Villéger (2015) and then using a bootstrapping approach because the number of samples differed among species (Cucherousset and Villéger 2015). A number of isotopic metrics (isotopic-dispersion, -divergence, -evenness, and -uniqueness) were calculated to bring information on the variability among individuals within species (Cucherousset and Villéger 2015). Isotopic similarity, corresponding to the ratio between the volume shared by the three species and the volume of the union of the three convex hulls, was then calculated among the three species by adapting the script produced by Cucherousset and Villéger (2015). The approach presented by Cucherousset and Villéger (2015; total convex hull area combined with a bootstrapping approach) was preferred to the Bayesian ellipse approach (Jackson et al. 2011) as it quantitatively assesses several aspects of isotopic diversity by providing abundance-weighted and unitless indices which can then be used to compare across ecosystems.
All statistical analyses were conducted using R v3.2.5 (R Team 2016). Significance level was set at 0.05.
Results
Adult biometrics
Males and females of the three species showed overlap in tarsus and all bill measurements (Table 1). However, Grey-headed Albatross males were significantly heavier, and had significantly greater depth at pre-tube, at gonys, and on tube than females (Table 1; all p < 0.011). The discriminant analysis conducted on bill measurements selected these last two measurements, depth at gonys and depth on tube, to assign 91.7% of Grey-headed Albatrosses to the correct sex (λwilks = 0.392, F(2,21) = 16.250, p < 0.001). Similarly, Sooty Albatross males were significantly heavier than females and showed greater depth on tube and at gonys; they also had a longer tube length and culmen (Table 1; all p < 0.05). Culmen length and depth on tube were selected by a discriminant analysis to assign correctly 93.3% of Sooty Albatrosses (λwilks = 0.370, F(2,12) = 10.230, p = 0.003). The small number of samples of Light-mantled Albatrosses precluded a comparison between male and female measurements of this species.
When both adults from the same nest were caught (2 pairs of Grey-headed Albatrosses, and 2 pairs of Sooty Albatrosses), no clear pattern was detected considering the seven measurements, with males not always exhibiting the bigger features within the pair.
Influence of sex on stable isotope ratios
The influence of sex on carbon and nitrogen stable isotope ratios of blood and feathers was investigated for adults and chicks of Grey-headed and Sooty Albatrosses but only among chicks of Light-mantled Albatrosses. No significant differences were found between males and females of either adults or chicks in any of the three species (Table 2).
Influence of age on stable isotope ratios
In contrast to sex, age did affect stable isotope values. During chick-rearing (blood data), Grey-headed Albatross chicks exhibited significantly higher δ15N than adults (Mann–Whitney test, U = 87, p = 0.0002), while their δ13C ratios were similar (t test, t = − 0.253, p = 0.801; Fig. 2a). On the other hand, Sooty Albatrosses showed the opposite, adults exhibited higher δ13C values than chicks (t test, t = 3.384, p = 0.002) but their δ15N were similar (Mann–Whitney test, U = 142.5, p = 0.494; Fig. 2a). No differences were found between Light-mantled Albatross adults and chicks for either δ13C (t test, t = − 0.356, p = 0.725) or δ15N (t test, t = − 1.126, p = 0.271; Fig. 2a).
In terms of stable isotope data from feathers, chicks of all three species exhibited significantly lower δ13C values than conspecific adults (Grey-headed Albatross: Mann–Whitney test, U = 49.5, p < 0.0001; Sooty Albatross: Mann–Whitney test, U = 1, p < 0.0001; Light-mantled Albatross: Mann–Whitney test, U = 19, p = 0.003; Fig. 2b). No differences were found between δ15N of adults and chicks of Grey-headed Albatross (Mann–Whitney test, U = 238.5, p = 0.767) or Light-mantled Albatrosses (Mann–Whitney test, U = 41.5, p = 0.070), but in Sooty Albatrosses, δ15N values were significantly lower for chicks than adults (t test, t = 8.296, p < 0.0001; Fig. 2b).
The variability among individuals, as reflected by the standard deviations, was similar between adults and chicks for blood (Fig. 2a) but not feathers (Fig. 2b). This reflects the fact that values for blood represent a common food source for the two age classes during the chick-rearing period, but separate foraging during feather growth. Variability among the feathers of conspecific adults was two to three times greater than among chick feathers for both δ13C and δ15N with the exception of δ15N values for Sooty Albatross feathers, which showed similar variability among adults and among chicks (Fig. 2b).
Influence of chick tissue on stable isotope ratios
Tissues had a significant influence on stable isotope ratios in 5 out of 6 comparisons (Fig. 3). δ13C values were significantly lower in blood than in feathers in all three species (Grey-headed Albatross: Paired t test, t = − 14.782, p < 0.0001; Sooty Albatross: Wilcoxon signed rank test, W = 231, p < 0.0001; Light-mantled Albatross: Paired t test, t = − 6.803, p < 0.0001). δ15N values were higher for feathers than blood, but this was significant only for Grey-headed and Light-mantled Albatrosses (Grey-headed Albatross: Paired t test, t = − 3.963, p = 0.0008; Sooty Albatross: Wilcoxon signed rank test, W = 130, p = 0.350; Light-mantled Albatross: Paired t test, t = − 3.590, p = 0.002). Trends between tissues were similar in the three species; however, the difference between tissues was not equal, ranging from 1.3‰ (Light-mantled Albatross) to 2.0‰ (Grey-headed Albatross) for δ13C and from 0.1‰ (Sooty Albatross) to 0.6‰ (Grey-headed and Light-mantled Albatrosses) for δ15N.
Influence of species on stable isotope ratios in chicks
Species had a significant influence on both δ13C and δ15N values measured in chick blood (Fig. 2a; δ13C: ANOVA, F(2,62) = 17.32, p < 0.0001; δ15N: Kruskal–Wallis test, H(2,62) = 29.44, p < 0.0001). Sooty Albatross chicks had significantly higher δ13C than both other species (both Tukey’s pairwise comparisons, p < 0.0004) but Light-mantled and Grey-headed Albatrosses exhibited similar values (p = 0.381). In contrast, δ15N values differed significantly among the chicks of all three species (all Mann–Whitney pairwise comparisons, p < 0.017). The total convex hull was three times bigger for Grey-headed Albatrosses (0.34‰2) than for Sooty Albatrosses (0.11‰2), with Light-mantled Albatrosses in the middle (0.23‰2) (Table 3). In general, Sooty Albatrosses exhibited the lowest indices compared to the two other species particularly in the case of isotopic uniqueness (0.16 compared to 0.28 and 0.34 for Grey-headed and Light-mantled Albatrosses, respectively; Table 3). Overlap among the three species considering δ13C and δ15N together (geographic area and trophic level of prey used to raise their chicks, respectively) reached 6% (Fig. 4a).
As with blood, Sooty Albatross feathers exhibited the highest δ13C, followed by Grey-headed Albatross and Light-mantled Albatross feathers (Kruskal–Wallis test, H(2,62) = 37.2, p < 0.0001; all Mann–Whitney pairwise comparisons, p < 0.024). δ15N values measured in Grey-headed Albatrosses were statistically lower than in the two fuliginous species (ANOVA, F(2,62) = 9.942, p = 0.0002; both Tukey’s pairwise comparisons, p < 0.005). Sooty and Light-mantled Albatross feathers exhibited similar δ15N values (Tukey’s pairwise comparisons, p = 0.705). Convex hull areas for Grey-headed and Sooty Albatrosses were the biggest (0.31‰2 and 0.23‰2) and more than twice as big as those for Light-mantled Albatross (0.14‰2) (Table 3). Contrary to blood data, Light-mantled Albatrosses exhibited the lowest isotopic indices, particularly for isotopic dispersion (0.35 vs. 0.51 for the other two species; Table 3) and isotopic uniqueness (0.29 vs. 0.39 and 0.53, for Light-mantled, Sooty and Grey-headed Albatrosses, respectively; Table 3). Considering δ13C and δ15N together, chick feather data suggested that the three species did not share any isotopic space (Fig. 4b).
Discussion
This work brings new insights into factors affecting stable isotope values in seabirds, by highlighting the potential effects of tissue, age, species, and sex of the birds on stable isotope values in three species of Southern Ocean albatrosses. In combination with data presented in Connan et al. (2014), the comparison of the three closely related species improves our understanding on how they share resources to facilitate co-existence, and it further clarifies the degree to which differences in stable isotope values are linked to intrinsic (genetic) or extrinsic (behavioural) factors.
Sexual isotopic segregation among breeding adults has been found in Grey-headed (Phillips et al. 2004, 2011) and Waved Phoebastria irrorata (Awkerman et al. 2007) Albatrosses as well as non-breeding Wandering (Jaeger et al. 2009; Phillips et al. 2009) and Grey-headed (Phillips et al. 2009) Albatrosses. On Marion Island, a few tracks from sexed Grey-headed Albatrosses showed distinctive differences in foraging behaviour during incubation and early chick-rearing but any effects on sexual dietary segregation remained unknown (Nel et al. 2000). Our blood stable isotope data suggest that during late chick-rearing, breeding females and males do not exhibit any isotopic (and thus dietary or latitudinal) segregation in any of the three species studied. Both parents of the three species take part in incubation shifts as well as feeding the chick (Weimerskirch et al. 1986). The observed sexual foraging segregation in early chick-rearing, with male Grey-headed Albatrosses favouring short foraging trips over long ones, may either disappear in late chick-rearing as the number of long foraging trips is likely to increase at the expense of short ones as the chicks grow older (Weimerskirch and Lys 2000), or else foraging segregation is not reflected by isotopic segregation. Costs of reproduction and carry-over effects have been highlighted in female Grey-headed Albatrosses (Crossin et al. 2017) but the mostly biennial breeding strategy of the species may allow the females to recover in the long term rather than displaying different foraging behaviour from males to allow rapid recovery within a single breeding season.
Stronger investment towards male chicks has been shown in dimorphic (Wandering Albatross; Weimerskirch et al. 2000) and monomorphic (Common Murre Uria aalge; Cameron-MacMillan et al. 2007) species which fledge only a single chick. Different diets between male and female chicks have also been observed in the slightly dimorphic Adélie Penguin Pygoscelis adeliae (Jennings et al. 2016). Our stable isotope data indicated that male and female chicks were fed with species originating from similar geographic areas and trophic levels. We have, however, no data about possible differences in the mass of prey that chicks received, nor are we aware of such data in other studies. Grey-headed, Sooty and Light-mantled Albatrosses can be considered monomorphic species (Table 1), so, unlike the Wandering Albatross, it is not necessary to favour male chicks so that they can attain a bigger size in the limited time available.
Sampling in April–May coincides with the end of chick-rearing for our three species (Berruti 1979, pers. obs.). Chicks were thus not in the intense growing phase (Ricketts and Prince 1981, Terauds and Gales 2006) that can affect stable isotope values in other seabirds such as the Rhinoceros Auklet Cerorhinca monocerata (Sears et al. 2009). When comparing chick blood and feathers within species, the trend observed by Quillfeldt et al. (2008a) was similar, with chick feathers generally exhibiting higher δ13C and δ15N values than chick blood. In addition, our data followed the linear regression between δ13C (or δ15N) blood against δ13C (or δ15N) feathers calculated mostly for adults from 31 species by Cherel et al. (2014). Differences between tissues mainly originate from tissue specific composition as explained in detail by Cherel et al. (2014). Our data reinforce the call for additional detailed studies about the relationships among these tissues (Cherel et al. 2014). Indeed, differences in δ15N values between tissues were as expected, higher δ15N values in feathers, in Grey-headed and Light-mantled Albatrosses but not in Sooty Albatrosses where no statistical differences were observed between blood and feathers (Fig. 3). So far, only two species (Cape Daption capense and Snow Pagodroma nivea Petrels) out of 31, did not follow the pattern with higher δ15N values measured in blood than in feathers (Cherel et al. 2014). This was linked to a change in diet between the diet integration information reflected in blood and feathers. The differences among the chicks of three albatross species observed in our study could thus be (i) the result of a slight change in diet between the time periods reflected in blood and feathers (Phillips and Hamer 2000; Evans Ogden et al. 2004), or (ii) a change in physiology that would artificially increase blood—or decrease feather—δ15N values in Sooty Albatross chicks.
Interestingly, overlaps among species differed between chick blood and chick feathers (Fig. 4a and 4b). One explanation may be linked to differing diet integration time between those two tissues and a change in diet as chick-rearing advances. Whole blood represent diet information for a few weeks prior to sampling (i.e. mid-March/April), while feathers represent diet at the time of their growth, which may have happen before mid-March/April as all chicks were completely feathered when we sampled them. In Northern Fulmar Fulmarus glacialis chicks, most of the feather production happens in the second third of their rearing period (Phillips and Hamer 2000). If this is the case for the three albatross species, then there may have been a slight time mismatch between blood and feather diet information. Differences in nutrient allocation among the three species are unlikely, considering the genetic relatedness of the three species, particularly the Sooty and Light-mantled Albatrosses (Chambers et al. 2009).
Comparison between adult and chick blood data within species indicated different patterns among the three albatross species. Light-mantled Albatrosses fed their chicks with prey from a similar geographic area (south of the Antarctic polar front; Cherel and Hobson 2007) and of similar trophic level to the prey they assimilated themselves (Fig. 2). Likewise breeding Grey-headed Albatrosses fed for themselves in similar areas to those where they caught food for their chicks (to the south or in the proximity of the Antarctic polar front; Jaeger et al. 2010b), but they seemed to feed their chicks with higher trophic level prey, for example squid rather than myctophid fish (Cherel et al. 2008), as indicated by higher δ15N in chicks (Fig. 2). The fatty acid analysis of stomach oils, which adults produce from prey eaten far from their breeding ground (Warham et al. 1976), supported this hypothesis: oils mainly originated from fish while stomach contents were mainly composed of a mix of fish and squid remains (Connan et al. 2014). Indeed, the lipid part of the prey is concentrated in the form of stomach oils to address the trade-off of expensive flight costs and energy-rich food for the chick. The oil is regurgitated to the chick, while the protein part is digested by the adult (Cheah and Hansen 1970; Clarke and Prince 1976). The situation for Sooty Albatrosses is slightly more complicated. Carbon stable isotope values suggested that breeding adults provisioned both themselves and their chicks from the sub-Antarctic zone (between the sub-Antarctic and Antarctic polar fronts; Cherel and Hobson 2007), but, surprisingly, in more southerly waters for their chicks than for themselves. Subsequent tracking of breeding adults from Marion Island (46°S) showed that some individuals forage as far north as 35°S while brooding/guarding chicks (Schoombie et al. 2017). This difference in geographical area is likely to affect the baseline in δ15N, because like carbon stable isotopes, nitrogen stable isotopes exhibit a gradient in the Southern Ocean with higher values observed in lower latitudes (Jaeger et al. 2010b). The trophic information provided by δ15N was therefore likely confounded with the geographical gradient of nitrogen stable isotopes in the Southern Ocean. This hypothesis could be verified using the compound specific isotope analysis approach. By analysing the nitrogen stable isotope values of specific source and trophic amino acids (e.g. phenylalanine and glutamic acid, respectively), this would allow one to disentangle the relative influence of variability at the base of the food web from trophic variability in nitrogen stable isotope values of adults and chicks of Sooty Albatrosses (e.g. Lorrain et al. 2009). Accepting the hypothesis of a confounding effect of δ15N baseline would suggest that Sooty Albatross chicks were fed with prey species of higher trophic levels than the adults preyed on for themselves. Altering the balance of chick diets in favour of squid over fish by Grey-headed and Sooty Albatrosses is difficult to understand from an energetic point of view as fish normally have a higher energy content (e.g. Cherel and Ridoux 1992). High δ15N could also be the result of seabird consumption (e.g. Salvin Prion, Pachyptila salvini, Soft-plumaged Petrel, Pterodroma mollis or else penguins likely from the genus Eudyptes) as seen particularly in the two fuliginous species (Cooper and Klages 1995; Connan et al. 2014).
Comparison of adult and chick feathers represents different timings with chick feathers being grown during chick-rearing and adult feathers during their moulting period which is usually outside of the breeding season in albatrosses (Warham 1996). δ13C values indicated that adults of all three species moulted mostly north of the Prince Edward Islands, though intra- and inter-individual variability was high (Connan et al. 2014). Chick feather data suggested that Light-mantled Albatross chicks were raised on prey originating from more southern waters than chicks from the two other species, corroborating the pattern seen in adults with Light-mantled Albatross being the most southerly feeding species of the three (Connan et al. 2014). Chick feather stable isotopes of Light-mantled and Sooty Albatrosses have also been studied at the Crozet Archipelago (Jaeger et al. 2010a). Our findings are in agreement with Jaeger et al. (2010a) who observed that Sooty Albatross chicks were fed with prey from more northern waters than Light-mantled Albatross chicks.
Conclusion
The three albatross species studied in this work are closely related genetically, especially the two fuliginous species (Chambers et al. 2009). The resource partitioning observed during both breeding and moulting within and between species (Connan et al. 2014; this study) is likely to facilitate their co-existence in the Prince Edward Islands. The fact that the three species do not react the same way, particularly in the differences between tissues, reinforces the hypothesis that phylogeny is not the only determinant of the blood and feather isotopic values (Cherel et al. 2014). Our work combined with tracking studies would reinforce the conclusion that behaviour plays a dominant role in shaping stable isotopes values in predators. Finally, the differences observed among the three species in our study may also be important in the context of climate change, particularly the predicted poleward movement of fronts in the Southern Ocean (Péron et al. 2012; Krüger et al. 2018). Understanding the effects of all possible intrinsic factors on the foraging ecology of these seabird species may help to understand if and how the species will adapt to future environmental changes and this will be key for their conservation.
References
Ansorge IJ, Lutjeharms JRE (2002) The hydrography and dynamics of the ocean environment of the Prince Edward Islands (Southern Ocean). J Mar Syst 37:107–127
Awkerman JA, Hobson KA, Anderson DJ (2007) Isotopic (δ15N and δ13C) evidence for intersexual foraging differences and temporal variation in habitat use in waved Albatrosses. Can J Zool 85:273–279
Barrett R, Camphuysen KCJ, Anker-Nilssen T, Chardine JW, Furness WR, Grarthe S, Hüppop O, Leopold MF, Montevecchi WA, Veit RR (2007) Diet studies of seabirds: a review and recommendations. ICES J Mar Sci 64:1675–1691
Berruti A (1979) The breeding biologies of the Sooty albatrosses Phoebetria fusca and P. palpebrata. Emu 79:161–175
BirdLife International (2016) IUCN red list for birds. http://www.birdlife.org. Accessed 9 Jan 2016
Burger AE, Shaffer SA (2008) Perspectives in ornithology: application of tracking and data-logging technology in research and conservation of seabirds. Auk 125:253–264
Burke CM, Montevecchi WA, Regular PM (2015) Seasonal variation in parental care drives sex-specific foraging by a monomorphic seabird. PLoS ONE 10:e0141190
Cameron-MacMillan ML, Walsh CJ, Wilhelm SI, Storey AE (2007) Male chicks are more costly to rear than females in a monogamous seabird, the common murre. Behav Ecol 18:81–85
Caut S, Angulo E, Courchamp F (2009) Variation in discrimination factors (δ15N and δ13C): the effect of diet isotopic values and applications for diet reconstruction. J Appl Ecol 46:443–453
Ceia FR, Phillips RA, Ramos JA, Cherel Y, Vieira RP, Richard P, Xavier JC (2012) Short- and long-term consistency in the foraging niche of wandering Albatrosses. Mar Biol 159:1581–1591
Ceia FR, Ramos JA, Phillips RA, Cherel Y, Jones DC, Vieira RP, Xavier JC (2015) Analysis of stable isotope ratios in blood of tracked wandering albatrosses fails to distinguish a δ13C gradient within their winter foraging areas in the southwest Atlantic Ocean. Rapid Commun Mass Spectrom 29:2328–2336
Chambers GK, Moeke C, Steel R, Trueman JWH (2009) Phylogenetic analysis of the 24 named albatross taxa based on full mitochondrial cytochrome b DNA sequences. Notornis 56:82–94
Cheah CC, Hansen IA (1970) Stomach oil and tissue lipids of the petrels Puffinus pacificus and Pterodroma macroptera. Int J Biochem 1:203–208
Cherel Y, Hobson KA (2007) Geographical variation in carbon stable isotope signatures of marine predators: a tool to investigate their foraging areas in the southern ocean. Mar Ecol Prog Ser 329:281–287
Cherel Y, Ridoux V (1992) Prey species and nutritive value of food fed during summer to king penguin Aptenodytes patagonicus chicks at possession island, crozet archipelago. Ibis 134:118–127
Cherel Y, Ducatez S, Fontaine C, Richard P, Guinet C (2008) Stable isotopes reveal the trophic position and mesopelagic fish diet of female southern elephant seals breeding on the Kerguelen Islands. Mar Ecol Prog Ser 370:239–247
Cherel Y, Fontaine C, Richard P, Labat J-P (2010) Isotopic niches and trophic levels of myctophid fishes and their predators in the southern ocean. Limnol Oceanogr 55:324–332
Cherel Y, Jaquemet S, Maglio A, Jaeger A (2014) Differences in δ13C and δ15N values between feathers and blood of seabird chicks: implications for non-invasive isotopic investigations. Mar Biol 161:229–237
Clarke A, Prince PA (1976) The origin of stomach oil in marine birds: analyses of the stomach oil from six species of subantarctic Procellariiform birds. J Exp Mar Biol Ecol 23:15–30
Connan M, Kelly CMR, McQuaid CD, Bonnevie BT, Barker NP (2011) Morphological versus molecular identification of Sooty (Phoebetria fusca) and Light-mantled (P. palpebrata) albatross chicks. Polar Biol 34:791–798
Connan M, McQuaid CD, Bonnevie BT, Smale MJ, Cherel Y (2014) Combined stomach content, lipid and stable isotope analyses reveal spatial and trophic partitioning among three sympatric albatrosses from the Southern Ocean. Mar Ecol Prog Ser 497:259–272
Cooper J, Klages NTW (1995) The diets and dietary segregation of sooty albatrosses (Phoebetria spp.) at subantarctic marion island. Antarct Sci 7:15–23
Corbisier TN, Petti MAV, Skowronski RSP, Brito TAS (2004) Trophic relationships in the nearshore zone of martel inlet (King George Island, Antarctica): δ13C stable-isotope analysis. Polar Biol 27:75–82
Crossin GT, Phillips RA, Lattin CR, Romero LM, Bordeleau X, Harris CM, Love OP, Williams TD (2017) Costs of reproduction and carry-over effects in breeding albatrosses. Antarct Sci 29:155–164
Cucherousset J, Villéger S (2015) Quantifying the multiple facets of isotopic diversity: new metrics for stable isotope ecology. Ecol Indic 56:152–160
Dalerum F, Angerbjörn A (2005) Resolving temporal variation in vertebrate diets using naturally occurring stable isotopes. Oecologia 144:647–658
DeNiro MJ, Epstein S (1976) You are what you eat (plus a few per mill): the carbon isotope cycle in food chains. Geol Soc Am Bull 8:834–835
Durgadoo JV, Ansorge IJ, Lutjeharms JRE (2010) Oceanographic observations of eddies impacting the Prince Edward Islands, South Africa. Antarc Sci 22:211–219
Evans Ogden LJ, Hobson KA, Lank DB (2004) Blood isotopic (δ13C and δ15N) turnover and diet-tissue fractionation factors in captive dunlin (Calidris alpina pacifica). Auk 121:170–177
François R, Altabet MA, Goericke R (1993) Changes in the δ13C of surface water particulate organic matter across the subtropical convergence in the SW Indian Ocean. Global Biogeochem Cy 7:627–644
Fridolfsson AK, Ellegren H (1999) A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol 30:116–121
Hanson NN, Wurster CM, Bird MI, Reid K, Boyd IL (2009) Intrinsic and extrinsic forcing in life histories: patterns of growth and stable isotopes in male Antarctic fur seal teeth. Mar Ecol Prog Ser 388:263–272
Hayward A, Gillooly JF (2011) The cost of sex: quantifying energetic investment in gamete production by males and females. PLoS ONE 6:e16557
Hilton GM, Thompson DR, Sagar PM, Cuthbert RJ, Cherel Y, Bury SJ (2006) A stable isotopic investigation into the causes of decline in a sub-Antarctic predator, the rockhopper penguin Eudyptes chrysocome. Glob Change Biol 12:611–625
Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes I: turnover of 13C in tissues. Condor 94:181–188
Hobson KA, Gibbs HL, Gloutney ML (1997) Preservation of blood and tissue samples for stable-carbon and stable-nitrogen isotope analysis. Can J Zool 75:1720–1723
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. J Anim Ecol 80:595–602
Jaeger A, Blanchard P, Richard P, Cherel Y (2009) Using carbon and nitrogen isotopic values of body feathers to infer inter- and intra-individual variations of seabird feeding ecology during moult. Mar Biol 156:1233–1240
Jaeger A, Connan M, Richard P, Cherel Y (2010a) Use of stable isotopes to quantify seasonal changes of trophic niche and levels of population and individual specialisation in seabirds. Mar Ecol Prog Ser 401:269–277
Jaeger A, Lecomte VJ, Weimerskirch H, Richard P, Cherel Y (2010b) Seabird satellite tracking validates the use of latitudinal isoscapes to depict predators’ foraging areas in the Southern Ocean. Rapid Commun Mass Spectro 24:3456–3460
Jaeger A, Jaquemet S, Phillips RA, Wanless RM, Richard P, Cherel Y (2013) Stable isotopes document inter- and intra-specific variation in feeding ecology of nine large southern Procellariiformes. Mar Ecol Prog Ser 490:255–266
Jennings S, Varsani A, Dugger KM, Ballard G, Ainley DG (2016) Sex-based differences in Adélie penguin (Pygoscelis adeliae) chick growth rates and diet. PLoS ONE 11:e0149090
Jouventin P, Weimerskirch H (1984) L’albatros fuligineux à dos sombre Phoebetria fusca, exemple de stratégie d’adaptation extrême à la vie pélagique. Rev Ecol (Terre Vie) 39:401–427
Karnovsky NJ, Hobson KA, Iverson SJ (2012) From lavage to lipids: estimating diets of seabirds. Mar Ecol Prog Ser 451:263–284
Kernaléguen L, Cazelles B, Arnould JPY, Richard P, Guinet C, Cherel Y (2012) Long-term species, sexual and individual variations in foraging strategies of fur seals revealed by stable isotopes in whiskers. PLoS ONE 7:e32916
Krüger L, Ramos JA, Xavier JC, Grémillet D, González-Solís J, Petry MV, Phillips RA, Wanless RM, Paiva VH (2018) Projected distribution of Southern Ocean albatrosses, petrels and fisheries as a consequence of climatic change. Ecography 41:195–208
Lorrain A, Graham B, Ménard F, Popp B, Bouillon S, van Breugel P, Cherel Y (2009) Nitrogen and carbon isotope values of individual amino acids: a tool to study foraging ecology of penguins in the Southern Ocean. Mar Ecol Prog Ser 391:293–306
Michener RH, Kaufman L (2007) Stable isotope ratios as tracers in marine food webs: An update. In: Michener R, Lajtha K (eds) Stable isotopes in ecology and environmental science. Blackwell Publishing Ltd, Singapore, pp 238–282
Michener R, Lajtha K (2007) Stable isotopes in ecology and environmental science. Blackwell Publishing Ltd, Singapore
Mizutani H, Fukuda M, Kabaya Y (1992) δ13C and δ15N enrichment factors of feathers of 11 species of adult birds. Ecology 73:1391–1395
Nel DC, Nel JL, Ryan PG, Klages NTW, Wilson RP, Robertson G (2000) Foraging ecology of grey-headed mollymawks at Marion Island, southern Indian Ocean, in relation to longline fishing activity. Biol Conserv 96:219–231
Péron C, Weimerskirch H, Bost C-A (2012) Projected poleward shift of king penguins’ (Aptenodytes patagonicus) foraging range at the Crozet Islands, southern Indian Ocean. Proc R Soc Lond B 279:2515–2523
Phillips RA, Hamer KC (2000) Postnatal development of northern fulmar chicks, Fulmarus glacialis. Physiol Biochem Zool 73:597–604
Phillips RA, Silk JRD, Phalan B, Catry P, Croxall JP (2004) Seasonal sexual segregation in two Thalassarche albatross species: competitive exclusion, reproductive role specialization or foraging niche divergence? Proc R Soc Lond B 271:1283–1291
Phillips RA, Bearhop S, McGill RAR, Dawson DA (2009) Stable isotopes reveal individual variation in migration strategies and habitat preferences in a suite of seabirds during the nonbreeding period. Oecologia 160:795–806
Phillips RA, McGill RAR, Dawson DA, Bearhop S (2011) Sexual segregation in distribution, diet and trophic level of seabirds: insights from stable isotope analysis. Mar Biol 158:2199–2208
Quillfeldt P, McGill RAR, Furness RW (2005) Diet and foraging areas of Southern Ocean seabirds and their prey inferred from stable isotopes: review and case study of Wilson’s storm-petrel. Mar Ecol Prog Ser 295:295–304
Quillfeldt P, Bugoni L, McGill RAR, Masello JF, Furness RW (2008a) Differences in stable isotopes in blood and feathers of seabirds are consistent across species, age and latitude: implications for food web studies. Mar Biol 155:593–598
Quillfeldt P, McGill RAR, Masello JF, Weiss F, Strange IJ, Brickle P, Furness RW (2008b) Stable isotope analysis reveals sexual and environmental variability and individual consistency in foraging of thin-billed prions. Mar Ecol Prog Ser 373:137–148
Quillfeldt P, Masello JF, McGill RAR, Adams M, Furness RW (2010) Moving polewards in winter: a recent change in the migratory strategy of a pelagic seabird? Front Zool 7:15–26
Ramos R, González-Solís J (2012) Trace me if you can: the use of intrinsic biogeochemical markers in marine top predators. Front Ecol Environ 10:258–266
Ricketts C, Prince PA (1981) Comparison of growth of Albatrosses. Ornis Scand 12:120–124
Schoombie S, Crawford RJM, Makhado AB, Dyer BM, Ryan PG (2016) Recent population trends of sooty and light-mantled albatrosses breeding on Marion Island. Afr J Mar Sci 38:119–127
Schoombie S, Dilley BJ, Davies D, Glass T, Ryan PG (2017) The distribution of breeding Sooty Albatrosses from the three most important breeding sites: gough, Tristan and the Prince Edward Islands. Emu 117:160–169
Sears J, Hatch SA, O’Brien DM (2009) Disentangling effects of growth and nutritional status on seabird stable isotope ratios. Oecologia 159:41–48
Team RC (2016) A Language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Terauds A, Gales R (2006) Provisioning strategies and growth patterns of Light-mantled Sooty Albatrosses Phoebetria palpebrata on Macquarie Island. Polar Biol 29:917–926
Trull TW, Armand L (2001) Insights into Southern Ocean carbon export from the δ13C of particles and dissolved inorganic carbon during the SOIREE iron release experiment. Deep-Sea Res II 48:2655–2680
Warham J (1996) The behaviour, population biology and physiology of the petrels. Academic Press, London and San Diego
Warham J, Watts R, Dainty RJ (1976) The composition, energy content and function of the stomach oils of Petrels (Order, Procellariiformes). J Exp Mar Biol Ecol 23:1–13
Weimerskirch H, Lys P (2000) Seasonal changes in the provisioning behaviour and mass of male and female wandering albatrosses in relation to the growth of their chick. Polar Biol 23:733–744
Weimerskirch H, Jouventin P, Stahl JC (1986) Comparative ecology of the six albatross species breeding on the Crozet Islands. Ibis 128:195–213
Weimerskirch H, Barbraud C, Lys P (2000) Sex differences in parental investment and chick growth in wandering albatrosses: fitness consequences. Ecology 81:309–318
Whitehead TO, Connan M, Ropert-Coudert Y, Ryan PG (2017) Subtle but significant segregation in the feeding ecology of sympatric penguins during the critical pre-moult period. Mar Ecol Prog Ser 565:227–236
Xavier JC, Trathan PN, Ceia FR, Tarling GA, Adlard S, Fox D, Edwards EWJ, Vieira RP, Medeiros R, De Broyer C, Cherel Y (2017) Sexual and individual foraging segregation in Gentoo penguins Pygoscelis papua from the Southern Ocean during an abnormal winter. PLoS ONE 12:e0174850
Acknowledgements
The authors thank PG Ryan, MGW Jones, B Dyer, and L Clokie for their valuable advices in the field. Isotope samples were analysed by I Newton at the Stable Light Isotope Laboratory of the University of Cape Town under the supervision of J Lanham. Funding and logistical support was provided by the Department of Environmental Affairs and Tourism through the South African National Antarctic Program and administered by the National Research Foundation. This work is based on research supported by the South African Research Chairs Initiative of the Department of Science and Technology and the National Research Foundation. The authors thank Prof Piepenburg, Prof Quillfeldt, and an anonymous reviewer for their valuable comments on an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international guidelines for the care and use of animals were followed. All procedures performed during the study were in accordance with the ethical standards of Rhodes University and were undertaken under an ethics clearance granted by its Animal Ethics committee.
Rights and permissions
About this article
Cite this article
Connan, M., Bonnevie, B. & McQuaid, C. Ontogeny, tissue, and species but not sex influence stable isotopic values of three albatross species. Polar Biol 41, 1175–1186 (2018). https://doi.org/10.1007/s00300-018-2276-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-018-2276-7