Abstract
The UV-susceptibility of zoospores of the lower sublittoral kelp Laminaria digitata was studied in the laboratory under varying fluence of spectral irradiance consisting of photosynthetically active radiation (PAR, 400–700 nm; = P), PAR + UV-A radiation (UV-A, 320–400 nm; = PA), and PAR + UV-A + UV-B radiation (UV-B, 280–320 nm; = PAB). In vivo absorption of phlorotannin, localisation of phlorotannin-containing physodes, structural changes, DNA damage and repair, photosynthesis and germination of zoospores were measured after exposure treatments and after 2–6 days of recovery in dim white light. Photodegradation of phlorotannins was observed after extended exposure to ultraviolet radiation (UVR). The UV-protective function of extra- and intracellular phlorotannins was, therefore, observed only after 8 h, but not after 16-h UVR exposure. The energetic cost of photoprotection may have caused the delay in ontogenic development of zoospores after 8-h exposure to PA and PAB treatment; longer exposure time corresponding to 16-h PA and PAB treatment eventually lead to cell degeneration at 6 days post-cultivation. The formation of cyclobutane–pyrimidine dimers (CPDs), as indicator of DNA damage, was not blocked by the UV-absorbing phlorotannins during the 16-h PAB exposure and the inability for DNA damage repair was likely responsible for low photosynthetic recovery and spore mortality. The higher sensitivity of L. digitata zoospores to UVR compared to other kelps such as Saccorhiza dermatodea and Alaria esculenta confirmed our hypothesis that the depth distribution of adult sporophytes in the field correlates to the sensitivity of their corresponding early life history stages to different stress factors in general and UVR in particular.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several studies showed that kelp zoospores and gametophytes are more sensitive to ultraviolet radiation (UVR) compared to their corresponding juvenile and adult sporophytes (e.g. Dring et al. 1996; Vèliz et al. 2006; Roleda et al. 2007 and references therein). Consequently, the unicellular, wall-less and 4-μm-large zoospores exposed to UVR suffer several adverse physiological, biochemical and ultrastructural damage (e.g. Huovinen et al. 2000; Swanson and Druehl 2000; Makarov and Voskoboinikov 2001; Wiencke et al. 2004; Roleda et al. 2005a; Steinhoff et al. 2008). After exposure to tolerable magnitude of UV dose, zoospores post-cultivated under low white light are capable of photosynthetic recovery and repair DNA damage leading to germination success (Roleda et al. 2005a, 2006a; Wiencke et al. 2007; Tala et al. 2007). In the field, propagules (e.g. spores and gametes) released and suspended within the euphotic zone can experience changes in both light quantity and quality. They can actively swim or passively sink, and settle under algal canopies or at depths where the low light environment is suitable for germination, and further development.
Among brown algae, phlorotannins are reported to serve as secondary metabolites of various functions. Aside from UV-screening, they also have various roles in reproduction, fertilisation, spore attachment and cell wall construction (Schoenwaelder 2002; Arnold and Targett 2003). However, the intracellular UV sunscreens in 1 to <10 μm size class organisms is suggested to afford benefits only at the expense of high energetic investment and with restricted efficiencies (Garcia-Pichel 1994).
The release of phlorotannins from kelp sporophytes into seawater via exudation, tissue erosion or cell damage has been reported to change the spectral characteristics of near shore waters, inhibiting the penetration of harmful UV-B radiation into the water column (Swanson and Druehl 2002 and references therein). Moreover, the release of clouds of spores with intracellular phlorotannin-containing physodes can screen each other, and together with phlorotannin exudates from the sorus can reduce the impact of UV-B exposure to UV-sensitive zoospores (Roleda et al. 2006b) and can compensate for the restricted efficiency of intracellular phlorotannin. Upon zoospore release, contact media show strong absorption in the UV region characteristic of phlorotannin (Roleda et al. 2005a, 2006a; Wiencke et al. 2007).
The impacts of global climate change have been manifested in high arctic environments. For example, the seasonal depletion of stratospheric ozone concentration over the polar regions initiated numerous research efforts to elucidate the biological consequences of the related increase in UV-B radiation reaching the biosphere. Kongsfjorden in Spitsbergen represents a model ecosystem for the Arctic to study the impact of UVR on a marine ecosystem. For example, detailed studies on the impact of UVR on different physiological, biochemical and structural responses in Arctic kelps have been reported in Saccorhiza dermatodea (Roleda et al. 2006a) and Alaria esculenta (Wiencke et al. 2007). The above studies showed species-specific response to the same set of controlled laboratory environmental factors.
The present study will look into the same aspects on another important kelp species Laminaria digitata. The photoprotective role of extracellular phlorotannin exudates available in the contact medium upon spore release and of intracellular phlorotannin-containing physodes relative to increasing UV dose will be discussed. Moreover, the results of this study will be discussed relative to the susceptibility of other arctic kelp (Alaria esculenta, Laminariales) and kelp-like (Saccorhiza dermatodea, Tilopteridales) species and its implication on depth distribution.
Materials and methods
Algal material
Fertile sporophytes of Laminaria digitata (Huds.) Lamour. were collected in June 2004 by SCUBA diving in Kongsfjorden (Spitsbergen, 78°55′N, 11°56′E). Blades with sori from five different individuals were cleaned of epiphytes, blotted dry with tissue paper and kept in darkness in a moist chamber at 0°C overnight or up to a maximum of 2 days. To induce zoospore release, sori were immersed in 5–10 ml filtered (0.2 μm pore size) seawater at ±15°C and exposed to natural sunlight close to a window (Wiencke et al. 2006). The initial zoospore density was estimated using a Neubauer chamber (Brand, Wertheim, Germany). Stock suspensions were diluted with filtered seawater to give spore densities appropriate for each experiment.
Light treatments
Two exposure desks were set up with fluorescent tubes 40 cm (shelf 1) and 30 cm (shelf 2) above the table. Photosynthetically active radiation (P) was provided by two white fluorescent tubes (L65 Watt/25S, Osram, Munich, Germany) and UVR by three UVA-340 fluorescent tubes (Q-Panel, Cleveland, OH). A third desk was set up with only two white fluorescent tubes to study subsequent recovery in dim white light (8 ± 2 μmol photons m−2 s−1). To study the effect of different wavelength ranges, the experimental units were covered with three different cut-off filters: Ultraphan transparent (280–700 nm, Digefra GmbH, Munich, Germany), Folanorm (320–700 nm, Folex GmbH, Dreieich, Germany) or Ultraphan URUV farblos (400–700 nm, Digefra GmbH) corresponds to the treatments PAB (PAR + UV-A + UV-B), PA (PAR + UV-A) and P (PAR), respectively. Ultraviolet irradiation was determined by use of a Kruse UV–visible spectrometer equipped with a cosine sensor (M. Kruse, Bremerhaven, Germany). The equivalent biologically effective doses (BEDs) were calculated using action spectra for DNA damage (280–320 nm; Setlow 1974). The available filter foils cut off wavelengths slightly differing from the definition of CIE (Commission Internationale De l’Éclairage, UV-B = 280–315 nm, UV-A = 315–400 nm). P was determined using a cosine quantum sensor attached to a LI-COR data logger (LI-1000, LI-COR Biosciences, Lincoln, NE). Weighted and unweighted irradiances are presented in Table 1. The maximum daily average irradiance in summer on Spitsbergen (June and July) is 790 μmol photons m−2 s−1 PAR, 17 W m−2 UV-A and 0.30 W m−2 UV-B in air (cf. Hanelt et al. 2001). The weighted UV-B radiation (UVery) at different water depths in Kongsfjorden was reported by Wiencke et al. (2006) with 1% UV-B penetration down to 6 m depth.
Absorption spectra
To determine the presence of UV-absorbing compounds, 80 ml zoospore suspension containing 3 × 105 spores ml−1, was filled into 85 × 15 mm culture dishes and exposed for 19 h to P, PA and PAB in shelf 2 (Table 1). A portion of the zoospore suspension was kept in the dark (dark control). After exposure, the spores were resuspended and filled into quartz cuvettes and scanned in the 250–700 nm waveband using UV 2401PC photometer (Shimadzu, Tokyo, Japan) equipped with an integrating sphere. The spectrophotometer was zeroed at 750 nm for each measurement to correct for turbidity and contaminating coloured compounds. Absorption spectra of the zoospore suspension, zoospores and the medium were determined using (1) zoospore suspension with seawater as reference, (2) zoospore suspension with filtrate as reference and (3) filtrate with seawater as reference, respectively. The filtrate was obtained by filtering the zoospore suspension through a 44-mm-diameter 1.0 μm pore size Nuclepore” polycarbonate membrane (Whatman, Maidstone, Kent, UK) using a vacuum pump at 400–600 mb to minimise damage to the cells. The experiment was performed three times with spores from different individuals.
Cell structure and physodes
Cell structure, presence and location of physodes of germinating zoospore germination were monitored after 4-, 8- and 16-h exposure to P, PA and PAB by light microscopy. Recovery of 8- and 16-h-exposed samples was studied after 2 and 6 days of cultivation under dim white light. Suspensions of freshly released zoospores from three individual sporophytes (n = 3) were diluted with filtered seawater to a concentration of 7 × 105 spores ml−1. Per sporophyte, 21 culture dishes (53 × 12 mm) containing a paper filter at the bottom and a cover slip (18 × 18 mm) on the paper filter were prepared and filled each with 10 ml zoospore suspension. After exposure under irradiance of shelf 2 (Table 1) at 7 ± 1°C, one-third of the samples was analysed immediately, while the rest was transferred under dim white light (8 ± 2 μmol photons m−2 s−1) for 2 or 6 days of recovery. At the sampling time, the cover slip with settled spores was removed from the filter, put on a slide, covered with another cover slip (22 × 22 mm) and examined in a light microscope (Axioplan imaging, Zeiss, Germany) using a high-magnification objective (numerical aperture 1.4). Micrographs were taken with a digital camera (Canon 70, Tokyo, Japan), and images were processed with Adobe Photoshop.
DNA damage and repair
DNA damage and its subsequent repair were determined after exposure for 1, 4, 8 and 16 h to PAB under shelf 1 and shelf 2 (Table 1) and at 2 days post-cultivation, respectively. For each experimental unit, 40 ml of the spore suspension (4 × 105 spores ml−1) was used. For each treatment, six experimental units were prepared. After exposure, three experimental units were processed immediately, while the other three were transferred under dim white light for recovery. Settled and germinated spores were gently resuspended by jetting the medium against the bottom of the culture dish using Eppendorf pipettes. Detachment of pigmented spores was visually observed. The remaining spores attached to the Petri dish was mechanically detached by rubbing slowly with the forefinger covered with latex gloves. The spore samples were filtered through 44 mm diameter, 1.0 μm pore size, Nuclepore” polycarbonate membrane. Filters were then placed separately into 2-ml Eppendorf tubes and frozen at −80°C for further analysis.
For DNA extraction, the filters with the frozen spore samples were incubated in cetyltrimethyl ammonium bromide (CTAB) extraction buffer, as described by van de Poll et al. (2001) and modified by Roleda et al. (2005a). After DNA extraction, the pellet obtained was dissolved in 0.2 ml Tris–EDTA buffer (10 mM Tris, 1 mM EDTA, pH 8.0), treated with RNAase (5 μl of 10 mg ml−1, 30 min, 37°C; Sigma, St Louis, MO) and stored at −20°C. The DNA concentration was quantified fluorometrically using the PicoGreen assay (Molecular Probes, Eugene, OR) and a Cary Eclipse Fluorescence Spectrophotometer (Variance Scientific Instrument, Palo Alto, CA). For calibration, a dilution series with a known amount of DNA (Serva, Heidelberg, Germany) was used.
Content of CPDs was determined using an immunoassay as described by van de Poll et al. (2001) and modified by Roleda et al. (2005a). Heat-denatured samples containing 50 ng DNA were transferred to a nitrocellulose membrane (Protran BA 79, pore size 0.1 μm, Schleicher and Schuell, Keene, NH) with a Minifold I SRC96 dot blot apparatus (Schleicher and Schuell). After a two-step antibody assay, the membrane was treated with ECLWestern blotting detection reagent (Amersham, Little Chalfont, Buckinghamshire, UK) and sealed in a transparent plastic folder. Subsequently, the films were exposed to photosensitive ECL films (Amersham) at different exposure times and then developed using X-ray film developer. Developed films were scanned using a Biorad imaging densitometer (Model GS-700, Bio-Rad, Hercules, CA). Quantification of the grey scale values was done by use of Multi-Analyst (Bio-Rad). A calibration series of UV-irradiated calf thymus DNA (Serva) supplemented with unexposed DNA was included giving 1 μg ml−1 DNA for each calibration point. The UV-irradiated DNA (45 min exposure to 2 TL-20W/12 lamps, Philips, Eindhoven, the Netherlands) was previously calibrated against UV-irradiated Hela DNA with known amounts of CPDs (kindly provided by A. Vink). CPDs were quantified by comparing the grey scales within the linear range of the film.
Maximum quantum yield of photosystem II
Photosynthetic efficiency of zoospores was measured as variable fluorescence of PSII by use of a water pulse amplitude modulation fluorometer connected to a PC with WINCONTROL software (HeinzWalz GmbH, Effeltrich, Germany). Immediately after adjustment of spore density (3 × 105 to 4 × 105 spores ml−1, not exceeding 1 h after spore release), the maximum quantum yield (F v/F m) was measured to determine initial photosynthetic efficiency at time zero (control, n = 5), as described by Roleda et al. (2006c). Controls measured at time zero were placed into cell culture dishes (35 × 10 mm) and exposed for 48 h to dim white light. For the treatments, fresh zoospore suspensions (5 ml) were filled into cell culture dishes and exposed for 8 and 16 h under shelf 1 and 2 to the three radiation treatments (Table 1; n = 5, per treatment) at 7 ± 1°C. After exposure, F v/F m was determined and the spore suspensions were exposed for 48 h to dim white light for recovery. The spores that settled and germinated in the meantime were gently resuspended by jetting the medium against the bottom of the culture dish using Eppendorf pipettes, and F v/F m was determined for a second time.
Germination
Culture dishes (53 × 12 mm) were filled with filtered seawater and two to five drops of zoospore suspension containing approximately 4 × 105 to 5 × 105 spores ml−1 from different sporophytes (n = 5) were added to each dish. The dishes were covered with cut-off filters and exposed for 8 and 16 h to P, PA and PAB under shelf 2 (Table 1). After exposure, the spores were transferred to dim white light for 3 or 6 days for recovery. Spores were scored as germinated or not germinated by counting 300 cells per replicate using light microscopes equipped with 20× seawater immersion objective. A spore was classified as germinated when at least a germ tube was formed. Dead and living cells were not differentiated.
Statistical analysis
To determine whether the data are homoskedastic and well-modelled by a normal distribution, they were tested for homogeneity (Levene Statistics) and normality (Kolmogorov–Smirnov test) of variance, respectively. Corresponding transformations were done to heteroskedastic and non-normal data. Response of dependent variables to varying irradiance and exposure time was tested using ANOVA (P < 0.05). All analyses were followed by Duncan’s multiple range test (P = 0.05). Statistical analyses were done using SPSS program (SPSS, Chicago, IL).
Result
Absorption characteristics
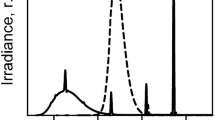
The zoospore suspensions (Fig. 1a), zoospores only (Fig. 1b) and the media (Fig. 1c) components showed a strong absorption in the ultraviolet range below 300 nm with in vivo absorption maxima at 265 nm characteristic of phlorotannins. DNA and proteins also absorb proximate to this wavelength region. In vivo absorption maxima at 675 and 440 nm are characteristic of chlorophyll a (Chl a) and the in vivo absorption maximum at 630 nm for chlorophyll c (Chl c). Brown algal fucoxanthin absorbs in vivo in the spectral range of 500–560 nm. β-carotene was less dominant and absorbed in vivo at 350–500 nm. The media (Fig. 1c) consist only of UV-absorbing compounds.
Absorption spectra in (a) zoospore suspension, (b) zoospores and (c) filtrate of Laminaria digitata exposed to 19 h of photosynthetically active radiation (PAR; P = 30 μmol photons m−2 s−1), PAR + UV-A (PA; UV-A = 7.0 W m−2), and PAR + UV-A + UV-B (PAB; UV-B = 0.6 W m−2). Absorption maxima at 675 and 265 nm are characteristics of chlorophyll a (Chl a) and phlorotannins (phl), respectively. Zoospore density is 3.0 × 106 spores ml−1
An increase in absorbance in the UV waveband was observed after 19 h of exposure of the zoospore suspension under P while a decrease in absorbance under PAB was observed compared with the dark control (Fig. 1a). The same trend was also observed in the zoospore-only fraction but with decrease in both PA and PAB treatment (Fig. 1b). Moreover, the impact of exposure to different radiation regimes was observed in the decrease of in vivo absorption of different pigments (Fig. 1b). For example, decrease in Chl a peak at 675 nm was 49, 25 and 12% under PAB, PA and P, respectively. UV-absorbing compounds in the medium were highest under P, followed by under PAB treatment compared with the dark control (Fig. 1c). Amount of UV-absorbing compounds in the medium after PA treatment is comparable to that of the dark control.
Changes in cellular structure
Freshly released zoospores of L. digitata were egg-shaped, 3–5 μm in diameter and possessed two flagella (blue arrows, Fig. 2a). One chloroplast per spore was observed during the time of release (green arrows, Fig. 2a). All cells were filled with numerous physodes (black arrows, Fig. 2a), appearing light to dark grey, often localised at the periphery of the cells. Furthermore, several vesicles, possibly phenolic bodies (grey arrows, Fig. 2a), were present in the medium of the zoospore suspension.
Laminaria digitata spores after release (a), 4 (b–d), 8 (e–g) and 16 (h–j) h exposure to PAR (b, e, h), PAR + UV-A (c, f, i), and PAR + UV-A + UV-B (d, g, j) on shelf 2. Black arrows are physodes appearing light to dark grey; grey arrows are phenolic bodies in the medium; red arrows are secretion of phenolic bodies into the medium; green arrows are chloroplasts appearing yellow to orange; blue arrows are flagella; white arrows are disintegrated cells. Scale bar = 5 μm
Exposure to P showed germling development within 16 h (left-hand side column, Fig. 2b, e, h). After 4 h of exposure to P (Fig. 2b), the majority of the zoospores were settled, circular in shape, and started to form germ tubes. After 4–8 h, physodes were located in the germ tubes (black arrows, Fig. 2b, e; see also Fig. 3c, f under PA). The chloroplasts became extremely elongated (green arrows, Fig. 2b, e; see also Fig. 2c under PA and Fig. 2g under PAB). After 8 h of exposure to P (Fig. 2e), germ tube formation and translocation of chloroplasts into the germ tubes were observed in many germlings. After 16 h of exposure to P (Fig. 3h), the young germlings had a characteristic pear-shape morphology. The original zoospore (embryospore) still contained most of the cell organelles, while the germ tube, typically containing several physodes (black arrows, Fig. 2h), became longer and thinner.
Laminaria digitata spores after 8- (a–f) and 16-(g–l) h exposure treatment to PAR (a, d, g, j), PAR + UV-A (b, e, h, k), and PAR + UV-A + UV-B (c, f, i, l) and corresponding 2 (a–c; g–i) and 6 (d–f; j–l) days of post-cultivation in low white light (8 μmol photons m−2 s−1). Black arrows are physodes appearing light to dark grey; grey arrows are phenolic bodies in the medium; green arrows are chloroplasts appearing yellow to orange; light blue arrows are empty embryospores; pink arrows are cross wall formation. Scale bar = 5 μm
After 4 and 8 h of exposure to PA or PAB, no apparent differences in zoospore development and cell structure were observed compared to the P treatment (Fig. 2b–d, e–g, respectively). Exocytosis of phenolic bodies was observed in PAB treatment (red arrows, Fig. 2d, g) not visible under P and PA. After 16 h of exposure in the PA or PAB treatment (Fig. 2i, j) drastic structural deformations were observed. Spores did not germinate; instead, disintegrated cells were observed (white arrows, Fig. 2i, j). Viable zoospores after 16 h of PA treatment were normally pigmented and rich in physodes (Fig. 2i), while the zoospores after 16 h of PAB treatment had lost their pigmentation, appeared pale and their chloroplasts appeared disintegrated. PAB-treated cells contained one or two enlarged physodes (black arrows, Fig. 2j).
At 2 and 6 days post-cultivation under dim white light, zoospores previously exposed to 8 and 16 h of “high” P treatment continued their normal development. Germlings with long swollen germ tubes enriched with numerous organelles were observed after 2 days (Fig. 3a, g); while the embryospores (light blue arrows, Fig. 3a, g) were devoid of organelles. Chloroplasts exhibited binary fissions (green arrows, Fig. 3a, g, see also Fig. 3b under PA). The length of germ tube doubled after 6 days post cultivation in both 8- and 16-h P-treated samples (Fig. 3d, j, respectively) and the first cross wall formations (pink arrows, Fig. 3d) were visible, resulting in 2-celled gametophytes. Several chloroplasts were located in the peripheral cytoplasm. Physode distribution within the gametophytes showed no regular pattern.
Zoospores exposed to 8 h of PA and PAB treatment showed a delay in ontogeny requiring 6 days to develop visible gametophytes (Fig. 3e, f, respectively). At 2-days post-cultivation, 8-h PA-treated zoospores (Fig. 3b) slowly developed into pear-shaped germlings, a stage already observed after 16-h exposure to P treatment (Fig. 2h). No germlings were observed after 2 days in 8-h PAB-treated samples (Fig. 3c). The 6-day-old gametophyte filaments of PA- and PAB-treated zoospores (Fig. 3e, f) were smaller in diameter compared to gametophytes of P-treated zoospores (Fig. 3d, j). No regeneration occurred among the 16-h PA- and PAB-treated zoospores post-cultivation. Remaining zoospores were completely bleached containing one or two enlarged physodes after 2 days (Fig. 3h, i, respectively), and showed autolysis after 6 days (Fig. 3k, l, respectively).
DNA damage and repair
Induction and accumulation of CPDs significantly increased with increasing biologically effective UV-B dose (BEDDNA) (Table 2). After 2 days of recovery in low white light, spores previously exposed to low fluence of BEDDNA were able to completely repair DNA damage. At higher fluence of BEDDNA, the remaining DNA damage after 2 days of recovery was directly proportional to the pre-exposure fluence treatment. ANOVA (P > 0.001) showed significant effect of dose on DNA damage and repair capacity (Table 3). Post-hoc analysis (DMRT, P = 0.05) showed that CPD accumulation was dose-dependent and highest at 920 J m−2 > 848 > 460 = 424 > 212 > 53 J m−2. Remaining CPD after 2 days of recovery was more at 920 J m−2 > 848 > 460 = 424 J m−2.
Maximum quantum yield of photosystem II
Initial F v/F m = 0.508 was measured after release (Table 2). After exposure to different fluence (J m−2, as a function of exposure time and irradiance) of light (P, PA, and PAB), photosynthesis was already photoinhibited by 76–85% under P. Photoinhibition increased under PA and PAB to 90–94 and 92–98%, respectively. Photosynthetic recovery was observed at 2 days post-cultivation in low white light with corresponding increase in F v/F m of 2–54% under PAB, 88–93% under PA and 98–99% under P. ANOVA showed significant effects of the three main factors (radiation, exposure time and intensity) and their interactive effects on photoinhibition of photosynthesis (Table 3). After 2 days post-cultivation, recovery of photosynthesis was significantly different in experimental units pre-exposed to different radiation (P, PA, PAB) and exposure time (8 and 16 h), and their interaction (Table 3). The interaction between different intensity (high and low) of radiation also has a significant effect on photosynthetic recovery.
Germination
Spore density used for this experiment was lower compared to the density used to monitor changes in cellular structure (above experiment). Despite this, UVR effect was evident between the two independent experiments. The magnitude of UVR effect was, however, observed to be density-dependent. Germination rate after 8- and 16-h exposure under P and 3 days post-cultivation was comparable ranging between 69 and 76% (Table 2). After additional post-cultivation for 3 days, the germination rate increased slightly to 85%. Under PA treatment, 54–57% germination was observed after 8 h of exposure and 3 days post-cultivation. Additional 15–21% increase in germination was observed at 6 days post-cultivation. Increasing PA exposure to 16 h resulted in <1% germination rate. Under PAB treatment, regardless of exposure time, germination rate was markedly suppressed. Repeated analysis of variance (RMANOVA, P > 0.001) showed significant effect of treatment and their interaction. Duncan’s multiple range test showed germination in P = PA > PAB after 8 h and P > PA = PAB after 16 h of exposure treatment.
Discussion
This study showed a dose-dependent UVR effect (as a function of exposure time) on the photosynthesis, cell structure, germination, as well as DNA damage and repair capacity in zoospores of Laminaria digitata. The UV-protective function of phlorotannins was observed after 8 h, but not after 16 h of UVR exposure. The energetic cost of photoprotection may have been responsible for the delay in ontogenic development of zoospores after 8 h of exposure to PA and PAB treatment; longer exposure time corresponding to 16 h of PA and PAB eventually lead to cell degeneration at 6-days post-cultivation. The accumulation of high amount of CPD was not blocked by the UV-absorbing phlorotannin during the 16-h PAB exposure and the inability for DNA damage repair was responsible for the spore mortality.
UV spectrophotometry for quantifying UV-absorbing compounds like phlorotannin and mycosporine-like amino acids (MAAs) have been routinely used (e.g. Laurion et al. 2003; Henry and van Alstyne 2004; Müller et al. 2009); however, this has to be interpreted with care since DNA and proteins also absorb in the proximate wavebands (Ragan and Craigie 1980; Ragan and Glombitza 1986). Our in vivo absorption spectra showed a decline in the UV-absorption peak, characteristic of phlorotannin, after extended exposure to PAB and PA treatment relative to the dark control; while exposure to P induced an increase in UV-absorption in the zoospore suspensions, the cells and the media. Production of UV-absorbing compounds, i.e. mycosporine-like amino acids and phlorotannins, are not only induced by UV-B radiation but also by photosynthetically active radiation (Karsten et al. 1998; Amsler and Fairhead 2006 and references therein).
Under UVR, intracellular and extracellular (contact medium) phlorotannin in L. digitata was apparently photodegraded. A similar UVR photodegradation of phlorotannin exudates from Macrocystis sporophytes has also been reported by Swanson and Druehl (2002), a phenomenon not observed in the zoospores and contact media of Arctic Saccorhiza dermatodea (Roleda et al. 2006a) and Alaria esculenta (Wiencke et al. 2007). Despite photodegradation, low concentrations of phlorotannin exudates in coastal waters can already reduce the impact of UV-B exposure to UV-sensitive kelp meiospore (Swanson and Druehl 2002). In the laboratory, extended exposure to UVR (>16 h), extra- and intracellular phlorotannin showed limited UV-protective function to L. digitata spores affecting germination rate. The same was observed in S. dermatodea (Roleda et al. 2006a) and A. esculenta (Wiencke et al. 2007). The adverse mechanistic effect of UV-B (in reference to non-ecologically relevant high UVR:PAR ratio) on germination capacity may be related to our experimental set-up. In the laboratory, our experimental units, spore suspension standing ~1 cm height inside the petri dishes were directly exposed under the artificial light sources. In field experiments, even when spore suspensions were incubated at depths receiving higher UV-B, UV-A and PAR doses (Wiencke et al. 2006), the germination capacity was not severely affected compared to our laboratory experiments. The UVR effect on germination capacity was comparable under low and high PAR in the laboratory and field experiments, respectively. On the other hand, the negative effects of UV-B radiation on the early life history stages of red macroalgae were reported on the cell division and growth of sporelings of Porphyra haitanensis (Jiang et al. 2007), but not on the germination capacity of spores of different ploidy in Gigartina skottsbergii (Roleda et al. 2008).
Our microscopic data showed the presence of vesicles (phenolic bodies) in the zoospore medium immediately after spore release (T 0 control), the origin of which, whether from the sorus or from the zoospore itself, cannot be categorically identified (e.g. also reported by Müller et al. 2009). However, under PAB treatment, the exocytosis of phenolic bodies into the medium was microscopically observed. The same phenomenon was reported in zoospores of Alaria esculenta under PA and PAB treatment (Wiencke et al. 2007), but not in zoospores of Saccorhiza dermatodea (Roleda et al. 2006a). Cell membranes can be damaged by UVR through lipid peroxidation or protein channel inhibition leading to increase cell permeability (Sobrino et al. 2008), which may have facilitated the release of phenolic bodies. A similar phenomenon was described in the eggs of the brown alga Durvillaea potatorum (Durvillaeales) where physodes (phlorotannin-containing vesicles) located just below the membrane undergo mass synchronised exocytosis, releasing phenolic bodies outside the membrane (Clayton and Ashburner 1994). Extended exposure to PAB and PA induced fusion of physodes resulting into one to two enlarged physodes per cell (this study). This phenomenon was described before in other brown algal zoospores and found before cell death (Wiencke et al. 2004, 2007; Roleda et al. 2006a).
The peripheral localisation of physodes in zoospores of Laminariales (e.g. this study, Wiencke et al. 2004, 2007), Dictyotales (Phillips et al. 1994), and eggs and zygotes of Fucales (Clayton 1992; Schoenwaelder and Wiencke 2000; Schoenwaelder et al. 2003) may not effectively protect a centrally-located chloroplast and nucleus. However, the higher physode abundance in Fucus spiralis compared to F. serratus was found to be correlated to the higher UVR tolerance of the former compared to the later species (Schoenwaelder et al. 2003). Therefore, the presence of extracellular phlorotannin may compensate for the limited protective function of cellular phlorotannin enclosed in small vesicles and randomly localised on periphery of the cells (Raven 1991; Garcia-Pichel 1994).
Consequently, the accumulation of DNA damage in Laminaria digitata spores was not prevented by the presence of (intra- and/or extra-cellular) UV-absorbing compounds after exposure to high fluence of UV-B radiation. The higher CPD accumulation in L. digitata may also be related to the photodegradation of phlorotannin. Compared to S. dermatodea and A. esculenta where no photodegradation was observed, lower CPD accumulation was observed under the same UV-B dose. Under the highest dose of UV-B treatment, the upper sublittoral Saccorhiza dermatodea acquired the lowest DNA damage compared to the mid- and lower sublittoral Alaria esculenta and L. digitata, respectively. Corresponding repair rate is, however, highest in A. esculenta and lowest in L. digitata. Sensitivity to UV-induced DNA damage and capacity for repair was observed to be related to the depth distribution pattern of the adult sporophytes (Fig. 4).
Comparative sensitivity of kelp zoospores to UV-B-induced DNA damage. Filled bars are the amount of CPDs after exposure to increasing UV-B dose as a function of exposure time. Empty bars are the corresponding remaining CPDs after 2 days of recovery in dim white light (8 μmol photons m−2 s−1). The biologically effective UV-B dose calculated using weighing function for DNA damage, BEDDNA (Setlow 1974), is shown on the x-axis. Composite and modified from Roleda et al. 2006a and Wiencke et al. 2007, and this study (data also in Table 2)
In large macroalgal thalli, CPD accumulation and repair rates are not directly related to the vertical distribution of the algae (van de Poll et al. 2002a). However, higher UV-tolerance is observed in littoral compared to sublittoral species (van de Poll et al. 2001). This could be related to morphological, functional life forms and pigment composition of the algae. Among young Laminariales sporophytes, accumulation of DNA damage is related not only to thallus thickness but also to their optical characteristic (Roleda et al. 2005b, 2006d, 2007).
DNA damage in Arctic L. digitata zoospores (this study) is comparable to that measured in temperate L. digitata zoospores (Roleda et al. 2005a), but the DNA damage repair rate was faster in the temperate population. Previously, CPD accumulation was also reported to be independent of temperature while the summer temperature in temperate regions facilitates repair of UV-induced DNA damage by light-dependent photolyases and/or light-independent nucleotide excision repair (Pakker et al. 2000; van de Poll et al. 2002b). A temperature effect is coherent since enzymes are involved in the repair of DNA and enzymatic reactions are decelerated in low temperatures.
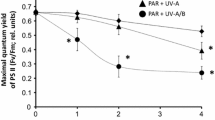
CPD accumulation may have been responsible for the inhibition of photosynthetic recovery and cell viability which eventually lead to spore mortality. Species-specific response to P, PA and PAB treatment was observed in the F v/F m after highest total fluence treatment (Fig. 5). The capacity for photosynthetic recovery was highest in L. digitata under P, and PA (both 83%) compared to S. dermatodea (58 and 62%, respectively) and A. esculenta (51 and 28%, respectively); however, photosynthetic recovery under PAB was lowest in L. digitata (0.3%) compared to A. esculenta (6%) and S. dermatodea (10%). When exposed to lower total fluence (up to 4 h exposure period), complete recovery of F v/F m in zoospores of S. dermatodea and L. digitata was observed between 24 and 48 h post-cultivation in low white light (Roleda et al. 2006c). Under the same PAB treatment, with BEDDNA of 2.12 × 102 J m−2, the remaining CPD was minimal to completely repaired (Fig. 4; Roleda et al. 2006a; Wiencke et al. 2007, this study).
Comparative sensitivity in the mean optimum quantum yield (log F v/F m) of zoospores and germlings after 16 h exposure to photosynthetically active radiation (PAR = P), PAR + UV-A (PA) and PAR + UV-A + UV-B (PAB) and after 2 days of recovery in low white light (8 μmol photons m−2 s−1) in Saccorhiza dermatodea (reported by Roleda et al. 2006a), Alaria esulenta (reported by Wiencke et al. 2007), and Laminaria digitata (this study). C1 and C2 are absolute mean F v/F m of controls at time after spore release and after post-cultivation at the same dim light condition with that of the treated samples, respectively
Furthermore, spore viability of L. digitata was adversely affected under high PA and PAB dose compared to S. dermatodea and A. esculenta (Fig. 6). The higher susceptibility of L. digitata to UVR was also observed in field experiments (Wiencke et al. 2006). Our data showed that the mechanistic effect of UVR under controlled laboratory condition was comparable to those observed in the field. The higher sensitivity of L. digitata zoospores to UVR compared to S. dermatodea and A. esculenta further confirm our hypothesis that the depth distribution pattern of adult sporophytes in the field correlates to the sensitivity of their corresponding early life history stages to different stress factors in general and UVR in particular. The presence of UV-absorbing compounds which can protect zoospores at a given maximum UV-dose and effective repair mechanisms are important to maintain cell viability. Spores laterally transported across the littoral zones may be able to settle under shaded environment (e.g. under algal canopy, rocks and boulders) which may permit survival of patches of juvenile and adult sporophytes of species, randomly distributed beyond their upper depth distribution limit.
Comparative sensitivity of kelp zoospores to UVR. Zoospore germination under UVR expressed as % of PAR after 8 and 16-h exposure to photosynthetically active radiation (PAR), PAR + UV-A (PA) and PAR + UV-A + UV-B (PAB) and after 3 days of recovery in low white light (8 μmol photons m−2 s−1) in Saccorhiza dermatodea (reported by Roleda et al. 2006a), Alaria esulenta (reported by Wiencke et al. 2007), and Laminaria digitata (this study)
References
Amsler CD, Fairhead VA (2006) Defensive and sensory chemical ecology of brown algae. Adv Bot Res 43:1–91
Arnold TM, Targett NM (2003) To grow and defend: lack of tradeoffs for brown algal phlorotannins. Oikos 100:406–408
Clayton MN (1992) Propagules of marine macroalgae: structure and development. Br Phycol J 27:219–232
Clayton MN, Ashburner CM (1994) Secretion of phenolic bodies following fertilisation in Durvillaea potatorum (Durvillaeales, Phaeophyta). Eur J Phycol 29:1–9
Dring MJ, Makarov V, Schoschina E, Lorenz M, Lüning K (1996) Influence of ultraviolet-radiation on chlorophyll fluorescence and growth in different life-history stages of three species of Laminaria (Phaeophyta). Mar Biol 126:183–191
Garcia-Pichel F (1994) A model for internal self-shading in planktonic organisms and its implications for the usefulness of ultraviolet sunscreens. Limnol Oceanogr 39:1704–1717
Hanelt D, Tüg H, Bischof K, Groß C, Lippert H, Sawall T, Wiencke C (2001) Light regime in an arctic fjord: a study related to stratospheric ozone depletion as a basis for determination of UV effects on algal growth. Mar Biol 138:649–658
Henry BE, van Alstyne KL (2004) Effects of UV radiation on growth and phlorotannins in Fucus gardneri (Phaeophyceae) juveniles and embryos. J Phycol 40:527–533
Huovinen PS, Oikari AOJ, Soimasuo MR, Cherr GN (2000) Impact of UV radiation on the early development of the giant kelp (Macrocystis pyrifera) gametophytes. Photochem Photobiol 72:308–313
Jiang H, Gao K, Helbling EW (2007) Effects of solar UV radiation on germination of conchospores and morphogenesis of sporelings in Porphyra haitanensis. Mar Biol 151:1751–1759
Karsten U, Franklin LA, Lüning K, Wiencke C (1998) Natural ultraviolet radiation and photosynthetically active radiation induce formation of mycosporine-like amino acids in the marine macroalga Chondrus crispus (Rhodophyta). Planta 205:257–262
Laurion I, Blouin F, Roy S (2003) The quantitative filter technique for measuring phytoplankton absorption: interference by MAAs in the UV waveband. Limnol Oceanogr Methods 1:1–9
Makarov MV, Voskoboinikov GM (2001) The influence of ultraviolet-B radiation on spore release and growth of the kelp Laminaria saccharina. Bot Mar 44:89–94
Müller R, Wiencke C, Bischof K, Krock B (2009) Zoospores of three Arctic Laminariales under different UV radiation and temperature conditions: exceptional spectral absorbance properties and lack of phlorotannin induction. Photochem Photobiol 85:970–977
Pakker H, Beekman CAC, Breeman AM (2000) Efficient photoreactivation of UVBR-induced DNA damage in the sublittoral macroalga Rhodymenia pseudopalmata (Rhodophyta). Eur J Phycol 35:109–114
Phillips JA, Clayton MN, Harvey AS (1994) Comparative studies on sporangial distribution and structure in species of Zonaria, Lobophora and Homoeostrichus (Dictyotales, Phaeophyceae) from Australia. Eur J Phycol 29:93–101
Ragan MA, Craigie JS (1980) Quantitative studies on brown algal phenols. IV. Ultraviolet spectrophotometry of extracted polyphenols and implications for measuring dissolved organic matter in sea water. J Exp Mar Biol Ecol 46:231–239
Ragan MA, Glombitza KW (1986) Phlorotannins, brown algal polyphenols. In: Round FE, Chapman DJ (eds) Progress in Phycological Research, vol 4. Biopress Ltd, Bristol, pp 129–241
Raven JA (1991) Responses of aquatic photosynthetic organisms to increased solar UVB. J Photochem Photobiol B Biol 9:239–244
Roleda MY, Wiencke C, Hanelt D, van de Poll WH, Gruber A (2005a) Sensitivity of Laminariales zoospores from Helgoland (North Sea) to ultraviolet and photosynthetically active radiation: implications for depth distribution and seasonal reproduction. Plant Cell Environ 28:466–479
Roleda MY, Hanelt D, Wiencke C (2005b) Growth kinetics related to physiological parameters in young Saccorhiza dermatodea and Alaria esculenta sporophytes exposed to UV radiation. Polar Biol 28:539–549
Roleda MY, Wiencke C, Lüder UH (2006a) Impact of ultraviolet radiation on cell structure, UV-absorbing compounds, photosynthesis, DNA damage, and germination in zoospores of Arctic Saccorhiza dermatodea. J Exp Bot 57:3847–3856
Roleda MY, Clayton MN, Wiencke C (2006b) Screening capacity of UV-absorbing compounds in spores of Arctic Laminariales. J Exp Mar Biol Ecol 338:123–133
Roleda MY, Hanelt D, Wiencke C (2006c) Exposure to ultraviolet radiation delays photosynthetic recovery in Arctic kelp zoospores. Photosynth Res 88:311–322
Roleda MY, Wiencke C, Hanelt D (2006d) Thallus morphology and optical characteristics affect growth and DNA damage by UV radiation in juvenile Arctic Laminaria sporophytes. Planta 223:407–417
Roleda MY, Wiencke C, Hanelt D, Bischof K (2007) Sensitivity of the early life stages of macroalgae from the Northern Hemisphere to ultraviolet radiation. Photochem Photobiol 83:851–862
Roleda MY, Zacher K, Wulff A, Hanelt D, Wiencke C (2008) Susceptibility of spores of different ploidy levels from Antarctic Gigartina skottsbergii (Gigartinales, Rhodophyta) to ultraviolet radiation. Phycologia 47:361–370
Schoenwaelder MEA (2002) The occurrence and cellular significance of physodes in brown algae. Phycologia 41:125–139
Schoenwaelder MEA, Wiencke C (2000) Phenolic compounds in the embryo development of several of several northern hemisphere fucoids. Plant Biol 2:24–33
Schoenwaelder MEA, Wiencke C, Clayton MN, Glombitza KW (2003) The effect of elevated UV radiation on Fucus spp. (Fucales, Phaeophyta) zygote and embryo development. Plant Biol 5:366–377
Setlow RB (1974) The wavelengths in sunlight effective in producing skin cancer: a theoretical analysis. Proc Natl Acad Sci USA 71:3363–3366
Sobrino C, Ward ML, Neale PJ (2008) Acclimation to elevated CO2 and ultraviolet radiation in the diatom Thalassiosira pseudonana: Effects on growth, photosynthesis and spectral sensitivity of photoinhibition. Limnol Oceanogr 56:494–505
Steinhoff FS, Wiencke C, Müller R, Bischof K (2008) Effects of ultraviolet radiation and temperature on the ultrastructure of zoospores of the brown macroalga Laminaria hyperborea. Plant Biol 10:388–397
Swanson AK, Druehl LD (2000) Differential meiospore size and tolerance of ultraviolet light stress within and among kelp species along a depth gradient. Mar Biol 136:657–664
Swanson AK, Druehl LD (2002) Induction, exudation and the UV protective role of kelp phlorotannins. Aquat Bot 73:241–253
Tala F, Vèliz K, Gòmez I, Edding M (2007) Early life stages of the South Pacific kelps Lessonia nigrescens and Lessonia trabeculata (Laminariales, Phaeophyceae) show recovery capacity following exposure to UV radiation. Phycologia 46:467–470
van de Poll WH, Eggert A, Buma AGJ, Breeman AM (2001) Effects of UV-B-induced DNA damage and photoinhibition on growth of temperate marine red macrophytes: habitat-related differences in UV-B tolerance. J Phycol 37:30–37
van de Poll WH, Hanelt D, Hoyer K, Buma AGJ, Breeman AM (2002a) Ultraviolet-B-induced cyclobutane–pyrimidine dimer formation and repair in Arctic marine macrophytes. Photochem Photobiol 76:493–501
van de Poll WH, Eggert A, Buma AGJ, Breeman AM (2002b) Temperature dependence of UV radiation effects in Arctic and temperate isolates of three red macrophytes. Eur J Phycol 37:59–68
Vèliz K, Edding M, Tala F, Gòmez I (2006) Effects of ultraviolet radiation on different life cycle stages of the south Pacific kelps, Lessonia nigrescens and Lessonia trabeculata (Laminariales, Phaeophyceae). Mar Biol 149:1015–1024
Wiencke C, Clayton MN, Schoenwaelder M (2004) Sensitivity and acclimation to UV radiation of zoospores from five species of Laminariales from the Arctic. Mar Biol 145:31–39
Wiencke C, Roleda MY, Gruber A, Clayton MN, Bischof K (2006) Susceptibility of zoospores to UV radiation determines upper depth distribution limit of Arctic kelps: evidence through field experiments. J Ecol 94:455–463
Wiencke C, Lüder UH, Roleda MY (2007) Impact of ultraviolet radiation on physiology and development of zoospores of the brown alga Alaria esculenta from Spitsbergen. Physiol Plant 130:601–612
Acknowledgment
We thank C. Daniel and A. Gruber for laboratory assistance, the scuba-divers of the Spitsbergen 2004 field campaign, especially M. Schwanitz for collecting fertile plant material. Moreover, we thank the International Arctic Environmental Research and Monitoring Facility at Ny Ålesund, Svalbard, for support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roleda, M.Y., Lüder, U.H. & Wiencke, C. UV-susceptibility of zoospores of the brown macroalga Laminaria digitata from Spitsbergen. Polar Biol 33, 577–588 (2010). https://doi.org/10.1007/s00300-009-0733-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-009-0733-z