Abstract
The effects of exposure to ultraviolet radiation (UVR), 280–400 nm, in different life histories and development stages of the kelps, Lessonia nigrescens and L. trabeculata, collected in the south-east Pacific coast (30°S) were evaluated in the laboratory. Germination and viability (motile zoospores, settled spores), diameter of the primary cell of the gametophytes, percentage of female gametophytes, fertility and sporophytes production were measured after exposure to three radiation treatments (PAR; PAR + UVA; PAR + UVA + UVB). The effects of UVR in young sporophytes (diploid stage) were evaluated as changes in maximal quantum yield of chlorophyll fluorescence of photosystem II (PSII) (F v/F m). A significant decrease in all variables was observed for the treatment that included UVB (PAR + UVA + UVB) after 2 and 4 h of exposure, in relation to the control. The motile spores were more sensitive to UVR exposure compared to settled spores and gametophytes, suggesting that along with an increase in ontogenetic development; there is an increase in the tolerance to UVR. In addition, it was observed that early stages of the intertidal L. nigrescens were more tolerant to UVR compared to the subtidal L. trabeculata. These results allow initially to infer that UVR may be regarded as an important environmental factor influencing the upper limit of distribution of these species, mainly through its detrimental effects on the early stages of the life cycle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early developmental stages of kelps (zoospores, gametophytes and small sporophytes) are shade-adapted organisms in contrast to adult thalli (sporophytes) (Kain 1964; Gómez and Wiencke 1996; Dring et al. 1996). The different photobiological performance associated with an alternation of heteromorphic phases has implications on kelp ecology: reproduction, metabolic performance (e.g., photosynthesis) and growth are seasonally synchronized, thus improving survival under changing environmental conditions in their natural habitat (Lüning 1990). Light adaptation not only is an attribute expressed on an ontogenic basis but also constitutes a very important functional character connecting the different stages of life cycle. With regard to the sensitivity to solar radiation, it is known that spores and early establishment stages of kelps are highly vulnerable, for example, to ultraviolet radiation (UVR), as compared to sporophytes (Han and Kain 1993; Dring et al. 1996). In general, small zoospores and gametes posses few structural or physiological mechanisms of photoprotection, exhibiting a very limited potential acclimation to light stress (Amsler and Neushul 1991). In different species of Laminariales from the Northern Hemisphere, the exposure to UVR has detrimental effects on the viability and germination of spores (Huovinen et al. 2000; Makarov and Voskoboinikov 2001), which has consequences on the further development of the gametophytes (Yabe et al. 1997; Swanson and Druehl 2000) and the bathymetrical distribution of sporophytes (Wiencke et al. 2000; Roleda et al. 2005).
Lessonia nigrescens Bory 1826 and L. trabeculata Villouta and Santelices 1986, which are harvested for phycocoloid extraction and feed for farmed abalone (Edding and Tala 2003; Edding et al. 1994), are two major representatives of the Laminariales order along the south-east Pacific coast (Santelices 1989; Edding et al. 1994). Both species share similar morphological organization and, in virtue of their large size and ecological function, are regarded as major contributors to coastal primary productivity. L. nigrescens is common in highly wave-exposed intertidal zones (Santelices and Ojeda 1984; Westermeier et al. 1994), while L. trabeculata inhabits subtidal locations (Villouta and Santelices 1986). Such depth distribution patterns intuitively suggest possible differences in the capacity of these algae to cope with enhanced solar UVR, which has not been examined so far for these species. Taking into account that spring/summer episodes of stratospheric ozone depletion can reach mid-latitudes in South America (Orce and Helbling 1997; Lovengreen et al. 2001), the knowledge of the potential effects of increased UVR on the life cycle will give new insights into the regulative mechanisms that act on southern cold-temperate Laminariales.
The aim of this study was to examine the influence of UVR on the germination of zoospores and subsequent development of gametophytes and sporophytes of L. nigrescens and L. trabeculata. Responses were evaluated at different stages and phases of development (motile spores, settled spores, gametophytes and young sporophytes) in order to cover their whole life cycle. Two major questions were addressed: (a) Are the distinct life cycle phases differentially affected by UVR? (b) Is the UV sensitivity of early developmental phases correlated to depth distribution of parental sporophytes (intertidal vs. subtidal)?
Materials and methods
Sampling and cultivation of early stages
Mature blades with sori were obtained from different adult thalli during March–April of 2003. L. nigrescens was collected from the rocky intertidal at Pampilla, Coquimbo (29°57′S, 71°22′W), while L. trabeculata was collected by SCUBA diving from 4 to 5 m depth in Tongoy Bay (30°15′S, 71°15′W). In the laboratory, around 10 fertile blades were selected, washed with tap water to remove epiphytes and finally dehydrated in the dark for 4 h at a constant temperature of 15±1°C (Fonck et al. 1998). Spores were released within opaque plastic boxes with filtered seawater (0.45 μm) for 90 min in the dark and counted in a haemocytometer (approximately 50,000 spores ml−1). In order to allow settlement the spores were incubated in Petri dishes (three replicates by treatment) for 24 h at 15±1°C and 25 μmol photons m−2 s−1. Thereafter, the water was replaced with Provasoli-enriched seawater (Starr and Zeikus 1993). Standard experimental conditions for both species were 15±1°C, photoperiod 12:12 (L:D) and illuminated with 60±5 μmol photons m−2 s−1 of photosynthetic active radiation (PAR) provided by fluorescent lamps (Daylight Phillips).
Light source and experimental design
For the experimental incubations, spores were exposed to a combination of UVB (280–315 nm), UVA (315–400 nm) and PAR (400–700 nm) irradiances provided by UVB 313 and UVA 340 lamps (Q-Panel Co., Cleveland, USA) and daylight fluorescent lamps (Philips 40 W, The Netherlands), respectively.
The Petri dishes were covered with cut-off filters to obtain a combination of two treatments of radiation: PAR + UVA + UVB (Ultraphan 295; Digefra GmbH, Munich, Germany) and PAR + UVA (Folex 320; Folex GmbH, Dreieich, Germany). Cultures exposed only to PAR were used as controls. Incident irradiances under the different combinations of lamps and cut-off filters are indicated in Table 1 and were measured with a spectroradiometer SUV-100 (Biospherical Instruments, San Diego, USA), and the PAR intensity was determined with a Li-190S quantum sensor connected to LI-250 radiometer (LI-COR Inst., USA). In order to estimate the wavelength-dependent effectiveness of the UV irradiances applied in each treatment, biologically effective dose (BED), spectral irradiances in the range between 280 and 400 nm were weighted, using two action spectra for well-known biological effects: generalized plant damage (Caldwell 1971) and photoinhibition of photosynthesis as normalized to unity at 300 nm (Jones and Kok 1966), following the calculations described by Gómez et al. (2001). BEDs resulting in a 50% decrease in biological activities (germination, survival, fertility and photosynthesis) were calculated by regression analysis of the dose–response data, using weighted irradiances.
Figure 1 summarises the experimental design and biological parameters used for the diagnosis of UV effects. To examine the response of the different stages of ontogenetic development, the cultures were irradiated separately after 3 h post-release (motile zoospores), after 24 h post-release zoospores (settled embryospores) and once the gametophytes had been developed (8 days post-release). Viability of motile and settled zoospores was established as number of spores with germination tube on the second day (percentage of a total of 100 spores per culture dishes). In addition, the diameter of the primary cell of 25 female gametophytes per culture dish was determined after day 7. This value was used to estimate the vegetative growth (Lüning 1980). Fertility in irradiated gametophytes and those irradiated at spore stages was evaluated after 15 days and expressed as a percentage of female gametophytes bearing oogonia of a total of 100 gametophytes. The number of sporophytes formed was determined after 25 days and expressed as percentage of a total of 100 female gametophytes + sporophytes per culture dish. The time span of exposure was not longer than 4 h because previous tests showed 100% mortality in both Lessonia species under exposure to UVR>4 h (data not shown). After exposure, the Petri dishes were placed back in the standard conditions of culture. Evaluation of the cultures was made utilizing random selected optical fields (0.6793 mm2) in an inverted phase contrast microscope (Nikon, Japan). In the case of young sporophytes (>10 cm), the effects of UVR were assessed as in vivo decreases in chlorophyll fluorescence of photosystem II (PSII) with a portable amplitude-modulated fluorometer (PAM-2000, Walz, Germany). The maximal quantum yield of fluorescence (F v/F m) was used as an indicator of physiological status (see Schreiber et al. 1994 for details).
Statistical analysis
The data sets for each species were analysed with a three-way ANOVA, with development stage (motile spores, settled spores, gametophytes and young sporophytes) as the first factor, radiation treatment (PAR; PAR + UVA; PAR + UVA + UVB) as the second factor and time exposure (0.5, 2 and 4 h) as the third factor. The normality (Kolmorov–Smirnov test) and homoscedasticity (Barlett test) of the percentage values were obtained using square-root arcsine transformation. A post hoc Dunnett test was applied, making comparisons with the control (Sokal and Rohlf 1995).
Results
The different developmental stages of microscopic phase of Lessonia under PAR condition are shown in Fig. 2. On the second day of culture all the spores in the control had germinated, which was evident from the observation of the germ tube (Fig. 2b). In the following days of culture, the spore and germination tube were empty and the first cell of the gametophyte was formed (Fig. 2c). The germination of spores varied in response to the two UV treatments (Fig. 3): under PAR + UVA, germination of motile and settled spores was similar as control, whereas under PAR + UVA + UVB, 100% germination was seen only after 0.5 h exposure. After 2 and 4 h, germination of motile spores of L. nigrescens decreased significantly by 20 and 100%, respectively (Dunnett, P<0.05). In L. trabeculata, decreases in germination rates after 2 h exposure were greater, reaching 80% by motile spores. After 4 h exposure, no germination of motile spores was detected in either of the species (Fig. 3). In the case of settled spores, germination rates after 4 h exposure to UVR was 40 and 20% for L. nigrescens and L. trabeculata, respectively.
Life-cycle stages of Lessonia: a settled spores 24 h post-release; b germinating spores 48 h post-release; c 8-day-old gametophytes with primary cell; d female and male gametophytes on day 15 of culture, arrow showing a zygote in division; e early sporophyte on day 25 of culture; f 4-month-old young sporophytes. Scale bar: a–e 10 μm, f 1 cm
Effects of two different radiation treatments on germination (percent of the control) of motile and settled spores. Measurements were performed 2 days after exposure to UV radiation. Asterisks denote significant differences from the control (PAR) (data not normalized to the control, Dunnett test, P<0.05). Mean germination rates of controls: L. nigrescens 92.5±3.2%; L. trabeculata 92.4±1.4%. Data are means ± SD; n=3
Ultraviolet radiation affected the viability of spores mainly due to cellular lyses, which were observed in both species (Fig. 4). In motile and settled spores of L. nigrescens, irradiated with the PAR + UVA + UVB treatment, survival was close to 20 and 15%, respectively (P<0.05). No decreases in survival relative to control were observed when gametophyte stage was irradiated. A similar response was seen in spores of L. trabeculata, in which survival was lower than 20% after 4 h exposure. In this species, however, survival of gametophytes decreased by 17% after 4 h UV exposure (P<0.05; Fig. 4). In neither of the species, the motile spores that survived 4 h exposure in the PAR + UVA + UVB treatment showed gametophytes development. Mean diameter of the female gametophytes primary cell in the control was 18.8±0.2 μm for L. nigrescens and 18.4±0.2 μm for L. trabeculata. After 2 h exposure to PAR + UVA + UVB the diameter of the gametophyte primary cell, for both motile and settled spores, averaged 15 μm (data not shown).
After 15 days female gametophytes attained 1–10 cells and zygotes in division were present (Fig. 2d). Male gametophytes were conformed by branched filaments of small cells (Fig. 2d). The percentage of female gametophytes fluctuated between 55 and 56% for L. nigrescens and L. trabeculata, respectively. UVB exposures of 2 and 4 h led to increases in the female/male ratios, particularly in motile and settled spores (Fig. 5). Fertility diminished significantly (Dunnett, P<0.05) in relation to the control in the cultures exposed to PAR + UVA + UVB in the three stages of development and in both species (Fig. 6). Similar to germination, an increase in fertility was observed for all treatments, which however, never reached the control values.
Effects of different radiation treatments on proportion of female gametophytes (percent of control) of L. nigrescens and L. trabeculata. Measurements were performed 15 days after exposure of motile spores, settled spores and gametophytes to UV radiation. Asterisks denote significant differences from the control (PAR) (Dunnett test, P<0.05). Control means: L. nigrescens 55.3±5.2%; L. trabeculata 56.6±1.8%. Data are means ± SD; n=3
Effects of different radiation treatments on fertility of female gametophytes (percent of control) of L. nigrescens and L. Trabeculata. Measurements were performed 15 days after exposure of motile spores, settled spores and gametophytes to UV radiation. Asterisks denote significant differences from the control (PAR) (Dunnett test, P<0.05). Control means: L. nigrescens 25.8±3.3%; L. trabeculata 21.1±2.1%. Data are means ± SD; n=3
The sporophyte formation was evaluated on day 25 of the culture with mean values lower than 20% for both species in control dishes. Morphologically, sporophytes were conformed by a monostromatic lamina (Fig. 2e). In all treatments, some sporophytes had begun to develop rhizoids. Under UVB irradiation, the formation of sporophytes was relatively lowered compared to the control, which was not directly related to the increase in dose (Fig. 7). After 2 h exposure under PAR + UVA + UVB, the proportion of sporophytes in motile and settled spores of both Lessonia species varied between 20 and 50% relative to the control.
Effects of different radiation treatments on formation of sporophytes (percent of control) in L. nigrescens and L. trabeculata. Measurements were performed 25 days after exposure of motile spores, settled spores and gametophytes to UV radiation. Asterisks denote significant differences from the control (PAR) (Dunnett test, P<0.05). Control means: L. nigrescens 15.0±2.6%; L. trabeculata 15.8±4.8%. Data are means ± SD; n=3
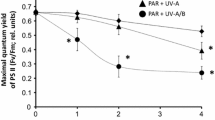
Photosynthesis measured as chlorophyll fluorescence of multi-stromatic sporophytes (>10 cm, Fig. 2f) was affected by treatments including UVB (P<0.05; Fig. 8). Although mean values of F v/F m (47%) were lower in sporophytes of L. trabeculata than L. nigrescens (57%), no significant differences between species could be detected. Exposures to PAR + UVA affected photosynthesis; however, these decreases did not exceed 27% (Fig. 8).
Effects of ultraviolet radiation treatments (PAR + UVA; PAR + UVA + UVB) on maximal quantum yield F v/F m) of young sporophytes of L. nigrescens and L. trabeculata. Algae were irradiated for 2 h with UV treatments. Data are means ± SD, n=10. Asterisks denote significant differences from the control (PAR)
Discussion
UV susceptibility of spores and consequences for further development of gametophytes
Our results indicated that effects of UVR were more detrimental in motile spores than in settled ones. It has been described earlier that motile spores do not posses a well-developed cellular wall and show a smaller cellular size than observed in spores after the settlement (Henry and Cole 1982). For example, the cellular diameter of motile spores of L. nigrescens is 5.5 μm, whereas the diameter of settled cells were close to 10 μm (Hoffman and Santelices 1982). In terms of their morpho-functional complexity, a motile spore would be regarded as the simplest stage in the life cycle: most of the physiological functions, e.g. the protein synthesis and the photosynthetic activity, are not yet well developed (Voskoboinikov and Kamnev 1991). According to Swanson and Druehl (2000), the spore size affects the capacity of different kelps to germinate and survive after exposure to UVR. In this sense, the surface area/volume ratio of single-celled organisms is an indicator of UV sensitivity (Karentz et al. 1991), as size influences the bio-optical characteristics of the cell and consequently the cross-section path length through the cytosol (Ramus 1978; Garcia-Pichel 1996). At this level, UVB radiation easily reaches key molecular targets, e.g. DNA, which can occur as early as during sporogenesis within the sporangia (Garman et al. 1994). Furthermore, nuclear division and translocation during germination can also be affected (Huovinen et al. 2000).
The question, whether the non-lethal UV damage at the spore levels is “transferred” to further developmental stages, has not commonly been addressed in this kind of studies. Our results indicated that exposure of motile and settled spores for 2 and 4 h to UVB radiation affected the development of primary cells (vegetative growth) and fertility of gametophytes. On the other hand, viability of spores stage was clearly more susceptible to UVR than gametophytes and in experiments where gametophytes (with one or two cells) were irradiated, decreases in fertility were less accentuated (Fig. 6). A decrease in the growth of the gametophytes was reported by Dring et al. (1996) in Laminaria saccharina and L. hyperborea irradiated with UVR for more than 1 h. Apparently, energy costs involved in DNA repair (Sancar and Sancar 1988), protein biosynthesis and turnover (Shelly et al. 2002), as well as a lower photosynthesis after damage of thylakoid proteins or photosystems (see Franklin and Foster 1997) can finally affect growth rates and algal survival (Aguilera et al. 1999; Roleda et al. 2006). Yabe et al. (1997) reported that growth of gametophytes of Laminaria religiosa was inhibited by 90% when parental zoospores were exposed to UVB dose close to 3 kJ m−2. Although in our study high spore mortality was observed after 4 h exposure to UVB radiation (80 and 89%), gametophytes formed from the surviving spores retained the capacity to reproduce and form viable sporophytes. Two major results can be emphasized: (a) the sporophyte formation was not affected by the UV dose applied in these species and (b) a recovery capacity of spores, with an increase in germination and fertility throughout time when the cultures were transferred to PAR conditions. These results agree with previous observations in three Laminaria species which exhibited an efficient DNA damage repair (removal of cyclobutane–pyrimidine dimers), recovery of photosynthetic efficiency and an enhancement of spore viability and germination capacity of UV-treated samples after maintaining spores in low PAR (Roleda et al. 2005). Apparently, this capacity to recover cell viability and germination depends on depth distribution. In fact, in a comparison of UV sensitivity of five Arctic species of Laminariales, Wiencke et al. (2004) demonstrated that the upper sublittoral Saccorhiza dermatodea exhibited a higher capacity of recovery of germination than algae from deeper zones, e.g., Laminaria digitata or L. solidungula.
Gametophytes of Laminariales are sexually dimorphic, with male gametophytes being characterized by several small cells that tend to branch, whereas female gametophytes attain larger cells (Druehl et al. 1989). Thus, if the cellular size is a characteristic determining the cell tolerance to UVR (Laurion and Vincent 1998; Swanson and Druehl 2000), then it is possible to argue that high female/male sex ratios may be favoured in terms of UVR tolerance. In our study, both species of Lessonia developed a higher proportion of female relative to male gametophytes when the spore stages were exposed to UVB radiation. However, this cannot be understood as a differential mortality due to morphological differences as at this time (3 and 24 h post-release) sexual dimorphism between gametophytes was not yet pronounced. Instead, other factors at cellular level rather than the spore size should be invoked in the diferential UVB response between male and female spores. For example, Druehl et al. (1989) suggest that male spores of Laminariales (Alaria marginata, Laminaria saccharina and Cymathere triplicata) have greater DNA compactness and/or nuclear protein content than female spores. When UVB radiation was applied to gametophytes the female/male ratio was similar to the control, indicating a null effect of UVB radiation on the sex ratio at this level. It may be argued that along with the development of gametophytes, DNA and other target molecules become less exposed to UVB radiation, mainly due to a greater scattering and lower transmission through filaments (self-shading). These findings may have implications for survival and recruitment success in algae that reproduce throughout the year, like Lessonia. Recent laboratory studies in L. trabeculata showed that gametophytes developed from “autumn spores” exhibit a better reproductive performance than gametophytes from other seasonal periods (Tala et al. 2004). In this sense, it is necessary to determine the existence of a potential seasonal variability in the answer to the early development stage to UVR exposure, which could be related to strategies of seasonal adaptive reproduction that assures successful recruitment of microscopic stage in the field under changing solar radiation conditions and other environmental factors.
Life cycle strategy and light adaptation of parental sporophytes
Figure 9 summarises the weighted doses (BEDs) required to cause 50% decrease in biological activities of the different life cycle stages of Lessonia. Based on these estimates, Caldwell-based BEDs of 2.18 and 4.22 kJ m−2 were necessary to cause 50% germination and fertility of spores and gametophytes of L. nigrescens, while in the subtidal L. trabeculata, these values were lower (1.41 and 2.29 kJ m−2). On the other hand, increasing morphological complexity increased the BEDs required to reduce biological activity, i.e. tolerance to UVB stress increase with ontogenetic development. Such findings agree well with previous studies carried out in microscopic stages of Laminaria (Dring et al. 1996; Hanelt et al. 1997) and may be related to a greater structural complexity of the gametophytes compared to spores, and probably to the action of more efficient mechanisms of protection (e.g. UV absorbing substances) and repair of the UV damage (Schoenwaelder et al. 2003). There are limited data on the mechanisms involved in photoprotection in early stages of Laminariales, in contrast to the adult stage. In Arctic Laminariales spores, Wiencke et al. (2004) reported a protective potential of phlorotannin (phenolics) containing physodes, which increase in number and size after UV exposure. The phlorotannins strongly absorb in the short wavelengths and their synthesis is inducible by UVB radiation, i.e. may be regarded as photoprotective substances (Pavia et al. 1997; Schoenwaelder 2002). On the other hand, Roleda et al. (2005, 2006) reported the capability of spores and young sporophytes of Laminariales to repair DNA damaged under photoreactivating light (which could either be mediated by light-dependent photolyses or light-independent nucleotide excision repair). The question whether other UVR-protective and repair mechanisms (e.g. xanthophyll cycle, enzymatic and antioxidants defense system) are active in early developmental stages of kelps has not been examined.
This study underlines the depth dependence of UVR sensitivity in spores and correlates well with the distribution patterns of parental sporophytes at this region in northern Chile. In the case of L. trabeculata, weighted UVB levels required for 50% reduction in germination were in the range estimated (0.5–3.4 kJ m−2) for kelps from the Arctic or deep locations (>20 m) in the Mediterranean (Wiencke et al. 2000). Our values estimated for L. nigrescens were, however, higher than determined in subtidal species. Latitudinal UVR–PAR measurements for South America indicated that maximal noontime irradiances between 23°S and 34°S (austral summer) average 4.46 and 3.99 W m−2 for UVB (280–320 nm) and 55.47 and 52.99 W m−2 for UVA (320–400 nm), respectively (Orce and Helbling 1997). With regard to the UVR penetration into coastal waters of northern Chile (30°S), Montecino and Pizarro (1995) reported that the 1% UVB irradiance penetration depth (Z UV308nm) reached a minimum of 11 m and maximum of 15 m, well in the range of the distribution of L. trabeculata (0.5–20 m; Vásquez 1989) and with mean extinction coefficients (k 308nm) of 0.349 m−1 in autumn and 0.289 m−1 in winter. Under these current levels of solar radiation, exposures to UVB for 6 and 10 min could decrease the germination and fertility by 50% in L. trabeculata. However, it must be emphasized that these UVB levels only occur at the upper fraction of the water column. In the case of L. nigrescens, the algae is exposed to high solar irradiances, e.g. for intertidal populations at the coast of Valdivia (39°), UVB values close to 2.3 W m−2 have been reported during cloudless summer days (Gómez et al. 2004). Under these conditions, germination and fertility would decrease by 50% with an UVB exposure for 10 and 20 min, respectively. Likewise, photosynthesis measured as chlorophyll fluorescence in young sporophytes was similar in both species, although BED necessary to cause 50% inhibition in photosynthesis was slightly higher in L. nigrescens compared to L. trabeculata (Fig. 9). Although the settlement of kelps spores takes place mainly under parental canopies where they find low-light environment, after release of sporangia, the zoospores become planktonic for variable period of time (Reed et al. 1992). During this time, zoospores should be directly exposed to environmental stress conditions with respect to solar radiation (including UVR), especially if vertical mixing transports cells to shallower water (Helbling et al. 1994).
Overall, the results of this study confirm that, in general, the early developmental stages of kelps are shade-adapted organisms (Kain 1969; Lüning and Neushul 1978). The sensitivity of the life cycle phases of Lessonia to UVR was related to the bathymetric distribution of parental sporophytes.
References
Aguilera J, Karsten U, Lippert H, Vögele B, Philipp E, Hanelt D, Wiencke C (1999) Effects of solar radiation on growth, photosynthesis and respiration of marine macroalgae from the Artic. Mar Ecol Prog Ser 191:109–119
Amsler CD, Neushul M (1991) Photosynthetic physiology and chemical composition of the zoospores of the kelps Macrocystis pyrifera, Nereocystis luetkeana, Laminaria farlowii, and Pterigophora californica (Phaeophyceae). J Phycol 27:26–34
Caldwell MM (1971) Solar ultraviolet radiation and the growth and development of higher plants. In: Giese A (ed) Photophysiology. Academic, New York, pp 131–177
Dring MJ, Makarov V, Schoschina E, Lorenz M, Lüning K (1996) Influence of ultraviolet radiation on chlorophyll fluorescence and growth in different life history stages of three species of Laminaria (Phaeophyta). Mar Biol 126:183–191
Druehl LD, Robertson BR, Button DK (1989) Characterizing and sexing Laminarialean meiospores by flow cytometry. Mar Biol 101:451–456
Edding ME, Tala F (2003) Development of techniques for the cultivation of Lessonia trabeculata Villouta et Santelices (Phaeophyceae: Laminariales) in Chile. Aquac Res 34:507–515
Edding ME, Fonck E, Macchiavello J (1994) Lessonia. In: Akatzuka I (ed) Biology of economic algae. SPB Academic Publishing, The Netherlands, pp 407–446
Fonck E, Venegas M, Tala F, Edding M (1998) Artificial induction of sporulation in Lessonia (Phaeophyta, Laminariales). J Appl Phycol 10:399–403
Franklin L, Foster R (1997) The changing irradiance environment: consequences for marine macrophyte physiology, productivity and ecology. Eur J Phycol 32:207–232
Garcia-Pichel F (1996) The absorption of ultraviolet radiation by microalgae: simple optic and photobiological implications. Sci Mar 60:73–79
Garman GD, Pillai MC, Goff J, Cherr GN (1994) Nuclear events during early development in gametophytes of Macrocystis pyrifera, and the temporal effects of marine contaminant. Mar Biol 121:355–362
Gómez I, Wiencke C (1996) Photosynthesis, dark respiration and pigment contents of gametophytes and sporophytes of the Antarctic brown alga Desmarestia menziesii. Bot Mar 39:149–157
Gómez I, Figueroa FL, Sousa-Pinto I, Viñegla B, Pérez-Rodríguez E, Maestre C, Coelho S, Felga A, Pereira R (2001) Effects of UV radiation and temperature on photosynthesis as measured by PAM fluorescence in the red alga Gelidium pulchellum (Turner) Kützing. Bot Mar 44:9–16
Gómez I, Figueroa FL, Ulloa N, Morales V, Lovengreen C, Huovinen P, Hess S (2004) Patterns of photosynthesis in 18 species of intertidal macroalgae from southern Chile. Mar Ecol Prog Ser 270:103–116
Han T, Kain JM (1993) Blue light photoreactivation in ultraviolet irradiated young sporophytes of Alaria esculenta and Laminaria saccharina (Phaeophyta). J Phycol 29:79–81
Hanelt D, Wiencke C, Karsten U, Nultsch W (1997) Photoinhibition and recovery after high light stress in different development and life history stages of Laminaria saccharina (Phaeophyta). J Phycol 33:387–395
Helbling EW, Villafañe V, Holm-Hansen O (1994) Effects of ultraviolet radiation on Antarctic marine phytoplankton photosynthesis with particular attention to the influence of mixing. Antarctic Res Ser 62:207–227
Henry EC, Cole KM (1982) Ultrastructure of swarmers in the Laminariales (Phaeophyceae). I. Zoospores. J Phycol 18:550–569
Hoffman AJ, Santelices B (1982) Effects of light intensity and nutrients on gametophytes and gametogenesis of Lessonia nigrescens Bory (Phaeophyta). J Exp Mar Biol Ecol 60:77–89
Huovinen P, Oikar A, Soimasuo M, Cherr G (2000) Impact of UV radiation on the early development of the giant kelp (Macrocystis pyrifera) gametophytes. Photochem Photobiol 72:308–314
Jones L, Kok B (1966) Photoinhibition of chloroplast reaction. I. Kinetics and action spectra. Plant Physiol 41:1037–1043
Kain JM (1964) Aspects of the biology of Laminaria hyperborean. III: survival and growth of gametophytes. J Mar Biol Assoc UK 44:415–433
Kain JM (1969) The biology of Laminaria hyperborean. V: comparison with early stages of competitors. J Mar Biol Assoc UK 49:455–473
Karentz D, Cleaver JE, Mitchell D (1991) Cell survival characteristics and molecular responses of Antarctica phytoplankton to ultraviolet-B radiation. J Phycol 27:326–341
Laurion I, Vincent WF (1998) Cell size versus taxonomic composition as determinants of UV-sensitivity in natural phytoplankton communities. Limnol Oceanogr 43:1774–1779
Lovengreen C, Fuenzalida H, Villanueva L (2001) Ultraviolet solar radiation at Valdivia, Chile (39.8° S). Atmos Environ 34:4051–4061
Lüning K (1980) Critical levels of light and temperature regulating the gametogenesis of three Laminaria species (Phaeophyceae). J Phycol 16:1–15
Lüning K (1990) Seaweeds: their environment, biogeography, and ecophysiology. Wiley, New York, 527 pp
Lüning K, Neushul M (1978) Light and temperature demands for growth and reproduction of Laminarian gametophytes in Southern and central California. Mar Biol 45:297–309
Makarov M, Voskoboinikov G (2001) The influence of ultraviolet-B radiation on spore release and growth of the kelp Laminaria saccharina. Bot Mar 44:89–94
Montecino V, Pizarro G (1995) Phytoplankton acclimation and spectral penetration of UV irradiance off the central Chilean coast. Mar Ecol Prog Ser 121:261–269
Orce VL, Helbling WE (1997) Latitudinal UVR-PAR measurement in Argentina: extent of the ‘ozone hole’. Glob Planet Change 15:113–121
Pavia H, Cervin G, Lindgren A, Aberg P (1997) Effects of UV-B radiation and simulated hervibory on phlorotannins in the brown alga Ascophyllum nodosum. Mar Ecol Prog Ser 157:139–146
Ramus J (1978) Seaweed anatomy and photosynthetic performance: the ecological significance of light guides, heterogeneous absorption and multiple scatter. J Phycol 14:352–362
Reed DC, Amsler CD, Ebeling AW (1992) Dispersal in kelps: factors affecting spore swimming and competency. Ecology 73:1577–1585
Roleda MY, Wiencke C, Hanelt D, van de Poll WH, Gruber A (2005) Sensitivity of Laminariales zoospores from Helgoland (North Sea) to ultraviolet and photosynthetically active radiation: implications for depth distribution and seasonal reproduction. Plant Cell Environ 28:466–479
Roleda MY, Hanelt D, Wiencke C (2006) Growth and DNA damage in young Laminaria sporophytes exposed to ultraviolet radiation: implication for depth zonation of kelp of kelps on Helgoland (North Sea). Mar Biol (in press), available online
Sancar A, Sancar GB (1988) DNA repair enzymes. A Rev Biochem 57:29–67
Santelices B (1989) Algas Marinas de Chile. Ediciones Universidad Católica de Chile, Santiago, 399 pp
Santelices B, Ojeda P (1984) Recruitment, growth and survival of Lessonia nigrescens (Phaeophyta) at various tidal levels in exposed habitats of Central Chile. Mar Ecol Prog Ser 14:165–173
Schoenwaelder ME (2002) The occurrence and cellular significance of physodes in brown algae. Phycologia 41:125–139
Schoenwaelder MEA, Wiencke C, Clayton MN, Glombitza KW (2003) The effect of elevated UV radiation on Fucus spp. (Fucales, Phaeophyta) zygote and embryo development. Plant Biol 5:366–377
Schreiber U, Bilger W, Neubauer C (1994) Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. Ecol Stud 100:49–70
Setlow RB (1974) The wavelengths in sunlight effective in producing skin cancer: a theoretical analysis. Proc Natl Acad Sci USA 71:3363–3366
Shelly K, Heraurd P, Beardall J (2002) Nitrogen limitation in Dunaliella tertiolecta (Chlorophyceae) leads to increased susceptibility to damage by UV-B radiation but also increased repair capacity. J Phycol 38:713–720
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. W.H. Freeman, New York, 807 pp
Starr R, Zeikus J (1993) UTEX—the culture collection of algae at the University of Texas at Austin. J Phycol 29:1–106
Swanson AK, Druehl LD (2000) Differential meiospore size and tolerance of ultraviolet light stress within and among kelp species along a depth gradient. Mar Biol 136:657–664
Tala F, Edding M, Vásquez J (2004) Aspects of the reproductive phenology of Lessonia trabeculata (Laminariales: Phaeophyceae) from three populations in northern Chile. N Z J Mar Freshw Res 38:255–266
Vásquez J (1989) Estructura y organización de huirales submareales de Lessonia trabeculata. PhD Thesis, Facultad de Ciencias, Universidad de Chile, Santiago, 261 pp
Villouta E, Santelices B (1986) Lessonia trabeculata (Phaeophyta) a new kelp from Chile. Phycologia 25:81–88
Voskoboinikov GM, Kamnev AN (1991) Morphofunctional changes of the chloroplasts during the seaweed ontogenesis. Nauka, Leningrad, 96 pp
Westermeier R, Müller DG, Gómez I, Rivera JP, Wenzel H (1994) Population biology of Durvillaea antarctica and Lessonia nigrescens (Phaeophyta) on the rocky shores of southern Chile. Mar Ecol Prog Ser 110:187–194
Wiencke C, Gómez I, Pakker H, Flores-Moya A, Altamirano M, Hanelt D, Bischof K, Figueroa FL (2000) Impact of UV radiation on viability, photosynthetic characteristics and DNA of brown algal zoospores: implications for depth zonation. Mar Ecol Prog Ser 197:217–229
Wiencke C, Clayton MN, Schoenwaelder M (2004) Sensitivity and acclimation to UV radiation of zoospores from five species of Laminariales from the Arctic. Mar Biol 145:31–39
Yabe K, Makino M, Suzuki M (1997) Growth inhibition on gametophytes of Laminaria religiosa induced by UV-B irradiation. Fish Sci 63:668–670
Acknowledgments
The research was supported by grants from the Universidad Católica del Norte (DGICT-2003 to M.E. and F.T.) and CONICYT (FONDECYT no. 1030343 to I.G.). The authors thank P. Huovinen for criticism and comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Kinne, Oldendorf/Luhe
Rights and permissions
About this article
Cite this article
Véliz, K., Edding, M., Tala, F. et al. Effects of ultraviolet radiation on different life cycle stages of the south Pacific kelps, Lessonia nigrescens and Lessonia trabeculata (Laminariales, Phaeophyceae). Mar Biol 149, 1015–1024 (2006). https://doi.org/10.1007/s00227-006-0301-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0301-9