Abstract
The presence of the two morphs, “typical” and “large mouth”, in the Antarctic fish species Trematomus newnesi (Perciformes, Notothenioidei) was recorded for the first time in nearshore waters of the South Shetland Islands (Potter Cove) and western Antarctic Peninsula (Petermann Island). The two morphs were distinguishable in specimens of 60–241 mm total length (TL); about 30% of the specimens constituted intermediate forms. In addition to the previously known characters separating the morphs, we found that the “relative size of the eye” can also be used to identify smaller and larger fish of the typical morph. The ecological significance of the two morphs remains unclear. Ratios of diagnostic characters for identification of the species at two size ranges (60–131 and 132–241 mm TL) are provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Discontinuous phenotypes in a population constitute the greatest variability in most species. When all members of a local population are products of the same gene pool and members of a single taxonomic unit, the existence of morphologically different individuals reflects intraspecific variation (Mayr and Ashlock 1991).
Ichthyological research on trophic polymorphism indicates that the differences between morphs are associated with food and habitat (Smith and Skúlason 1996). In general, morphs differ in size and shape of body, head, fins or other characters. This widespread evolutionary phenomenon is known for fish in different fresh water and marine environments. Examples include river cichlids Cichlasoma spp. in Cuatro Ciénagas, Mexico (Sage and Selander 1975; Dunnigan et al. 1994); cichlids in Lake Tanganyika (Koblmüller et al. 2004); Arctic charr Salvelinus alpinus in lakes of Iceland and Northern Norway (Peres-Neto and Magnan 2004); Scandinavian white fish Coregonus spp. in nine Norwegian lakes (Whiteley 2007) and serranid marine fish Hypoplectrus spp. in Puerto Rico, U.S. Virgin Islands, Curaçao, Honduras and Belize (Whiteman et al. 2007).
The first case of phenotypic plasticity in Antarctic fish was described by Eastman and DeVries (1997) for the species Trematomus newnesi Boulenger 1902 from McMurdo Sound, documenting differences between the “typical morph” and a “large mouth morph”. T. newnesi is a coastal circumpolar Antarctic fish of the family Nototheniidae (Perciformes, Notothenioidei), widely distributed in waters of the southern Scotia Arc (South Orkney Islands and South Shetland Islands), the West Antarctic Peninsula and the High Antarctic Zone (Gon and Heemstra 1990). Although its depth distribution may reach 400 m (Tiedtke and Kock 1989), it is more abundant in shallow inshore waters in the range 20–50 m, primarily on rocky bottoms with macroalgae beds (Moreno et al. 1982; Barrera-Oro 2002).
The main differences between the two morphs recognised by Eastman and DeVries (1997) are the wide and U-shaped head in the large mouth morph and the more tapered and V-shaped head in the typical morph. According to Eastman and DeVries (1997), the holotype described by Boulenger (1902) was the typical morph and the form exemplified in Fischer and Hureau (1985) and DeWitt et al. (1990) corresponds to the large mouth morph. Further descriptions of polymorphism in Antarctic fish are scarce and are found in other members of the genus Trematomus: two colour morphs of T. bernacchii (white blotch and brown) in McMurdo Sound (Bernardi and Goswami 1997) and morphism in chromosome number and morphology in T. eulepidotus and T. hansoni (Ozouf-Costaz et al. 1997; Pisano et al. 1998).
Until 1990 the maximum total length (TL) known for T. newnesi was 200 mm (Gon and Heemstra 1990). More recent studies reported larger values, occasionally in the range 230–250 mm TL (Vacchi and La Mesa 1995; La Mesa et al. 2000; Casaux et al. 2003). The sample from McMurdo Sound, where the two morphs in question were found, included specimens from 113 mm to a maximum size of 280 mm TL (Eastman and DeVries 1997).
In Potter Cove, South Shetland Islands, several ichthyological studies have been carried out by means of trammel nets with mesh sizes that allowed capture of specimens over 150 mm TL (reviewed in Barrera-Oro and Casaux 2008). Recently, the scope of research at this locality was expanded by the use of bottom trawling with nets of smaller mesh capable of sampling earlier life history stages of fish, including T. newnesi, down to approximately 40 mm TL (Barrera-Oro and Piacentino 2007).
Through analysis of T. newnesi specimens collected mostly at Potter Cove, the primary aim of this study is to report on the occurrence of the typical and large mouth morphs for first time, and in smaller specimens (≥60 mm TL), in the South Shetland Islands and western Antarctic Peninsula region (Fig. 1). Furthermore, we found that the character “eye diameter” sometimes separates the two morphs. Because analysis of morphometric measurements throughout ontogeny is a useful tool in taxonomic studies, our second objective is to provide new diagnostic characters for two size ranges of T. newnesi to aid in its identification.
Materials and methods
Samples of T. newnesi were obtained primarily at Potter Cove, King George Island, South Shetland Islands, close to the scientific station Jubany (62°14′S; 58°40′W) by means of a bottom trawl (mouth 1 m2, length 2 m, mesh 4 mm) and trammel nets (length 25 m, inner mesh 25 mm) at 8–40 m depth. The abiotic features and biotic components of this area are described in Casaux et al. (1990). In addition, five individuals (Museo Argentino de Ciencias Naturales B. Rivadavia 5729) from Petermann Island, western Antarctic Peninsula, were also analysed. Some specimens (n = 29) were preserved in collection (MACN 9225 and MACN 9226). The sampling data and fish examined are summarised in Table 1, with measurements following Hubbs and Lagler (1958). Wilcoxon Matched-Pairs non-parametric test was used to test the null hypothesis that there were no differences in relative measurements between the two size classes.
Within the size range of the fish collected for study of ontogenetic variation, many length intervals were not well represented or even absent (e.g., seven 1 cm length classes with N < 3). Hence it was not possible, for instance, to recognise gradual ontogenetic variation of selected characters and to divide the whole sample into several, well represented, length classes that putatively represent different cohorts. Instead, we arbitrarily separated the fish sample in two main size ranges: specimens within the size ranges of 60–131 mm TL [13–32 mm head length (HL)] and 132–241 mm TL (33–59 mm HL) are defined as of small and large fish, respectively.
To analyse the degree of allometry, the allometric growth function (y = ax b) was fitted to the values of some morphometric characters (with x being the HLs). Values of b < 1 and b > 1 indicate negative and positive allometry, respectively. Values of b that are not significantly different from 1 indicate isometric growth.
The specimens investigated were collected during four intervals over the past 38–39 years and it was our original intent to study only ontogenetic morphometric variation in this species. However, upon learning of the existence of phenotypic plasticity or morphism in T. newnesi (Eastman and DeVries 1997) and recognising that this phenomenon was also present in our samples, we retrospectively expanded the scope of our study to include a documentation of the incidence of the various morphs. Thus the size of the sample we employed for the analysis of morphometric variation was larger (180 specimens) than that used for the study of the morphism component (70 specimens). This is because the specimens from the earlier collections were not preserved in a museum collection and thus could not be examined for morphism.
Results
Identification of morphs
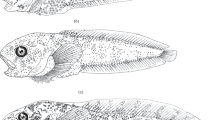
Of the total fish examined for identification of morphs (n = 70), 41% corresponded to the typical morph, 29% to the large mouth and the remaining 30% were intermediate forms (Fig. 2).
Morphometric measurements
Ratios of fish length and HL to a number of common diagnostic characters for both small and large specimens of T. newnesi are shown in Table 2. Values are expressed as ratios instead of relative sizes (as shown in Figs. 3, 4, 5, 6), to make them directly comparable with those characters described in the literature for the diagnosis of species of genus Trematomus (Dewitt et al. 1990).
Ontogenetic growth patterns
In T. newnesi the growth pattern is isometric for some characters. Their relative sizes varied from 29.16 to 43.33% for HL/SL from 37.93 to 59.61% for the length of snout to anal fin origin/SL and from 37.67 to 69.39% for the length of anal fin origin to caudal fin base/SL.
The growth pattern is also isometric (Table 3) for relative upper jaw length (Fig. 3b), relative mandible length (Fig. 4b) and relative gape width (Fig. 5b). The values for small and large fish ranged from 37.54 to 57.77% and from 42.85 to 58.82%, respectively, for relative upper jaw length; from 30.67 to 44.44% and from 36.4 to 48.6%, respectively, for relative mandible length from 28.33 to 45.18% and from 32.08 to 50.98%, respectively, for relative gape width. For relative mandible length and relative gape width, two discrete morphologies were observed (Figs. 4a, 5a). Eye diameter shows a slight negatively allometric growth (Table 3), with percentage ratios of 21.82–33.33% in small fish and 19.04–30.77% in large fish (Fig. 6b). The growth pattern is positively allometric for interorbital width/HL and for body depth/SL. The relative interorbital widths range from 18.75 to 31.81% in small fish and from 22.8 to 34.61% in large fish. For relative body depth, the values range from 16.36 to 24.77% in small fish and from 18.93 to 49.56% in large fish.
Discussion
The two distinct morphs described by Eastman and DeVries (1997) were also identified by external characters within the fish collected in the South Shetland Islands/western Antarctic Peninsula region (mainly at Potter Cove), including smaller fish (from 60 mm TL), which were not present in the sample from McMurdo Sound (113–280 mm TL). This indicates that this phenomenon is evident in juveniles and is not confined to later periods of ontogeny. In dorsal and ventral views, the head was more pointed, V-shaped in the typical morph and more wide, U-shaped and darker in the large mouth morph. In addition to this distinct colouration and morphology of the head, originally pointed out by Eastman and DeVries (1997), we observed that the pigmentation of the head and body was orange-brownish in the typical morph and dark grey in the large mouth morph (Fig. 2). We also noted that the relative size of the eye was, in some cases (n = 21) with the reservations noted below, a useful new character in separating morphs, with a larger eye diameter in the typical morph and smaller in the large mouth morph (Fig. 2). Nevertheless, despite the general validity of these characters, approximately 30% of the T. newnesi specimens analysed were intermediate forms, which could not be assigned to any of the morphs regardless of size. This could be a reflection of the variability of the phenotypic characters analysed. Intermediate morphs have also been collected recently in McMurdo Sound (J. T. Eastman, personal communication).
In the present study we observed, within fish of the same length interval, variability in eye diameter and projection of the maxillary relative to the eye. Many smaller and larger specimens had a larger eye diameter and the maxillary projected in some cases beyond the anterior one-third of the eye. This could be due to heterochrony, because this pattern remained constant with increasing fish size. Similar variability was also observed by Boulenger (1902) and by Eastman and DeVries (1997) for sizes of 116–239 mm SL.
Based on morphology and measurements Eastman and DeVries (1997) suggested that the large mouth morph could be more benthic than the typical semipelagic morph, as a response to a different diet. Two studies on the diet of T. newnesi have been carried out at Potter Cove. The most recent showed that early juvenile stages (4–154 mm TL) fed on a few benthic-demersal taxonomic groups associated with algae communities, with no differences in feeding habits between fish sizes (Barrera-Oro and Piacentino 2007). In the earlier study, larger juveniles and adult fish (123–233 mm TL) preyed on both benthic-demersal and pelagic organisms, becoming secondary plankton/water-column feeders (Casaux et al. 1990). These studies did not give conclusive evidence for niche diversification, since the dietary analysis was carried out over the total fish sample without separation of the two distinct morphs. The information in Barrera-Oro and Piacentino (2007) does not support this hypothesis because either differences in habitat or diet between juvenile fish was found in a sample that was composed of specimens of both morphs. We share the view of Eastman and DeVries (1997) on the need to clarify this hypothesis by a representative trophic analysis. Thus this phenotypic polymorphism is not yet a documented example of trophic polymorphism.
It is necessary to make clear that our samples were not processed to see strict morphometric differences between the two morphs (except for a comparison between eye sizes of a limited number of fish), but to see ontogenetic changes of T. newnesi without separation into morphs (see “Material and methods”). Hence, Figs. 3, 4, 5, 6 and Tables 2, 3 refer to morphometric differences between the two size ranges of the species and not to differences between the morphs.
In the morphometric analysis of T. newnesi the ratios of the diagnostic characters used for taxonomic identification (Table 2) extend the range of values given in the literature for nototheniids in general (DeWitt et al. 1990). Although it was not possible to divide the whole sample into several length classes and gradually follow the ontogenetic variation in the selected characters, the data presented for two main size ranges reflect size-related changes in growth of the species.
The joint analysis of mandible length, gape width and upper jaw length with increasing fish size indicate that the gape of the mouth was determined by the last character. Upper jaw lengths are proportionally larger in smaller specimens (Fig. 3b) allowing for a larger mouth opening, up to a position of the maxillary perpendicular to the dentary. This morphology was not observed in fish larger than 132 mm TL and could be interpreted as an adaptation in smaller fish for an effective ambush feeding on some relatively large prey, such as amphipods and krill (Barrera-Oro and Piacentino 2007).
Likewise, the characters mandible length and gape width in relation to HL separate the investigated specimens in a nearly bimodal fashion (each mode putatively being representative of a different morph) (Figs. 4a, 5a, respectively), similarly to the description in Eastman and DeVries (1997) for the relative upper jaw length and gape width of T. newnesi from McMurdo Sound. Because T. newnesi is a polymorphic species with intermediate morphs, some characters did not exhibit a clear bimodal distribution, as was the case in our data for upper jaw length and eye diameter (Figs. 3a, 6a, respectively). Quantification and further analysis of the occurrence of the typical and large mouth morphs of T. newnesi at Potter Cove will be the aim of a future work.
References
Barrera-Oro ER (2002) The role of fish in the Antarctic marine food web: differences between inshore and offshore waters in the southern Scotia Arc and west Antarctic Peninsula. Antarct Sci 14:293–309
Barrera-Oro ER, Casaux RJ (2008) General ecology of coastal fish from the South Shetland Island and west Antarctic Peninsula areas. Ber Polar Meeresforsch 571:95–110
Barrera-Oro ER, Piacentino GLM (2007) Feeding habits of juvenile Trematomus newnesi (Pisces, Nototheniidae) at Potter Cove, South Shetland Islands, Antarctica. Polar Biol 30:789–796
Bernardi G, Goswami U (1997) Molecular evidence for cryptic species among the Antarctic fish Trematomus bernacchii and Trematomus hansoni. Antarct Sci 9:381–385
Casaux RJ, Mazzotta AS, Barrera-Oro ER (1990) Seasonal aspects of the biology and diet of nearshore nototheniid fish at Potter Cove, South Shetland Islands, Antarctica. Polar Biol 11:63–72
Casaux RJ, Barrera-Oro ER, Baroni A, Ramón A (2003) Ecology of inshore notothenioid fish from the Danco Coast, Antarctic Peninsula. Polar Biol 26:157–165
DeWitt HH, Heemstra PC, Gon O (1990) Nototheniidae. In: Gon O, Heemstra PC (eds) Fishes of the Southern Ocean. JLB Smith Institute of Ichthyology, Grahamstown, pp 279–331
Dunnigan B, McElroy DM, Douglas ME (1994) Sexual dimorphism and morphological variation in Cichlasoma minckleyi from the Cuatro Ciénegas basin, México. Proc Des Fish Counc XXV:45–46 did trophic differences. These re the hypothesis that C. minckleyi
Eastman JT, DeVries AL (1997) Biology and phenotypic plasticity of the Antarctic nototheniid fish Trematomus newnesi in McMurdo Sound. Antarct Sci 9:27–35
Fischer W, Hureau JC (eds) (1985) FAO species identification sheets for fishery purposes. Southern Ocean (Fishing areas 48, 58 and 88) (CCAMLR Convention Area), vol 2. FAO, Rome, pp 233–470
Gon O, Heemstra PC (eds) (1990) Fishes of the Southern Ocean. JLB Smith Institute of Ichthyology, Grahamstown, pp 462
Hubbs CL, Lagler KF (1958) Fishes of the Great Lakes Region. Cranbrook Institute of Science, Bloomfield Hills
Koblmüller S, Duftner N, Katongo C, Phiri H, Sturmbauer C (2004) Ancient divergence in bathypelagic Lake Tanganyika deepwater cichlids: mitochondrial phylogeny of the tribe bathybatini. J Mol Evol 60:297–314
La Mesa M, Vacchi M, Sertorio TZ (2000) Feeding plasticity of Trematomus newnesi (Pisces, Nototheniidae) in Terra Nova Bay, Ross Sea, in relation to environmental conditions. Polar Biol 23:38–45
Mayr E, Ashlock PD (1991) Principles of systematic zoology. McGraw-Hill, New York
Moreno CA, Zamorano JH, Duarte WE, Jara HF (1982) Abundance of Antarctic juvenile fishes on soft-bottom substrates: the importance of the refuge. Cybium 6:37–41
Ozouf-Costaz C, Pisano E, Thaeron C, Hureau JC (1997) Antarctic fish chromosome Banding, significance for evolutionary studies. Cybium 21:399–409
Peres-Neto PR, Magnan P (2004) The influence of swimming demand on phenotypic plasticity and morphological integration: a comparison of two polymorphic charr species. Oecologia 140:36–45
Pisano E, Ozouf-Costaz C, Prirodina V (1998) Chromosome diversification in Antartic fish (Notothenioidei). In: di Prisco G, Pisano E, Clarke A (eds) Fishes of Antarctica: a biological overview. Springer-Verlag Italia, Milan, pp 275–285
Sage RD, Selander RK (1975) Trophic radiation through polymorphism in cichlid fishes. Proc Natl Acad Sci USA 72:4669–4673
Smith TB, Skúlason S (1996) Evolutionary significance of resource polymorphisms in fishes, amphibians, and birds. Annu Rev Ecol Syst 27:111–133
Tiedtke JE, Kock KH (1989) Structure and composition of the fish fauna around Elephant Island. Arch Fisch Wiss 39:143–169
Vacchi M, La Mesa M (1995) The diet of the Antarctic fish Trematomus newnesi Boulenger, 1902 (Nototheniidae) from Terra Nova Bay, Ross Sea. Antarct Sci 7:37–38
Whiteley AR (2007) Trophic polymorphism in a riverine fish: morphological, dietary, and genetic analysis of mountain whitefish. B J Linn Soc 92:253–267
Whiteman EA, Côté IM, Reynolds JD (2007) Ecological differences between hamlet (Hypoplectrus: Serranidae) colour morphs: between-morph variation in diet. J Fish Biol 71:235–244
Acknowledgments
We thank C. Bellisio for his help in field activities, M. Canevari for his assistance in photograph procedures and A. Gonzalez for providing the map of the Antarctic Peninsula. We are particularly grateful to Prof. J. Eastman for his valuable comments on the manuscript. This paper was also kindly reviewed by an anonymous referee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Piacentino, G.L.M., Barrera-Oro, E. Phenotypic plasticity in the Antarctic fish Trematomus newnesi (Nototheniidae) from the South Shetland Islands. Polar Biol 32, 1407–1413 (2009). https://doi.org/10.1007/s00300-009-0651-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-009-0651-0