Abstract
During their evolution and speciation in the Antarctic waters, notothenioid fish occupied a variety of habitats and ecological niches. The diversification led to important variations in several morphological features related to particular aspects of their ecologies. We investigated the feeding structures and biomechanics of three phylogenetically related species (family Nototheniidae) with different ecologies: the bentho-pelagic Antarctic toothfish Dissostichus mawsoni, the pelagic Antarctic silverfish Pleuragramma antarctica, and the benthic emerald rockcod Trematomus bernacchii. The suction index (SI), the mechanical advantage in jaw closing (MA), and 14 morphological traits related to their feeding activity were analyzed. Significant differences among the species were found for all the parameters considered, supporting a high level of specialization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The dominance and diversity of Notothenioidei in the Antarctic ichthyofauna are the result of a unique history of evolutionary change (Eastman and Clarke 1998; Eastman 2000, 2005; Rutschmann et al. 2011; Mintenbeck et al. 2012). Able to survive in the cold marine waters surrounding the Antarctic continent owing to the emergence of the ability to synthesize antifreeze glycoproteins (AFGPs) (Cheng and Detrich 2007), the Antarctic notothenioids underwent an important and rapid diversification during the late Miocene cooling (11.6–5.3 Ma). Such an amazing example of a species flock (Lecointre et al. 2013), rare in the marine environment, was favored by the local extinction of most of the previous fish species, especially the near-shore and continental shelf benthic fauna highly impacted by the advancing polar conditions and the increased ice activity (Near et al. 2012). Due to the reduced competition and novel ecological opportunities, the Antarctic notothenioids were able to occupy semipelagic, cryopelagic, and pelagic niches, despite being ancestrally devoid of a swim bladder (Klingenberg and Ekau 1996; Near et al. 2012; Eastman et al. 2014).
Pelagic habitat utilization in some lineages (secondary pelagization) has been accompanied in those species by important morphological and physiological modifications (Klingenberg and Ekau 1996). Pelagic notothenioid lineages show adaptive modifications that span from compensatory changes in body density (DeVries and Eastman 1978; Eastman 1993; Hagen and Kattner 2017; Voskoboinikova et al. 2017) to morphological adaptations for water column foraging (reviewed in La Mesa et al. 2004a).
In fishes, three broad methods of prey capture are known: suction feeding, ram feeding, and manipulation (Liem 1980). The particular feeding mode is primarily determined by the functional morphology of the feeding apparatus (reviewed in Sonnefeld et al. 2014).
Suction feeding is based on the generation of negative pressure in the buccal cavity prior to mouth opening; then mouth expansion produces a pressure gradient and causes the prey to be moved towards the open mouth. Fish using this feeding mechanism are characterized by having a small gape, low mechanical advantage for jaw closing, powerful force-generating capability of jaw-opening muscles, and high suction ability (Wainwright and Bellwood 2002). Suction feeding is the most common foraging method in predators of mobile animals (Wainwright and Bellwood 2002), and it is used by most teleosts as the primary method of prey capture (Lauder 1982; Wainwright and Bellwood 2002; Westneat 2006).
Ram feeding is a method consisting of ingestion of free-swimming prey by forward movement of the predator or protruding jaws (Wainwright and Bellwood 2002). The feeding mechanism of ram-feeding fishes is characterized by non-robust oral jaws, large gape, moderate suction ability, low mechanical advantage for jaw closing, and moderate force-generating capability of the muscles (Wainwright and Bellwood 2002).
Pure suction and ram feeding are relatively rare in nature; a combination of both modes is the most commonly used strategy (Norton and Brainerd 1993).
Fairly uncommon among teleosts is the manipulation method, based on the direct application of the jaws to prey to crush or remove it from its substrate. A manipulation feeder’s mouth apparatus is therefore characterized by robust oral jaws, small gape, high mechanical advantage for jaw closing, and powerful force-generating capability of the adductor mandibulae (Liem 1980; Wainwright and Bellwood 2002).
Regardless of the method, teleost feeding activity involves a large number of moving elements, with more than 20 major skeletal components set in motion by approximately 40 muscles in all modes of prey capture (Barnett et al. 2006). However, the analysis of a reduced number of key morphological traits, such as some attributes of the head, jaw regions, and gill rakers, provides valuable information on feeding performance and potential resource usage in several fish (Wainwright and Richard 1995; Barnett et al. 2006; Sonnefeld et al. 2014).
Here we analyze the feeding structures and biomechanics of three closely related Antarctic notothenioid species: the Antarctic silverfish Pleuragramma antarctica, a holopelagic species feeding exclusively on zooplankton; the Antarctic toothfish Dissostichus mawsoni, a large demersal predator, feeding mainly on fish and cephalopods; and the benthic generalist emerald rockcod Trematomus bernacchii, feeding mainly on benthic invertebrates, but also occasionally on planktonic prey. The main aim of our work is to characterize each species’ feeding mode by the use of an ecomorphological approach. Interspecific variation in mechanical advantage (MA) and suction index (SI) for the jaws is reported. In order to infer each species’ potential for filter feeding, gill raker morphology is also considered. Until now, despite a relatively good knowledge of Antarctic notothenioid fish diet composition, ecomorphological information was available only on the feeding capabilities of the nototheniid T. bernacchii (Bansode et al. 2014).
Materials and methods
Sampling
Three species of nototheniids were analyzed: D. mawsoni (n = 20), P. antarctica (n = 27), and T. bernacchii (n = 14), collected in the Ross Sea.
Dissostichus mawsoni samples were collected onboard the FV Janas in June and July 2016 during New Zealand winter survey on the seamounts of the Subarea 88.1 SSRUs B-C, at a depth of about 1500 m. Specimens were collected by bottom longline during commercial fishing (Stevens et al. 2016). Measurements were performed on board on fresh specimens.
Pleuragramma antarctica were caught on 23 February 2015 during a New Zealand survey conducted onboard of the RV Tangaroa. Specimens were collected by midwater trawl at a depth of 540 m (O’Driscoll and Double 2015). Fish were fixed and stored in 70% ethanol for later analyses.
Trematomus bernacchii were collected in November and December 2005 during the Italian Antarctic Expedition 2005/2006 (Bottaro and Vacchi 2006). Sampling was performed by fishing line through the pack-ice in Tethys Bay and was authorized by the Italian National Antarctic Research Programme (PNRA) on behalf of the Italian Ministry of Foreign Affairs. Specimens were frozen and stored at − 20 °C.
In order to minimize bias in this study, at the intra-specific level related to ontogeny, only adult specimens were analyzed (La Mesa et al. 2004a; La Mesa and Eastman 2012; Hanchet et al. 2015). Accordingly, ranges of Standard Length (SL) are as follows: D. mawsoni 980–1480 mm, P. antarctica 151–200 mm, T. bernacchii 164–246 mm.
Biomechanical indices calculation
Two metrics were assessed: the suction index (SI) and the mechanical advantage (MA). After cutting off the connection between muscles and bones, measurements were recorded to the nearest 0.02 mm using a vernier caliper for P. antarctica and T. bernacchii and to the nearest millimeter using a ruler for D. mawsoni.
SI is applied to evaluate suction-feeding capability based on the specific ability to rapidly expand the buccal cavity, creating a pressure gradient between this space and the area around the head (Wainwright and Bellwood 2002). The model for SI is based on the transmission of force from the epaxialis muscle to the buccal cavity, generating negative pressure that allows the predator to engulf its prey.
Following Carroll et al. 2004, SI was calculated as
where CSAepax is the cross-sectional area of the epaxialis, Lin is the moment arm of the epaxialis, and Lout is the moment arm of the buccal cavity. Gape width and buccal length were measured to estimate the area of the buccal cavity.
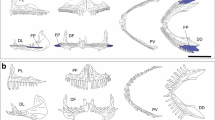
The cross-sectional areas of the ellipse-shaped epaxialis were calculated by the measurements of their axes. The major axis was measured from the supracleithrum-posttemporal (S-PT) joint to the dorsal margin of the epaxialis; the minor as the lateral width of the epaxialis. Lin (LinSI) was calculated as the vertical distance between the centroid of the epaxialis muscles’ cross section and the S-PT, Lout (LoutSI) was measured from the S-PT joint to the middle of the buccal cavity. Gape width in a maximally opened mouth was measured as the distance between the left and right coronoid processes of the mandible, buccal length (BL) as the distance between the anterior tip of the mandible and the depression in the sternohyoideus (Collar and Wainwright 2006).
MA indicates the capability of the fish to produce force during the closing of the lower jaw. The mandible of an actinopterygian fish is a third-order lever system for both opening and closing mechanisms, the fulcrum of the lower jaw being the quadrate/articular joint through which the jaw rotates open and is pulled closed during feeding (reviewed in Westneat 2004). Herein we consider the closing lever associated with jaw-closing systems. Accordingly, MA was calculated as the ratio of the jaw-closing in-lever (LinMA) to the jaw-closing out-lever (LoutMA). LinMA was measured as the distance from the quadrate-articular joint to the point of insertion of the adductor mandibulae muscle on the lower jaw, and LoutMA as the distance from the quadrate-articular joint to the anterior-most tooth of the lower jaw (Bansode et al. 2014).
Gill rakers morphological analysis
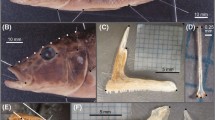
In P. antarctica and T. bernacchii, gill arches were observed under an Olympus SZX7 stereo microscope, and microphotographs were taken through a Nikon “DS-L3” digital camera, and measurements taken to the nearest 0.01 mm. In D. mawsoni, gill arch measurements were made by the use of ruler and caliper, to the nearest millimeter.
Measurements were made on the first left branchial arch. For each specimen, the length of the gill arch (LA), the number of gill rakers (NG), the length of gill rakers (LG), and the spacing between subsequent gill rakers (SG) were measured. The width of gill rakers (WG) was measured for five long gill rakers in the middle of the gill arch (Tanaka et al. 2006). To obtain more accurate measurements, the gill arch was mounted with the gill rakers perpendicular to the base of the arch (Amundsen et al. 2004).
Statistical analysis
Statistical analyses were performed using the program R 3.2.2 (R Development Core Team 2015).
After transforming data in arcsin \(\sqrt {\frac{p}{100}}\) and testing normality and homoskedasticity of the distributions with Shapiro-Wink and Levene tests, two univariate analyses of variance (ANOVAs) were conducted to test differences in mechanical advantage (MA) and suction index (SI). Tukey post hoc tests were employed to detect significant differences among species. Significance for all statistical analyses was determined at α = 0.05.
Principal component analysis (PCA) was developed on morphological traits to determine which morphometric traits explain the greatest variation among the three species. A total of 14 variables were taken into account: the morphological traits of the feeding apparatus used for the SI and MA metrics, the length of the head, gill rakers, and gill arch metrics.
As all the specimens were adults, we assumed that no intra-specific allometric variations occurred in the morphological traits in the range of body size of our samples. To compare individuals and species of different total length, morphological measurements were standardized to the standard length of each individual (Barnett et al. 2006). Since the measures were made on bones or hard elements of the head, or the insertion points of muscles, we assumed that the small possible alterations of sizes due to the different methodologies used to preserve the samples did not influence our results.
Results
ANOVA (F(2,58) = 1255, p < 0.0001) and Tukey’s post hoc test indicate that mean SI was significantly different in the three species. Among the studied species, T. bernacchii had the highest SI (Table 1); SI values in P. antarctica and D. mawsoni were significantly lower (Fig. 1).
MA ratios were also significantly different among the species (ANOVA, F(2,58) = 18.47, p < 0.0001) with P. antarctica having lower MA ratio on average than D. mawsoni and T. bernacchii (Table 1). A Tukey test confirmed the significance of the difference between MA of P. antarctica and those of D. mawsoni and T. bernacchii (Fig. 1).
Table 2 shows the mean values of the 14 variables of the three species that were taken into account to develop the PCA to assess what morphological traits were the most important in the diversification in their feeding structures. The relative measurement of the length of the head (HL) of T. bernacchii was significantly lower than those of the other two species. The value of gape width (GW) observed in P. antarctica was the lowest among the species studied. The buccal length (BL) was similar between P. antarctica and D. mawsoni, while it was lower in T. bernacchii, similar to that of HL. The values of the width and the height of the cross section of the epaxialis muscle measured at the S-PT joint (Wepax and Hepax) were highest for T. bernacchii. These two factors determine the higher suction index (SI) of this species. The cross section of the epaxialis muscle of P. antarctica was narrower and higher than that of D. mawsoni. The levers of the morphological pressure linked to the suction index (LinSI and LoutSI) had higher values in T. bernacchii. While high values of LinSI lead to high values of SI, the opposite holds for LoutSI. P. antarctica showed intermediate values of SI among the species in this study, while the low value of LinSI of D. mawsoni combined with its relatively high value of LoutSI resulted in a low total value of SI of this species. Focusing on the measurements of the lower jaw levers involved in the mechanism of mechanical advantage (MA), LinMA and LoutMA, the measure that separated the species was the LoutMA value of P. antarctica, which was higher than those of the other two species. LoutMA is negatively linked to the MA, which for the P. antarctica was significantly lower than those of the other two nototheniids.
The morphological analysis of the gill rakers revealed the presence of numerous elongated and little spaced gill rakers in P. antarctica (Fig. 2a; Table 2). Barely distinguishable and short gill rakers were observed in D. mawsoni and T. bernacchii, respectively (Fig. 2b, c; Table 2).
PCA explained 79.447% of the variance on the first two axes (Table 3). Investigation of the variables driving the two components resulted that above all the factors were important in determining the function. The ordination of PC1 axis is driven by variables linked to the SI value and by gill rakers’ characteristics. PC2 is instead driven by the lower jaw levers, from which we calculate MA. Overall, PCA clearly segregate the three species regarding the morphological traits of the feeding structures (Fig. 3).
Discussion
Ecomorphological studies, integrating morphological and ecological data, hold enormous potential to clarify form–function relationships, thus providing new elements to our understanding of the ecology of fish group and their evolution. A central assumption of ecomorphology is that the covariation that we observe today between morphological and environmental characteristics is the cumulative result of previous adaptations. Conversely, current morphological traits of an organism do not only hold a taxonomic and phylogenetic fingerprint, but they can in turn play a role in the evolution and adaptation of the organism, affecting its capability to adapt to different scenarios.
The rapid adaptive radiation leading notothenioid fish to occupy many ecological niches (Eastman 2005) makes this group of fish particularly suitable for investigating ecomorphological relationships between feeding structures and the ecology of the species to understand their capabilities to adapt to future environmental change. Klingenberg and Ekau (1996) applied this approach to analyze the divergence of nine nototheniid species into different habitats. A comparative morphometric analysis on the feeding structures was performed within the family Artedidraconidae by taking into consideration two morphometric measures of the mouth apparatus (the mouth width and mouth length, as well as other measurements pertaining to sensory systems (Lombarte et al. 2003). A more comprehensive study, focusing specifically on the feeding capabilities of Antarctic fish using a biomechanics approach, includes among others the nototheniid T. bernacchii (Bansode et al. 2014).
We compared the morphology and biomechanics of the feeding structures of three Antarctic notothenioid species. These species, all belonging to the family Nototheniidae, show a remarkable diversity in the diet and trophic niche spanning from feeding on benthic and epi-benthic invertebrate prey in T. bernacchii feeding (Moreno 1980; Vacchi et al. 1999; La Mesa et al. 2004b), to zooplanktivory in the pelagic P. antarctica (Pinkerton 2017), and to fish and squid feeding of the demersal D. mawsoni, the only piscine top predator of the Antarctic ecosystem (Stevens et al. 2014).
The structure and diversification of cranial and buccal skeletons in Nototheniidae has already been deeply investigated (Voskoboynikova 1993, 1994; Voskoboynikova et al. 1994; Balushkin 2000). Here we step forward and encapsulate morphometric information in biomechanical metrics (SI and MA), trying to relate form to function, to explore the feeding attitudes and preferential methodology of prey capture of the three studied species and, ultimately, to gain insights in these species’ feeding mode specialization vs flexibility.
Previous SI and MA calculation in T. bernacchii (Bansode et al. 2014) suggested a degree of plasticity in the feeding mode for this species. The high MA average value indicates high manipulation-feeding capability, confirmed by the available diet information as well as by its observed foraging behavior on the bivalve mollusk Adamussium colbeckii (Vacchi et al. 2000). However, owing to the relatively high SI, a suction-feeding mode is also proposed for the species, enabling foraging on mobile benthic and pelagic prey, as reported in the literature (Moreno 1980). Bansode et al. (2014) suggest that this species can also utilize suction strikes or ram suction strikes to capture prey.
T. bernacchii had the highest SI and MA values among the three species studied. High SI implies capability of rapid movements of the jaws, made possible in T. bernacchii by the relatively short head, short buccal length, and well-developed epaxialis muscle. This mouth conformation reduces the size of the buccal cavity, and its sub-ambient buccal pressure, opposite to the rotation of the neurocranium and the expansion of the mouth (Carroll et al. 2004; Collar and Wainwright 2006), while the development of the epaxialis muscle drives the dorsal rotation of the neurocranium. All these characteristics are consistent with T. bernacchii occasionally foraging by selective capture of individual prey, either benthic or planktonic. This species’ food items include isopods, pteropods, copepods, gammarids, tanaiids, mysids, euphausiids, and hyperiids, all planktonic prey that can be visually selected from the water column and engulfed by rapid suction in a particle-feeding mode fashion. Smaller prey items might be captured through filter feeding, but that would imply the presence of entrapment structures in the mouth apparatus such as gill rakers, capable of retaining the planktonic prey. Although present on the branchial arch, gill rakers in T. bernacchii are small and the space between sequential rakers is too large to support filter feeding. Small prey items are likely incidentally engulfed during the capture of larger prey.
The average MA of T. bernacchii is the highest among the three species studied, although not significantly different from that of D. mawsoni. In T. bernacchii, a powerful jaw-closing pressure might assist this fish in durophagous feeding. Indeed, in some areas, this species is known to feed on the Antarctic scallop (Vacchi et al. 2000), the hard shells of which can be broken owing to high MA and mouth conformation and also to strong conical jaw teeth with flattened tips of some that suggest wear (see Bansode et al.2014). Overall, high MA ratio supports the possibility of manipulation feeding in T. bernacchii.
Conversely, D. mawsoni, is a known bentho-pelagic predator observed to feed on demersal and pelagic prey (e.g., fish and squids, Stevens et al. 2014). This species is also reported to feed on scavenged items from the sea floor, such as the large squids, offal, penguins, and petrels (Fenaughty et al. 2003; Petrov and Tatarnikov 2011; Roberts et al. 2011). Such a scavenging foraging strategy is supported by well-developed olfaction capability, as suggested by the gross morphology and size of its olfactory organ and bulb (Ferrando et al. unpublished data). Feeding on large prey, both by active water column predation and by seafloor scavenging, requires a powerful bite in D. mawsoni. High MA ratio and well-developed dentition (DeWitt et al. 1990) are biomechanical and morphological evidences of this. The fact that D. mawsoni can display a labriform locomotion, moving with a rigid body and using pectoral fins for slow propulsion when undisturbed, but also able to swim fast by vigorous undulation of the trunk and caudal fin (Fuiman et al. 2002), suggests ram feeding for this species, associated with powerful bite capability. The finding of very low SI values in D. mawsoni, the lowest among all the fish species studied until now (Collar and Wainwright 2006), and the presence of rudimentary gill rakers on the arch confirm the previously described feeding strategies, and exclude any kind of filter feeding in this demersal fish.
Among the three species studied, the planktivore P. antarctica is the only one having both low MA ratio and a low SI index. Adaptation to planktophagy in this fish has already been demonstrated by skeletal analyses (reviewed in Voskoboinikova et al. 2017); however, no information is available on the species’ foraging strategy. Planktivorous fish might use two distinct feeding modes: particulate feeding and filter feeding (reviewed in Lazzaro 1987). The MA ratio in P. antarctica is significantly lower than those of the other two species, indicative of poor capability to produce force with the jaws. The reason for such biomechanic characteristics is that the silverfish’s elongated lower jaw makes the out-lever of its closing mechanism longer compared to that of the other two nototheniids. Besides resulting in poor bite capability, low MA ratio also provides ability to perform rapid movements with the lower jaw (Westneat 2006). This, associated with the occurrence in the silverfish of moderately protractile jaws (DeWitt and Hopkins 1977; Pinkerton 2017), supports particulate feeding as the prevalent planktivory foraging mode. In particular, the dentition of this species, with 1–3 enlarged teeth near the symphysis of upper jaw and 3–4 enlarged teeth about midway in length of lower jaw (DeWitt et al. 1990), allows P. antarctica to grab relatively large prey items, such as euphausiids. The morphology of P. antarctica’s gill rakers, numerous, long, and narrowly spaced, suggests the possibility for the species to rely on filter feeding as an alternative foraging mode. The role of gill rakers as entrapment structures to retain planktonic prey from a volume of engulfed water is common in planktivorous filter feeder fish (Lazzaro 1987; Gerking 1994). Although having a SI significantly higher than that of D. mawsoni, the poor capability of P. antarctica to create an area of low pressure that draws prey into the mouth makes suction feeding unlikely. Tow-net filter feeding is more probable, where prey is not detected a priori but a volume of water containing food items is rapidly engulfed by swimming acceleration coupled with fully agape mouth. Tow-net filter feeding mode by P. antarctica is consistent with the morphology of the mouth apparatus (specifically presence of numerous long and dense gill rakers), swimming mode, and with the observed diet of small planktonic organisms, such as copepods (Pinkerton 2017). Overall, although specialized for planktivory, P. antarctica has significant plasticity to switch from particulate to filter-feeding modes. The ability to change feeding mechanism is common throughout ontogeny, but it may also be a response to change in prey availability. Studies conducted on a number of planktivorous fishes, from various taxonomical orders including perciformes, demonstrate that particulate feeding is favored when prey are large or occur in low concentrations, whereas filter feeding prevails when prey are small and/or present at high concentration (Gibson and Ezzi 1992). The possibility of switching from one feeding mode to another might then be an evolutionary advantage that allows P. antarctica to maintain its energy intake under changing environmental and prey availability conditions.
The data presented here highlight morphological specialization of the feeding structures that are strikingly different in the three species, and that underlie the respective feeding modes. Feeding structure involves many morphological traits. However, although adaptive evolution has driven the three nototheniid species to a high degree of trophic specialization, a certain degree of feeding plasticity has been detected in at least two of the species, T. bernacchii and P. antarctica, that allow them to switch foraging mechanisms in response to changing environmental conditions and prey availability.
References
Amundsen PA, Bøhn T, Våga GH (2004) Gill raker morphology and feeding ecology of two sympatric morphs of European whitefish (Coregonus lavaretus). Ann Zool Fenn 41:291–300
Balushkin AV (2000) Morphology, classification, and evolution of notothenioid fishes of the Southern Ocean (Notothenioidei, Perciformes). J Ichthyol 40(Suppl 1):S74–S109
Bansode MA, Eastman JT, Aronson RB (2014) Feeding biomechanics of five demersal Antarctic fishes. Polar Biol 37(12):1835–1848. https://doi.org/10.1007/s00300-014-1565-z
Barnett A, Bellwood DR, Hoey AS (2006) Trophic ecomorphology of cardinalfish. Mar Ecol Prog Ser 322:249–257
Bottaro M, Vacchi M (2006) Rapporto sulla Campagna Antartica Estate-Australe 2005–2006. 21° Spedizione. P.N.R.A. Programma Nazionale di Ricerche in Antartide, Progetto 2004/8.4, Final Report, Roma
Carroll AM, Wainwright PC, Huskey SH, Collar DC, Turingan RG (2004) Morphology predicts suction feeding performance in centrarchid fishes. J Exp Biol 207(22):3873–3881
Cheng CH, Detrich HW III (2007) Molecular ecophysiology of Antarctic notothenioid fishes. Philos Trans R Soc Lond B Biol Sci 362(1488):2215–2232
Collar DC, Wainwright PC (2006) Discordance between morphological and mechanical diversity in the feeding mechanism of centrarchid fishes. Evolution 60(12):2575–2584
DeVries AL, Eastman JT (1978) Lipid sacs as a buoyancy adaptation in an Antarctic fish. Nature 271:352–353
DeWitt HH, Hopkins TL (1977) Aspects of the diet of the Antarctic silverfish, Pleuragramma antarcticum. In: Llano GA (ed) Adaptations within Antarctic ecosystems. Proceedings 3rd SCAR Symp Antarct Biol. Smithsonian Institution, Washington, pp 557–567
DeWitt HH, Heemstra PC, Gon O (1990) Nototheniidae. Fishes of the southern ocean. In: Gon O, Heemstra PC (eds) Fishes of the southern ocean. JLB Smith Institute of Ichthyology, Grahamstown, pp 279–331
Eastman JT (1993) Antarctic fish biology: evolution in a unique environment. Academic Press, San Diego
Eastman JT (2000) Antarctic notothenioid fishes as subjects for research in evolutionary biology. Antarct Sci 12(03):276–287
Eastman JT (2005) The nature of the diversity of Antarctic fishes. Polar Biol 28:93–107. https://doi.org/10.1007/s00300-004-0667-4
Eastman JT, Clarke A (1998) A comparison of adaptive radiations of Antarctic fish with those of nonAntarctic fish. In: di Prisco G, Pisano E, Clarke A (eds) Fishes of Antarctica: a biological overview. Springer, Milan, pp 3–26
Eastman JT, Witmer LM, Ridgely RC et al (2014) Divergence in skeletal mass and bone morphology in Antarctic notothenioid fishes. J Morphol 275(8):841–861
Fenaughty JM, Stevens DW, Hanchet SM (2003) Diet of the Antarctic toothfish (Dissostichus mawsoni) from the Ross Sea, Antarctic (Sub-area 88.1). CCAMLR Sci 10:113–123
Fuiman LA, Davis RW, Williams TM (2002) Behavior of midwater fishes under Antarctic ice: observations by a predator. Mar Biol 140:815–822
Gerking SD (1994) Feeding Ecology of Fish. Academic Press, San Diego
Gibson RN, Ezzi IA (1992) The relative profitability of particulate-and filter-feeding in the herring, Clupea harengus L. J Fish Biol 40(4):577–590
Hagen W, Kattner G (2017) The role of lipids in the life history of the Antarctic silverfish Pleuragramma antarctica In. In: Vacchi M, Pisano E, Ghigliotti L (eds) the Antarctic silverfish. A keystone species in a changing ecosystem. Springer, Cham
Hanchet S, Dunn A, Parker S, Horn P, Stevens D, Mormede S (2015) The Antarctic toothfish (Dissostichus mawsoni): biology, ecology, and life history in the Ross Sea region. Hydrobiologia 761(1):397–414
Klingenberg CP, Ekau W (1996) A combined morphometric and phylogenetic analysis of an ecomorphological trend: pelagization in Antarctic fishes (Perciformes: Nototheniidae). Biol J Linnean Soc 59(2):143–177
La Mesa M, Eastman JT (2012) Antarctic silverfish: life strategies of a key species in the high-Antarctic ecosystem. Fish Fish 13(3):241–266. https://doi.org/10.1111/j.1467-2979.2011.00427.x
La Mesa M, Eastman JT, Vacchi M (2004a) The role of notothenioid fish in the food web of the Ross Sea shelf waters: a review. Polar Biol 27(6):321–338. https://doi.org/10.1007/s00300-004-0599-z
La Mesa M, Dalú M, Vacchi M (2004b) Trophic ecology of the emerald notothen Trematomus bernacchii (pisces, nototheniidae) from Terra Nova Bay, Ross Sea, Antarctica. Polar Biol 27(11):721–728. https://doi.org/10.1007/s00300-004-0645-x
Lauder GV (1982) Patterns of evolution in the feeding mechanism of actinopterygian fishes. Am Zool 22(2):275–285
Lazzaro XA (1987) A review of planktivorous fishes: their evolution, feeding behaviours, selectivities, and impacts. Hydrobiologia 146:97. https://doi.org/10.1007/BF00008764
Lecointre G, Ameziane N, Boisselier M-C et al (2013) Is the species flock concept operational? The Antarctic shelf case. PLoS ONE 8:e68787. https://doi.org/10.1371/journal.pone.0068787
Liem KF (1980) Acquisition of energy by teleosts: adaptive mechanisms and evolutionary patterns. In: Ali MA (ed) Environmental physiology of fishes. Plenum Press, NewYork, pp 299–334
Lombarte A, Olaso I, Bozzano A (2003) Ecomorphological trends in the Artedidraconidae (Pisces: Perciformes: Notothenioidei) of the Weddell Sea. Antarct Sci 15(2):211–218
Mintenbeck K, Barrera-Oro ER, Brey T, Jacob U, Knust R, Mark FC, Moreira E, Strobel A, Arntz WE (2012) Impact of climate change on fishes in complex Antarctic ecosystems. Adv Ecol Res 46:351–426
Moreno CA (1980) Observations on food and reproduction in Trematomus bernacchii (Pisces: Nototheniidae) from the Palmer Archipelago, Antarctica. Copeia 1:171–173
Near TJ, Dornburg A, Kuhn KL et al (2012) Ancient climate change, antifreeze, and the evolutionary diversification of Antarctic fishes. Proc Nat Acad Sci USA 109:3434–3439
Norton SF, Brainerd EL (1993) Convergence in the feeding mechanics of ecomorphologically similar species in the Centrarchidae and Cichlidae. J Exp Biol 176(1):11–29
O’Driscoll R, Double M (2015) Voyage Report TAN1502: New Zealand-Australia Antarctic Ecosystems Voyage. NIWA Client Report WLG2015-21
Petrov AF, Tatarnikov VA (2011) Results of investigation of the diet of Antarctic toothfish Dissostichus mawsoni (Nototheniidae) in the Lazarev Sea. J Ichthyol 51:131–135
Pinkerton MH (2017) Diet and trophic ecology of adult Antarctic silverfish (Pleuragramma antarctica). In: Vacchi M, Pisano E, Ghigliotti L (eds) The Antarctic silverfish. A keystone species in a changing ecosystem. Springer, Cham
R Development Core Team (2015) R: a language and environment for statistical computing. R Foundation for statistical computing. Vienna, Austria. www.R-project.org
Roberts J, Xavier JC, Agnew DJ (2011) The diet of toothfish species Dissostichus eleginoides and Dissostichus mawsoni with overlapping distributions. J Fish Biol 79:138–154
Rutschmann S, Matschiner M, Damerau M, Muschick M, Lehmann MF, Hanel R, Salzburger W (2011) Parallel ecological diversification in Antarctic notothenioid fishes as evidence for adaptive radiation. Mol Ecol 20(22):4707–4721. https://doi.org/10.1111/j.1365-294X.2011.05279.x
Sonnefeld MJ, Turingan RG, Sloan TJ (2014) Functional morphological drivers of feeding mode in marine teleost fishes. Adv Zool Bot 2:6–14. https://doi.org/10.13189/azb.2014.020102
Stevens DW, Dunn MR, Pinkerton MH, Forman JS (2014) Diet of Antarctic toothfish (Dissostichus mawsoni) from the continental slope and oceanic features of the Ross Sea region, Antarctica. Antarct Sci 26(05):502–512
Stevens DW, Di Blasi, Parker S (2016) Results of first winter longline survey to the northern Ross Sea region to investigate toothfish reproductive life history. Working Document WG-FSA 16/37 CCAMLR, Hobart, Australia
Tanaka H, Aoki I, Ohshimo S (2006) Feeding habits and gill raker morphology of three planktivorous pelagic fish species off the coast of northern and western Kyushu in summer. J Fish Biol 68(4):1041–1061
Vacchi M, Pisano E, La Mesa G (1999) Cold-Adapted Organisms. Ecological features of antarctic fishes. Springer, Berlin, pp 219–238
Vacchi M, Cattaneo-Vietti R, Chiantore M, Dalù M (2000) Predator–prey relationship between the nototheniid fish Trematomus bernacchii and the Antarctic scallop Adamussium colbecki at Terra Nova Bay (Ross Sea). Antarct Sci 12(01):64–68
Voskoboinikova OS, Tereshchuk O, Kellermann A (1994) Osteological development of the antarctic silverfish (Pleuragramma antarcticum (Nototheniidae). Cybium 18(3):251–271
Voskoboinikova O, Detrich HW III, Albertson C et al (2017) Evolution reshaped life for the water column: the skeleton of the Antarctic silverfish Pleuragramma antarctica Boulenger, 1902. In: Vacchi M, Pisano E, Ghigliotti L (eds) the Antarctic silverfish. A keystone species in a changing ecosystem. Springer, Cham
Voskoboynikova OS (1993) Evolution of the visceral skeleton and phylogeny of Nototheniidae. J Ichthyol 33(7):23–47
Voskoboynikova OS (1994) Rates of individual development of the bony skeleton of eleven species of the family Nototheniidae. J Ichthyol 34(8):108–120
Wainwright PC, Bellwood DR (2002) Ecomorphology of feeding in coral reef fishes. In: Sale PF (ed) Coral reef fishes: dynamics and diversity in a complex ecosystem. Academic Press, San Diego, pp 33–55
Wainwright PC, Richard BA (1995) Predicting patterns of prey use from morphology of fishes. Environ Biol Fish 44:97–113
Westneat MW (2004) Evolution of levers and linkages in the feeding mechanisms of fishes. Integr Comp Biol 44:378–389
Westneat MW (2006) Skull biomechanics and suction feeding in fishes. In: Shadwick RE, Lauder GV (eds) Fish biomechanics. Elsevier Academic Press, San Diego, pp 29–75
Acknowledgements
The study was supported by the Italian National Programme for Antarctic Research (PNRA) projects 2013/AZ1.18 (RAISE) and 2015/B1.02 (DISMAS), and contributes to the SCAR Scientific Research Program AnT-ERA (Antarctic Thresholds - Ecosystem Resilience and Adaptation). The New Zealand-Australian Antarctic Ecosystems voyage on Tangaroa was jointly funded by Antarctica New Zealand, Australian Antarctic Division, NIWA, and the New Zealand Ministry for Business, Innovation and Employment. The voyage to collect D. mawsoni in the northern Ross Sea was funded by the New Zealand Ministry for Primary Industries under contract ANT201501.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The samplings of P. antarctica and D. mawsoni were carried out in accordance with permit AMLR14/04/Tangaroa/ZMFR and permit AMLR/15/01/Janas/ZMTW issued by the New Zealand government under the Antarctic Marine Living Resources (AMLR) Act 1981. The sampling of T. bernacchii was conducted in compliance with the ‘‘Protocol on Environmental Protection to the Antarctic Treaty’’, Annex II, Art. 3, to provide specimens for scientific activity, referring to the PNRA Research Project.
Rights and permissions
About this article
Cite this article
Carlig, E., Di Blasi, D., Ghigliotti, L. et al. Diversification of feeding structures in three adult Antarctic nototheniid fish. Polar Biol 41, 1707–1715 (2018). https://doi.org/10.1007/s00300-018-2310-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-018-2310-9