Abstract
Part of the Larsen A Ice Shelf (64°15′S to 74°15′S) collapsed during January 1995. A first oceanographic and biological data set from the newly free waters was obtained during December 1996. Typical shelf waters with temperatures near and below the freezing point were found. A nutrient-rich water mass (max: PO4 3− 1.80 μmol L−1 and NO3 − 27.64 μmol L−1) was found between 70 and 200 m depth. Chlorophyll-a (Chl-a) values (max 14.24 μg L−1) were high; surface oxygen saturation ranged between 86 and 148%. Diatoms of the genera Nitzschia and Navicula and the prymnesiophyte Phaeocystis sp. were the most abundant taxa found. Mean daily primary production (Pc) estimated from nutrient consumption was 14.80 ± 0.17 mgC m−3 day−1. Pc was significantly correlated with total diatom abundance and Chl-a. Calculated ΔpCO2 (difference of the CO2 partial pressure between surface seawater and the atmosphere) was –30.5 μatm, which could have contributed to a net CO2 flux from the atmosphere to the sea and suggests the area has been a CO2 sink during the studied period. High phytoplankton biomass and production values were found in this freshly open area, suggesting its importance for biological CO2 pumping.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The steady rise of atmospheric greenhouse gases such as CO2 has very much stimulated investigation during the last decades, because of their effects on climate (Takahashi et al. 2002; Hoppema 2004). Climate warming has been evidenced in the Southern Ocean (SO), especially along the western Antarctic Peninsula (Meredith and King 2005; Turner et al. 2005). Ice shelves and glaciers are sensitive to global warming and their melting is of concern because of the potential increase in sea level it might produce (Rignot et al. 2004) but also because of their importance in trapping CO2 below the ice surface as noted by Stephens and Keeling (2000) for the Last Glacial Maximum. The SO plays a key role in the regulation of climate through its influence on gas exchange processes between atmosphere and ocean. Particularly, in the Weddell Sea, due to its physical characteristics, sinking of CO2 might be favored (Hoppema 2004). The Weddell Sea is the main source of the Antarctic Bottom Water (AABW). This and other dense water masses such as those in the North Atlantic contribute to thermohaline ocean circulation (Orsi et al. 1993). Ventilation of the deep ocean depends on it. The interaction between coastal currents, ice shelves, sea ice and shelf waters controls freshwater fluxes in the Weddell Sea.
Marginal Ice Zones (MIZ) in the Weddell Sea have been long considered as high primary production areas (El-Sayed and Taguchi 1981; Smith and Nelson 1986; Fryxell and Kendrick 1988; Sullivan et al. 1988) and are limited to coastal and ice-edge zones (Rubin et al. 1998). Polar planktonic systems are conditioned, among others, by the variability in ice extension, macro- and micronutrients concentrations, hydrodynamics and the light regime (Smith and Nelson 1986). Several investigations have shown elevated phytoplankton standing stocks in the vicinity of the ice edge due to enhanced vertical stratification (Garrison and Buck 1989; Castro et al. 2002; Krell et al. 2005). MIZ have been recognized as sites of intense phytoplankton blooms formation and primary production (Lizzote 2001).
Atmospheric temperature increase has important consequences for the Antarctic ecosystems (de la Mare 1997; Domack et al. 2005; among others). Small temperature differences can have large effects on the extension and the thickness of sea ice, on the stability of the ice shelves, and on the decline in the sea-ice extent, which in turn have ecological consequences. Among them, changes in phytoplankton community diversity (Moline et al. 2004), the decline in the abundance of some key species such as the Antarctic krill (Euphausia superba) (Smetacek and Nicol 2005; Nicol 2006) and the increase in salps (Pakhomov et al. 2002; Atkinson et al. 2004) have been observed. CO2 exchange between the ocean and the atmosphere is partly influenced by the biological activity. Planktonic organisms remove CO2 and nutrients, which in turn drives CO2 transfer from the air to deeper layers of the ocean. This is commonly called the biological pump (Hoppema et al. 1999; Sarmiento and Gruber 2006).
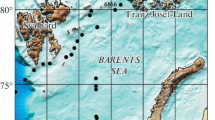
The Larsen Ice Shelf (LIS) was the largest floating ice body on the Antarctic Peninsula (Fig. 1a), and it extended from 64°15′S to 74°15′S (Skvarca 1994). Waters just beyond ice shelves might be regarded as MIZ. They act as an extension of continental glaciers (200–1,000 m thick extension) and are sensitive to climate (van den Broeke 2005). In the past 30 years, ice shelves from the Antarctic Peninsula have decreased in area by approximately 13,500 km2 (Vaughan and Doake 1996). A series of fractures, disintegrations and retreat events have been occurring in parts of the ice shelf north of Robertson Island. A 2°C warming of the lower Antarctic Peninsula atmosphere since 1979 has forced the limit for ice shelves southward (van den Broeke 2005). During austral summer 1995 big areas of the Larsen A Ice Shelf (LIS A) section (1,600 km2) have collapsed (Skvarca et al. 1999; Scambos et al. 2004). As a consequence, a new large ice-free area of the ocean was exposed to both incident irradiance and gas exchanges with the atmosphere. Under adequate light and mixing conditions this new coastal zone could harbor intense biological production. The biogeochemical processes that take place in the ocean could make the area an additional important sink of anthropogenic CO2. Here, we analyze historical information obtained from a series of oceanographic sampling stations covering around 1,400 km2 in the vicinity of the area previously occupied by a section of the LIS A in the northwestern Weddell Sea. Our measurements are the first source of oceanographic and biological information on the water column for this area. We aim to determine phytoplankton primary production in the area, and discuss its importance in pCO2 dynamics.
a Map of the Antarctic Peninsula showing the study area, Jubany and Matienzo Stations, and the ice shelf edges: the dashed black line corresponds to the limit of the LIS during the 1960s; the dash-dotted black line corresponds to the LIS A edge after the 1995 collapse; full black line corresponds to LIS edges at the moment of sampling. b The study area, showing the position of the stations: full line represents Transect N; dotted line represents Transect S
Materials and methods
Oceanographic sampling
Fourteen stations were carried out on board the Argentinean icebreaker ARA “Almirante Irizar” between 10 and 14 December 1996 close to the Argentinean Station Matienzo, in the vicinity of the LIS (Fig. 1). Samples and water column data were taken along two northeast–southwest transects (Fig. 1b) using a CTD profiler (Neil Brown MKIII b) with an attached rosette equipped with 5-L Niskin bottles. The southern transect (S) with a mean depth of 306 m, harbored the stations 1, 2, 3, 4, while the northern transect (N), with a mean depth of 395 m, included the stations: 6, 7, 8, 9, 12, 13 and 14. Both transects were located entirely over the continental shelf and registered a maximum depth of 487 m. Water samples were collected at 5, 15, 20 and 25 m depth. In addition, extra samples from maximum and intermediate depths were taken at some stations (Tosonotto et al. 2000). These samples were used for chemical and biological analyses and for CTD data calibration. Temperature was calibrated using a Richter-Wiesse reversal thermometer and salinity was measured with a Beckman RS9 inductive salinometer. pH was measured with a Beckman Model GS pH-meter to a 0.01 precision using a KH2PO4 standard buffer. Dissolved oxygen concentrations were determined on duplicate 125 mL samples following the Winkler method using a Metrohm Model 665 Dosimat titrator. The average coefficient of variation for replicate samples was 0.5. Chlorophyll a (Chl-a) analyses were performed on board by filtering 1 L seawater through 45 mm Whatman GF/F filters. Pigments were extracted in 90% acetone and measured with a Shimadzu visible UV spectrophotometer. Chl-a concentration was calculated following Strickland and Parsons (1972) and corrected for phaeopigments with a final precision of 0.05 μg L−1. Filtered sea water samples (60 mL) were used to determine nitrate and phosphate with a Technicon II Autoanalyzer, following Strickland and Parsons (1972). Samples for nutrient estimations have been stored at –20°C before analysis, which was done no longer than 2 months after the cruise. Alkalinity was calculated according to the formulation by Lee et al. (2006) for SO waters, using salinity and temperature data. Results were further contrasted with other alkalinity estimations, obtained from the relation between alkalinity and salinity described by Anderson et al. (1991). Total CO2 (TCO2) and seawater pCO2 were then calculated using the CO2SyS program (Lewis and Wallace 1998). There was a good agreement between alkalinity values calculated with both equations. However, TCO2 values obtained when using alkalinity data after Anderson et al. (1991) were somewhat higher than those reported for Weddell Sea waters, which were obtained with the equation of Lee et al. (2006). Therefore, this was the one we finally used. Atmospheric pCO2 concentration was measured during the study period at Jubany station (62°14′S and 58°40′W; 312.42 km from sampled area). The difference between seawater and atmospheric pCO2 (ΔpCO2) was then calculated. Even if atmospheric pCO2 was obtained at some distance of the sampling area, the seasonal and geographical variation of surface-water pCO2 is much greater than that of atmospheric pCO2, so that ΔpCO2 is mainly regulated by the oceanic pCO2 (Takahashi et al. 2002).
Data analyses
Dissolved oxygen saturation (%O2) was calculated following Murray and Riley (1969) using salinity and temperature data obtained from the CTD measurements. Primary production based on nitrate depletion was estimated following Hoppema et al. (2002). This method estimates the net nutrient consumption as the difference in nutrient concentration between the surface layer (0–30 m) and the remnant winter layer below it, as a representation of the net nutrient uptake until the moment of sampling. In this study, winter nutrient concentrations were estimated considering the maximum nitrate value recorded at minimum temperature depth (as in Hoppema et al. 2007) for all the studied stations. Surface nutrient concentrations were calculated as the average between 0 and 30 m. In order to avoid the effect of dilution due to freshwater additions from melting waters, nutrient concentrations were normalized to a constant value of salinity of 34.5 according to c norm = c · (34.5/S) (Hoppema et al. 2007). Carbon consumption was then estimated using Redfield et al. (1963) ratio (molar C:N ratio = 6.6 mol/mol), which has been shown to be adequate for the surface waters of the western Weddell Sea (Hoppema and Goeyens 1999). These production values (Pc) correspond to the time integrated change from the winter until the moment of sampling (Hoppema et al. 2007). Our Pc values were estimated for a period of 70 days, from the start of the spring bloom reported for the Weddell Sea (Bianchi et al. 1992) to the moment of sampling. In addition, phytoplankton bloom production was integrated over the surface layer of the water column (PcI).
Phytoplankton quantification
Two hundred and fifty milliliter samples were taken from the surface layer at the same depth as those for chemical analyses, fixed with acidic Lugol and kept in the dark at 4°C. Estimates of phytoplankton cell numbers were done following Utermöhl (1958) using an inverted microscope (Iroscope SI-PH) equipped with phase contrast. Phytoplankton groups were divided into two different categories: phytoflagellates and diatoms. Both were identified to the genus level. A phytoplankton taxon was considered dominant when cell numbers was >50% of total cells present in the sample.
Linear regressions and correlations were computed by means of the STATISTICA (Statsoft) program following Sokal and Rohlf (1995). Mean values are presented ± standard error unless otherwise indicated.
Results and discussion
Physical characterization of the water column
Waters in the upper 50 m at all stations corresponded to Antarctic Surface Waters according to the description of Weddell Sea waters by Hoffmann and Klinck (1998), showing a range of temperature between −1.70 and 0.83°C and salinity from 34.05 to 34.44 psu. The lowest temperatures and salinities were measured at stations 1, 2, 13 and 14, which are the farthermost from the coast. Positive temperatures were found in the surface waters at stations 7, 8, 9 and 12. Temperatures near or below the freezing point were observed between 150 and 300 m, close to the bottom, at stations 3, 6 and 7. This could show the influence of waters flowing from below the ice platform (Rack et al. 1999). Below these depths at stations 1, 2, 9, 12 and 13, temperature was around −1.87°C.
Stabilization of the upper water column mixed layer generated by the retreat of the winter pack ice plays a role in controlling the primary production of the ice edge blooms (Rubin et al. 1998). A marked vertical stratification in salinity and temperature was evident in surface waters (<50 m) at all stations on transect S (Fig. 2a, b). Stratification was enhanced close to the coast. On transect N, this pattern was less evident than on transect S (Fig. 2c), where waters presented a continuous vertical stratification up to 100 m depth. Northern stations (stations 12, 13 and 14) showed a stratification pattern similar to transect S (Fig. 2d).
Nutrients and Chl-a distributions
Nitrate (NO3 −) and phosphate (PO4 3−) concentrations in the surface layer showed similar patterns (Figs. 3a, b, 4a, b). Vertical distribution showed lower values in surface waters than at greater depths, ranging from 0.29 to 1.98 μmol L−1 PO4 3− and 0.68 to 27.64 μmol L−1 NO3 −. Minimum concentrations were measured at 5 m depth at station 3 (Transect S), while the maximum value was registered at 15 m depth at station 14 (Transect N). Between 70 and 200 m, high concentrations were generally found (NO3 − 24 μmol L−1, PO4 3− 1.8 μmol L−1), which were within the range described by other authors during 1992 austral winter and 1998 austral autumn (e.g., Hoppema et al. 2002). Chl-a concentration throughout the study area ranged from 1.09 to 14.24 μg L−1 (Table 1) with an average of 7.46 ± 0.05 μg L−1. Transect S showed a Chl-a maximum of 13.33 μg L−1 at station 3 between 10 and 15 m depth (Fig. 3c). Nutrients could have been consumed in surface waters, allowing a high biomass build-up. This biomass is then found at deeper depths, retained above the pycnocline (around 19 m; Fig. 2a). On transect N, high Chl-a values were measured at stations 9 and 13 (14.24 and 14.07 μmol L−1, respectively; Fig. 4c), coinciding with relatively low nitrate and phosphate concentrations (0–6 and 0–0.7 μmol L−1, respectively). On station 12, low values of Chl-a (<7 μg L−1) as well as low nutrient concentrations were found from 0 to 15 m depth. These low values (Fig. 4) could be related to freshwater dilution (Fig. 2d). These results are typical for phytoplankton bloom situations in the Weddell Sea (e.g., Semeneh et al. 1998; El-Sayed and Taguchi 1981), as well as in other MIZs of both northern and southern polar oceans (e.g., Smith and Nelson 1986; Nelson et al. 1987; Smetacek and Nicol 2005). Although the Redfield ratio of 16:1 is not universal, it represents a variety of phytoplankton growth strategies for diverse oceanic conditions (Arrigo 2005). In our study the average N:P ratio was 11.48, which is similar to the 11.7 value found by Weiss et al. (1979) for the Weddell Sea in the late summer. The low N:P ratio found seems to point to a diatom-dominated phytoplankton community that could deplete NO3 − relative to PO4 3− reducing the N:P ratio for the surface waters (a “bloomer” community, Arrigo 2005). The N:P ratio found is indicative of exponential phytoplankton growth, and appears to be in agreement with previous phytoplankton studies in the Weddell Sea (Krell et al. 2005; Hoppema et al. 2007).
Vertical and longitudinal distribution of a NO3 − (μmol L−1), b PO4 3− (μmol L−1), c Chl-a (μg L−1) on transect S. Arrows in the upper line show the position of the stations and black dots on the figure indicate the depth of discrete sampling. The stripped surface in the middle of the figure indicates that no data are available for that area
Vertical and longitudinal distribution of a NO3 − (μmol L−1), b PO4 3− (μmol L−1), c and Chl-a (μg L−1) on transect N. Arrows in the upper line show the position of the stations and black dots on the figure indicate the depth of discrete sampling. The stripped surface in the middle of the figure indicates that no data are available for that area
Percentages of oxygen saturation at the surface layer evidenced mostly super-saturation (with regard to theoretical values according to water salinity and temperature), with an average of 111% O2, and ranging between 86 and 148% (Table 1). Undersaturated waters were mainly found in stations 4 and 14. This will be discussed below. In general, maximum and mean %O2 results suggest an intense biological activity at the moment of sampling.
TCO2 and ∆pCO2 estimations
TCO2 concentrations showed a vertical increase toward the bottom at all stations. Changes in the surface layer are primarily caused by biological uptake, but also due to physical processes such as advection and vertical mixing. Mean TCO2 was 2,212.6 ± 1.5 μmol L−1. Bouquegneau et al. (1992) have found similar values (2,360–2,440 μmol L−1) in the surrounding areas of the Larsen marginal ice shelf (between 61° and 63°S) during 1988 spring. More recently, Hoppema (2004) has observed TCO2 values of 2,270.6 ± 0.9 μmol L−1 for the surface layer (≤100–150 m) of the western Weddell Sea.
pCO2 mean values calculated for the surface layer were lower than those measured in the atmosphere at the time of this study. The average to the upper 5 m depth of sea-water pCO2 for all stations across the study area was 327.75 ± 15.6 μatm, ranging between 147.5 and 636.5 μatm, while the atmospheric mean value was 358.25 μatm. The flux of CO2 between the ocean and the atmosphere strongly depends on the difference between seawater and atmospheric pCO2 (Hoppema 2004; Schloss et al. 2007). The difference in pCO2 values between the sea and atmosphere (∆pCO2), −30.5 μatm in this case would contribute to a net sequestration of CO2 from the atmosphere to the ocean. The seasonal amplitude of surface water pCO2 at a given location is regulated by the biological utilization of CO2 and temperature (Takahashi et al. 2002). A pCO2 undersaturation accompanying a %O2 supersaturation has been related to an active photosynthetic activity (Schloss et al. 2007). Therefore, photosynthesis could be responsible for the ∆pCO2 registered as it was shown for other areas (Takahashi et al. 2002; Hoppema 2004; Schloss et al. 2007). The existing negative ∆pCO2 is consistent with the pattern of TCO2 of the surface layer of the Weddell Sea (Hoppema et al. 1999). Whereas nitrate and phosphate are consumed by phytoplankton activity, CO2 is not only utilized, but also released by phytoplankton as well as by heterotrophs. In this sense, it would be necessary to know the heterotrophic production of CO2 in order to better delimit phytoplankton production.
Estimation of primary production (Pc and PcI)
Based on nutrient depletion and to a conversion of nutrients into carbon, the highest Pc values (>16 mgC m−3 day−1) were measured at stations 3, 7, 8, 9 and 12 (Table 2). The mean for all the study area was 14.80 ± 0.17 mgC m−3 day−1, ranging from 6.35 to 19.76 mgC m−3 day−1 (stations 14 and 8, respectively). Transect N showed a somewhat higher mean Pc value than transect S (15.13 ± 0.32 and 14.40 ± 0.37 mgC m−3 day−1, respectively). It is worth noticing that stations on transect N also presented the highest values of dissolved oxygen (>8 ml L−1) and Chl-a (>9 μg L−1).
The mean integrated primary production (PcI) was 426.54 ± 6.63 mgC m−2 day−1, ranging between 172.63 and 588.09 mgC m−2 day−1 (maximum and minimum corresponding to stations 14 and 12, respectively). This might be an underestimation, since the euphotic depth is usually >30 m (67 m average, Smith and Nelson 1990) in the MIZ of the Weddell Sea. During austral summer 1992–1993 Hoppema et al. (2000) found values higher than these offshore the LIS, ranging from 570 to 1,140 mgC m−2 day−1, while in the central Weddell Sea, primary production was estimated around to 100 mgC m−2 day−1 in the same work. Using satellite-based Chl-a estimations from 37 series of coastal polynya systems in the SO for the 1997–2002 period, Arrigo and van Dijken (2003) calculated an annual primary production at the LIS zone of 38.2 ± 53.6 gC m−2 and a daily production during the bloom of 0.18 ± 0.44 gC m−2 day−1. Smith and Nelson (1990) have estimated the new production in the western Weddell Sea to be 490 mgC m−2 day−1, which is in agreement with the present results. Other studies near the study area have shown values ranging between 230 and 410 mgC m−2 day−1 (El-Sayed and Taguchi 1981; Jennings et al. 1984). Our estimated primary production is also consistent with these estimations and with the notion that an important phytoplankton bloom with characteristics similar to those described in previous studies (El-Sayed and Taguchi 1981; Hoppema et al. 2000) developed in the area. This suggests that the ecosystem of the former LIS, under its new conditions, would be a biologically productive system. Furthermore, the decrease in surface layer TCO2 concentration was well correlated with Pc as evidenced by their significant negative linear relation (R 2 = 0.35; P < 0.05; Fig. 5).
Although Pc was elevated, we found some excess nutrients in the area, indicating that some more potential uptake could still occur. This might be interpreted as a low efficiency in the biological pump (Sarmiento and Gruber 2006) but, in the present case, it is probably indicating that some more potential uptake could still occur, especially if we consider that during the 1997–2002 period, the production in the area peaked in January (Arrigo and van Dijken 2003).
Based on our data, the yearly production of the area (1,400 km2) was estimated to be 0.07 TgC year−1, considering 120 days as an approximate duration of phytoplankton growth season at the latitude of our study. This value represents only 0.015% of the estimated mean production of the entire Weddell Sea (477 TgC year−1; Arrigo et al. 2008). This percentage, in turn, is even smaller than the spatial share of the study area (0.05%), suggesting that the annual production in the investigated LIS A region, although being in the range of those reported from other similar regions in the Weddell Sea (Hoppema et al. 2007), contributes less to total Weddell Sea production than other zones, such as for instance the MIZ. All these numbers, however, are only gross estimates and should thus be interpreted with due caution.
In addition, Pc calculations might be underestimated because of organic matter degradation as well as other heterotrophic processes by planktonic consumers and decomposers. However, information is not available for determining the influence of zooplankton, nor grazing pressure can be estimated. Bacterial metabolism was not quantified, so that our results represent the balance between photosynthesis and respiration at the community level. Although sampling was done during early summer, Pc calculations might be additionally underestimated if a source of nitrogen other than nitrate (e.g., ammonia) had been used for primary producer’s biomass build-up. The ammonium consumption is an important metabolism process at the end of the growth season (Krell et al. 2005), but as we did not measure it, we could not estimate its importance in terms of Pc estimations.
A significant linear relationship between Pc and %O2 was found (Fig. 6) (R 2 = 0.82; P < 0.05). This increase in Pc accompanied by a rise in %O2 seawater saturation values suggest that the depletion of CO2 in surface waters can be attributed to biological activity. The nutrient depletion-based Pc estimations were calculated on the phytoplankton assemblages as a whole. As mentioned by several authors (Arrigo et al. 1999; DiTullio et al. 2000), different phytoplankton groups have different carbon uptake rates; a changing community composition could therefore bias our estimations.
Phytoplankton composition
In terms of N:P disappearance ratios, Arrigo et al. (1999) found that disappearance ratios of NO3:PO4 were significantly different for samples dominated by either Phaeocystis antarctica or diatoms. The ratio for P. antarctica (19.2 ± 0.61) was nearly twice as high as that for diatoms (9.69 ± 0.33). The maximum standing crop attainable by these two phytoplankton taxa would be determined by the availability of different nutrients (NO3 in the case of P. antarctica and PO4 for diatoms). They explained that the variation reflected taxonomic differences in nutrient drawdown and not chemical differences in the water masses.
Phaeocystis sp. dominated phytoplankton composition (in terms of cell numbers) over the whole study area, although its concentrations were higher on Transect S (mean value around 18.26 × 106 cells L−1) than on Transect N (around 7.26 × 106 cells L−1, without considering station 6, which was similar in phytoplankton composition to stations of Transect S). On transect N diatoms reached high cell abundances (average: 9.26 × 105 and 4.43 × 105 cells L−1 for transects N and S, respectively; Table 3). This is consistent with the previously discussed nutrient limitation: a relatively lower N:P ratio was determined for transect S (N:P = 11.8) than for transect N (15.2), suggesting that both groups were intensely consuming the limiting nutrient. Stations with high Chl-a and Pc estimations on transect N, were clearly dominated by diatom species of the genera Nizstchia spp. and Navicula spp. Although as mentioned above the relative importance of diatoms and autotrophic flagellates varied between the sampled transects (Fig. 7), the low N:P ratio calculated point to a diatom-dominated uptake of nutrient (Hoppema et al. 2007). Both diatoms and the prymnesiophyte Phaeocystis sp. form major phytoplankton blooms during the summer season in many regions of Antarctica (Buck and Garrison 1983; Fryxell and Kendrick 1988; Arrigo et al. 1999; DiTullio et al. 2000). Phytoplankton assemblages dominated by diatoms are usually present in nutrient-rich areas. Previous studies at coastal zones close to the the LIS in the southwestern Weddell Sea were characterized by a pennate diatom-dominated phytoplankton community (Semeneh et al. 1998). Moreover, although Pc estimations were similar along both transects, we noted a strong relationship between diatom abundances and Pc (Fig. 8; R2 = 0.56; P < 0.05). Significant linear correlations between TCO2 and Pc and between TCO2 and %O2 were only evident for stations and depths where diatoms were relatively more abundant. This relation was not evident when diatoms were less abundant and mostly Phaeocystis sp. cells were present (R2 = 0.14; P = 0.07).
The relation of diatom-dominated assemblages with seawater pCO2 had been established for other areas. In the southwestern Atlantic Ocean, Schloss et al. (2007) have shown that seawater pCO2 was correlated with primary production when the phytoplankton community was dominated by diatoms. The dominance of diatoms during the high production events favors the establishment of an herbivorous food web (as in Legendre and Rassoulzadegan 1996), which could lead the area to acts as a CO2 sink (Schloss et al. 2007). Although in the present study no significant relation was found between Phaeocystis sp. and pCO2, Phaeocystis sp. dominated communities have been identified as active CO2 consumers. However, both diatom and Phaeocystis sp. dominated phytoplankton communities have been identified as active CO2 consumers. DiTullio et al. (2000) have reported that blooms of colonial P. antarctica in seasonal ice zones are rapidly exported during early bloom conditions, representing an important link in the carbon cycles. The net CO2 changes in the surface layer are caused mainly by the exported production during and after these blooms (Hoppema 2004).
A TCO2 decrease in surface waters during the present study was also negatively correlated (P < 0.05) with diatom abundances (Fig. 9) although correlation was low (R 2 = 0.35). This could be probably due to the generalization that is implicit in the “diatom” group: morphological and metabolic differences within the diverse species that are part of these diatom-dominated groups could be responsible for this low correlation.
Southern Ocean waters actively exchange heat and dissolved gases (CO2 and O2) with the atmosphere. An improved understanding of the biological and non-biological processes regulating these exchanges is important for predicting the future trend of climate change (Rubin et al. 1998). A barrier collapse event represents a unique opportunity to examine deep environments of a sub-ice shelf setting out. The continental shelf of the Weddell Sea harbors a complex and great benthic community mainly dominated by benthic suspension feeder distribution (Gutt and Starmans 2003) which deserve special attention because of the unique characteristics and the long recovery times. The disappearance of ice shelves changes the characteristics of coastal zones previously regarded as MIZs and biological production in those areas concomitantly. The question remains of whether other ice shelf collapses will result in similar changes in phytoplankton production, richness and abundance, and on their role in the ocean carbon cycle.
Summary and conclusions
We present some historical information from an area previously covered by the LIS (i.e., the first hydrographical data set after the LIS collapse). Data presented in this paper show the importance of high latitude oceans as areas of intense primary production and hence as potential sink for atmospheric CO2.
-
1.
The high water column stratification due to seasonal ice melting favors phytoplankton development in the area. High Chl-a concentrations (max 14.24 μg L−1) and Pc (max 13.83 ± 5.06 mgC m−3 day−1) calculated from nitrate consumption, and mainly supersaturated %O2 values were evident, in agreement with the phytoplankton bloom observed during the cruise. These observations lead us to consider new ice free zones as areas of high primary production and, therefore, potential carbon export zones.
-
2.
Community composition was dominated by species of the Nitszchia spp., Phaeocystis sp. and Navicula spp. genera. Significant correlations between diatoms-biomass and diatoms-Pc signal this phytoplankton group as a key element in the carbon intake processes. Although the phytoplankton assemblage is dominated by siliceous species, given its high abundances, Phaeocystis sp. undoubtedly has an important role in the biological pump despite there was no significant correlation with Pc during the present study.
-
3.
Seawater CO2 uptake from the atmosphere occurs during summer because the surface layer CO2 undersaturation, primarily due to biological utilization (Hoppema et al. 2007). During the studied period, atmospheric pCO2 was higher than that estimated for the surface water. Flux between air and sea is proportional to the ∆pCO2; the studied area could therefore acts as a sink of CO2, as estimated by Hoppema et al. (1999).
Polar regions are sensitive to climate change. Ice shelves collapses increase free water areas that could host, as shown in the present work, an elevated primary production. In turn, according to the dominant phytoplankton group, this can be related to CO2 dynamics. Understanding the relations between primary production and the different phytoplankton groups might help to give insight on their role in CO2 dynamics in the ocean.
References
Arrigo KR (2005) Marine microorganisms and global nutrient cycles. Nature 437:349–355. doi:10.1038/nature04159
Arrigo KR, van Dijken GL (2003) Phytoplankton dynamics within 37 Antarctic coastal polynya systems. J Geophys Res 108:L3271. doi:10.1029/2002JC001739
Arrigo KR, Robinson DH, Worthen DL, Dunbar RB, DiTullio GR, VanWoert M, Lizotte MP (1999) Phytoplankton community structure and the drawdown of nutrients and CO2 in the Southern Ocean. Science 283:365–367. doi:10.1126/science.283.5400.365
Arrigo KR, van Dijken GL, Bushinsky S (2008) Primary production in the Southern Ocean, 1997–2006. J Geophys Res 113:C08004. doi:10.1029/2007JC004551
Atkinson A, Siegel V, Pakhomov E, Rothery P (2004) Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432:100–103. doi:10.1038/nature02996
Bianchi F, Boldrin A, Cioce F, Dieckmann G, Kuosa H, Larsson AM, Nöthig EM, Sehlstedt PI, Socal G, Syvertsen EE (1992) Phytoplankton distribution in relation to sea ice, hydrography and nutrients in the northwestern Weddell Sea in early spring 1988 during EPOS. Polar Biol 12:225–235. doi:10.1007/BF00238264
Bouquegneau JM, Gieskes WWC, Kraay GW, Larsson AM (1992) Influence of physical and biological processes on the concentration of O2 and CO2 in the ice-covered Weddell Sea in the spring of 1988. Polar Biol 12:163–170. doi:10.1007/BF00238256
Buck KR, Garrison DL (1983) Protists from the ice-edge region of the Weddell Sea. Deep Sea Res 30:1261–1277. doi:10.1016/0198-0149(83)90084-5
Castro CG, Ríoz AF, Doval MD, Perez FF (2002) Nutrient utilization and chlorophyll distribution in the Atlanctic sector of the Southern ocean during austral summer 1995–96. Deep Sea Res Part II Top Stud Oceanogr 49:623–641. doi:10.1016/S0967-0645(01)00115-1
de la Mare WK (1997) Abrupt mid-twentieth-century decline in Antarctic sea-ice extent from whaling records. Nature 389:57–60. doi:10.1038/37956
DiTullio GR, Grebmeier JM, Arrigo KR, Lizotte MP, Robinson DH, Leventer A, Barry JP, VanWoert ML, Dunbar RB (2000) Rapid and early export of Phaeocystis antartica blooms in the Rose Sea, Antarctica. Nature 404:595–598. doi:10.1038/35007061
Domack E, Ishman S, Leventer A, Sylva S, Willmott V, Huber B (2005) A chemotrophic ecosystem found beneath Antarctic ice shelf. EOS 86:269–272. doi:10.1029/2005EO290001
El-Sayed SZ, Taguchi S (1981) Primary production and standing crop of phytoplankton along the ice-edge in the Weddell Sea. Deep Sea Res 28:1017–1032. doi:10.1016/0198-0149(81)90015-7
Fryxell GA, Kendrick GA (1988) Austral spring microalgae across the Weddell Sea ice edge: spatial relationships found along a northward transect during AMERIEZ 83. Deep Sea Res A 35:1–20. doi:10.1016/0198-0149(88)90054-4
Garrison DL, Buck KR (1989) The biota of Antarctic pack ice in the Weddell sea and Antarctic Peninsula regions. Polar Biol 10:211–219. doi:10.1007/BF00238497
Gutt J, Starmans A (2003) Patchiness of the megabenthos at small scales: ecological conclusions by examples from polar shelves. Polar Biol 20:276–278
Hoffmann EE, Klinck JM (1998) Hydrography and Circulation of the Antarctic continental shelf: 150°E to the Greenwich meridian. Sea 11:997–1042
Hoppema M (2004) Weddell Sea turned from source to sink for atmospheric CO2 between pre-industrial time and present. Global Planet Change 40:219–231. doi:10.1016/j.gloplacha.2003.08.001
Hoppema M, Goeyens L (1999) Redfield behavior of carbon, nitrogen, and phosphorus depletions in Antarctic surface water. Limnol Oceanogr 44:220–224
Hoppema M, Fahrbach E, Stoll MHC, de Baar HJW (1999) Annual uptake of atmospheric CO2 by the Weddell Sea derived from a surface layer balance, including estimations of entrainment and new production. J Mar Syst 19:219–233. doi:10.1016/S0924-7963(98)00091-8
Hoppema M, Goeyens L, Fahrbach E (2000) Intense nutrient removal in the remote area off Larsen Ice Shelf (Weddell Sea). Polar Biol 23:85–94. doi:10.1007/s003000050012
Hoppema M, de Baar HJW, Bellerbyc RGJ, Fahrbach E, Bakker K (2002) Annual export production in the interior Weddell Gyre estimated from a chemical mass balance of nutrients. Deep Sea Res Part II Top Stud Oceanogr 49:1675–1689. doi:10.1016/S0967-0645(02)00006-1
Hoppema M, Middag R, de Baar HJW, Fahrbach E, van Weerlee EM, Thomas H (2007) Whole season net community production in the Weddell Sea. Polar Biol 31:101–111. doi:10.1007/s00300-007-0336-5
Jennings JC Jr, Gordon LI, Nelson DM (1984) Nutrient depletion indicates high primary productivity in the Weddell Sea. Nature 309:51–54. doi:10.1038/309051a0
Krell A, Schnack-Schiel SB, Thomas DN, Kattner G, Zipan W, Dieckmann GS (2005) Phytoplankton dynamics in relation to hydrography, nutrients and zooplankton at the onset of sea ice formation in the eastern Weddell Sea (Antarctica). Polar Biol 28:700–713. doi:10.1007/s00300-005-0733-6
Lee K, Tong LT, Millero FJ, Sabine CL, Dickson G, Goyet C, Park G, Wanninkhof R, Feely RA, Ke RM (2006) Global relationships of total alkalinity with salinity and temperature in surface waters of the world’s oceans. Geophys Res Lett 33:L19605. doi:10.1029/2006GL027207
Legendre L, Rassoulzadegan F (1996) Food-web mediated export of biogenic carbon in oceans: hydrodynamic control. Mar Ecol Prog Ser 145:179–193. doi:10.3354/meps145179
Lewis E, Wallace DWR (1998) Program Developed for CO2 System Calculations. ORNL/CDIAC-105. Carbon Dioxide Information Analysis Center. Oak Ridge National Laboratory, US, Department of Energy, Oak Ridge
Lizzote MP (2001) The contribution of sea ice algae to Antarctic marine primary production. Am Zool 41:57–73. doi:10.1668/0003-1569(2001)041[0057:TCOSIA]2.0.CO;2
Meredith MP, King JC (2005) Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys Res Lett 32:L19604. doi:10.1029/2005GL024042
Moline MA, Claustre H, Frazer TK, Schofield O, Vernet M (2004) Alteration of the food web along the Antarctic Peninsula in response to a regional warming trend. Glob Change Biol 10:1973–1980. doi:10.1111/j.1365-2486.2004.00825.x
Murray CN, Riley JP (1969) The solubility of gases in distilled water and seawater—II. Oxygen. Deep Sea Res 16:311–320
Nelson DM, Smith WO Jr, Gordon LI, Huber BA (1987) Spring distributions of density, nutrients and phytoplankton biomass in the ice edge zone of the Weddell-Scotia Sea. J Geophys Res 92C:7181–7190. doi:10.1029/JC092iC07p07181
Nicol S (2006) Krill, currents, and sea ice: Euphausia superba and its changing environment. Bioscience 56:111–120. doi:10.1641/0006-3568(2006)056[0111:KCASIE]2.0.CO;2
Orsi A, Nowlin W Jr, Whitworth IIIT (1993) On the circulation and stratification of the Weddell Gyre. Deep Sea Res Part I Oceanogr Res Pap 40:169–203. doi:10.1016/0967-0637(93)90060-G
Pakhomov EA, Froneman PW, Perissinotto R (2002) Salp/Krill interactions in the southern Ocean: spatial segregation and implications for the carbon flux. Deep Sea Res Part II Top Stud Oceanogr 49:1881–1907. doi:10.1016/S0967-0645(02)00017-6
Rack W, Rott H, Siegel A, Skvarca P (1999) The motion field of northern Larsen Ice Shelf, Antarctic Peninsula, derived from satellite imagery. Ann Glaciol 29:261–266. doi:10.3189/172756499781821120
Redfield AC, Ketchum BH, Richards FA (1963) The influence of organisms on the composition of sea-water. Sea 2:26–77
Rignot E, Casassa G, Gogineni P, Krabill W, Rivera A, Thomas R (2004) Accelerated ice discharge from the Antarctic Peninsula following the collapse of Larsen B ice shelf. Geophys Res Lett 31:LI8401. doi:10.1029/2004GL020697
Rubin SI, Takahashi T, Chipman DW, Goddard JG (1998) Primary productivity and nutrient utilization ratios in the Pacific sector of the Southern Ocean based on seasonal changes in seawater chemistry. Deep Sea Res Part I Oceanogr Res Pap 45:1211–1234. doi:10.1016/S0967-0637(98)00021-1
Sarmiento JLS, Gruber N (2006) Ocean biogeochemical dynamics. Princeton University Press, Princeton
Scambos TA, Bohlander JA, Shuman CA, Skvarca P (2004) Glacier acceleration and thinning after ice shelf collapse in the Larsen B embayment, Antarctica. Geophys Res Lett 31:LI8402. doi:10.1029/2004GL020670
Schloss IR, Ferreyra GA, Ferrario ME, Almandoz GO, Codina R, Bianchi AA, Balestrini CF, Ochoa HA, Ruiz Pino D, Poisson A (2007) Role of plankton communities in sea–air variations in pCO2 in the SW Atlantic Ocean. Mar Ecol Prog Ser 332:93–106. doi:10.3354/meps332093
Semeneh M, Dehairs F, Elskens M, Baumann MEM, Kopczynska EE, Lancelot C, Goeyens L (1998) Nitrogen uptake regime and phytoplankton community structure in the Atlantic and Indian sectors of the Southern Ocean. J Mar Syst 17:159–177. doi:10.1016/S0924-7963(98)00036-0
Skvarca P (1994) Changes and surface features of the Larsen Ice Shelf, Antarctica, derived from Landsat and Kosmos mosaics. Ann Glaciol 20:6–12
Skvarca P, Rack W, Rott H (1999) 34-Year satellite time series to monitor characteristics, extent, and dynamics of Larsen B Ice Shelf, Antarctic Peninsula. Ann Glaciol 29:255–260. doi:10.3189/172756499781821283
Smetacek V, Nicol S (2005) Polar ocean ecosystems in a changing world. Nature 437:362–368. doi:10.1038/nature04161
Smith WO Jr, Nelson DM (1986) Importance of ice edge phytoplankton production in the Southern Ocean. Bioscience 36:251–257. doi:10.2307/1310215
Smith WO Jr, Nelson DM (1990) Phytoplankton growth and new production in the Weddell Sea marginal ice zone in the austral spring and autumn. Limnol Oceanogr 35:809–821
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn edn. WH Freeman, New York
Stephens BB, Keeling RF (2000) The influence of Antarctic sea ice on glacial–interglacial CO2 variations. Nature 404:171–174. doi:10.1038/35004556
Strickland JDH, Parsons TR (1972) A practical handbook of sea-water analysis, 2nd edn. J Fish Res Bd Can
Sullivan CW, McClain CR, Comiso JC, Smith WO Jr (1988) Phytoplankton standing crops within an Antarctic ice edge assessed by satellite remote sensing. J Geophys Res 93:12487–12498. doi:10.1029/JC093iC10p12487
Takahashi T, Sutherland SC, Sweeney C, Poisson A, Metzl N, Tilbrook B, Bates N, Wanninkhof R, Feely RA, Sabine C, Olafsson J, Nojiri Y (2002) Global sea–air CO2 flux based on climatological surface ocean pCO2, and seasonal biological and temperature effects. Deep Sea Res Part II Top Stud Oceanogr 49:1601–1622. doi:10.1016/S0967-0645(02)00003-6
Tosonotto GV, Gallo J, Comes R (2000) CTD in an area previously occupied by the Larsen Ice Shelf, Western Weddell Sea. Contribución No. 506, Dirección Nacional del Antártico, IAA, Buenos Aires
Turner J, Colwell SR, Marshall GJ, Lachlan-Cope TA, Carleton AM, Jones PD, Lagun V, Reid PA, Lagovinka S (2005) Antarctic climate change during the last 50 years. Int J Climatol 25:279–294. doi:10.1002/joc.1130
Utermöhl H (1958) Zur Vervolkommung der quantitativen phytoplankton. Mitt Int Verein Limnol 9:1–13
van den Broeke M (2005) Strong surface melting preceded collapse of Antarctic Peninsula ice shelf. Geophys Res Lett 32:L12815. doi:10.1029/2005GL023247
Vaughan DG, Doake CSM (1996) Recent atmospheric warming and retreat of ice shelves on the Antarctic Peninsula. Nature 379:328–331. doi:10.1038/379328a0
Weiss RF, Östlund HG, Craig H (1979) Geochemical studies of the Weddell Sea. Deep Sea Res 26:1093–1120. doi:10.1016/0198-0149(79)90059-1
Acknowledgments
We would like to thank Lic. Gabriela Tosonotto for her generosity in sharing data from this cruise with us, as well as the team of the Departamento de Ciencias del Mar from the Instituto Antártico Argentino and the crew from “Almirante Irizar” during the 1996 cruise. Our thanks also to Silvia Rodriguez for the phytoplankton counts and her constant help, to Drs H. Pizarro, R. Casaux, N. Coria, G. Ferreyra for their critical readings of the thesis and the manuscript, to M. Pratto for helping us with the graphic’s design and to X. Wang for advice with data. Finally, we especially thank Drs. M. Hoppema and L. Miller, as well as an anonymous reviewer, whose valuable suggestions greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bertolin, M.L., Schloss, I.R. Phytoplankton production after the collapse of the Larsen A Ice Shelf, Antarctica. Polar Biol 32, 1435–1446 (2009). https://doi.org/10.1007/s00300-009-0638-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-009-0638-x