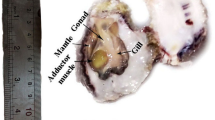
Abstract
The gametogenic cycle of the pearl oyster, Pinctada radiata, from the Zarat region (Gulf of Gabès) in Tunisia was investigated monthly during a 1-year study of the reproductive cycle. Gametogenic activity was determined based on histological analysis of the gonads during the study period. P. radiata was found to have a clearly defined annual reproductive cycle, with two spawning periods per year. The first spawning period was observed in June and August for both sexes, and the second one was occurred from September to December for males and from September to November (except October) for females. The observed index shows that the main periods of gametogenesis and spawning around the year were from September to December for males and from September to November (except October) for female. Two types of hermaphroditism were observed following gonad ripening. The sex ratio showed a clear relationship with the size, with males the dominate sex among smaller individuals (shell height [SH] < 65 mm) and females predominant in larger size classes (SH > 65 mm). The condition index proved to be a strong indication of the gonad cycle as it illustrates the reserve accumulation during gametogenesis, maximum maturity and gamete emission. Maturity is roughly synchronous between sexes. The beginning of reproduction seems to be controlled by sea surface temperature rather than salinity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Gulf of Gabès is situated in south-eastern Tunisia and the southern part of the Mediterranean Sea. Its coastline extends for 750 km from Chebba (35°N) to the Libyan border. The gulf is notable for its shallow waters, elevated levels of temperature and high salinity. The maritime zone includes large continental shelves known for the presence of large beds of the seagrass Posidonia oceanica (Ben Othman 1973; Ben Brahim 2005). The main characteristic of substrates in the inshore and midshore areas is muddy sand, with extensive beds of the seagrasses Cymodocea nodosa and Zostera noltii in some regions of the inshore area and thick coverage of P. oceanica in some regions of the midshore area (Ben Brahim 2005). However, in the deep sea, the bedrock consists mainly of reef barrier and dead shellfish.
Due to its topographical and biological features, this deep sea area is an important area for natural stocks of bivalves for both local and national fisheries. For local fisheries, this region is the main fishing region, with > 50% of the fishing fleet of Tunisia active here, and it provides around 94% of the total Tunisian clam production (Ruditapes decussatus), of which 99% is sent overseas, i.e. Europe (CRDA 2002). Among the edible shellfish species, the pearl oyster Pinctada radiata is abundant along the Gulf of Gabès. The total biomass of this species in the coastal region of Medenine has been reported to be 44.68 tons (Derbali et al. 2009). Pinctada radiata (Leach, 1814) belongs to the Pteriidae and is an Indo-Pacific species that was reported for the first time in the Gulf of Gabès in 1891 (Bouchon-Brandely and Berthoule 1891). This pintadine had been confined to this area and has reproduced there, forming very dense populations (Tlig-Zouari 1993). It is considered to be both the major species for producing cultivated pearls (Gervis and Sims 1992; Urban 2000) and a biovigilance fauna for the levels of heavy metal in the maritime environment (Al-Madfa et al. 1997). Only the inhabitants of a few coastal regions in Tunisia, namely the Kerkennah Islands, Djerba Island and Bizerte, are major consumers of P. radiata (Tlig-Zouari et al. 2010).
In Tunisia, this species is collected from wild sites and is not cultured. Therefore, a detailed understanding of the reproductive biology of P. radiata is an important tool for managing the renewal of natural stocks of this species. Since the 1950s, the reproduction of P. radiata has been studied by Uemoto (1958) in Japan and Tranter (1959) in Australia. Following its propagation in Tunisian waters, P. radiata has been the subject of much research on its biology, morphology and breeding (Zouali 1978; Soufi-Kechaou and Aloui-Bejaoui 2004; Irathni 2007; Dkhili 2008). However, to our knowledge only two studies, namely those of Tlig-Zouari and Zaouali (1994) and Derbali et al. (2009), have been conducted on its reproductive cycle in Tunisian waters. In this context, the aim of our study was to collect fundamental information on its reproductive biology by evaluating its reproductive cycle and sex ratio during a 1-year cycle. During this study we followed the stages of the reproductive maturity index and condition index at monthly intervals, and we determined the influence of two important environmental factors, i.e. temperature and salinity, on the progress of the reproductive cycle of P. radiata.
Materials and methods
Study site
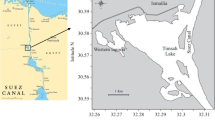
Samples (n = 10) of P. radiata were collected monthly from March 2012 to March 2013 (with the exception of July 2012 due to difficulty in sampling) by divers at depths of 5–7 m in the Zarat region (Gulf of Gabès, south-eastern Tunisia) (Fig. 1). The site is located 20 km to the east of Zarat (33°40′4.27″ N/10°21′1.78″ E). This area is characterized by low water depth, low current speed and high salinity and temperature. Moreover, the ecological conditions in the Zarat region are favorable for the growth and reproduction of many bivalves (Rabaoui et al. 2013).
Environmental parameters
The sexual cycle was studied in relation to changes in two environmental parameters: temperature and salinity. These two parameters were determined monthly using a thermometer (model 700260; Bibby Sterilin Ltd., Staffordshire, UK) and a salinometer (model MET 51302611; Mettler Toledo Greifensee, Switzerland–Mettler Toledo), respectively.
Condition index
The total weight was determined by weighing each oyster (P. radiata). We then separated the somatic tissues from the shells and weighed each part separately. The condition index (CI), determined according to the recommendation of the French Association for Standardization (Association Française de Normalisation) AFNOR NF V 45,056, (September 1985), was then calculated as:
Histological examination
After dissection, the gonads were embedded in aqueous Bouin’s solution for 48 h, following which these samples were dried in four consecutive baths of increasing alcohol content (70, 80, 95, 100 %), then passed through three consecutive baths of toluene and finally fixed in paraffin (melting point 57 °C). These samples were cut into 3-μm-thick slices on a microtome (model RM 2135; Leica Microsystems, Wetzlar, Germany), stained with hematoxylin and eosin, and placed under a Pertex coverslip. The aim of microscopic examination under a light microscope (model MCX 300; MICROS Austria, Vienna, Austria) was to determine the phases of the gametogenic cycle. The reproductive phases of gonad development were based on the description by Guillon et al. (1990).
Maturity index
Numbers from 1 to 5 were given to classify the different stages of the reproductive status of the oysters were classified on a scale of 1–5, where a1 = indifferent; a2 = early development; a3 = development; a4 = ripe; a5 = emission (Guillou et al. 1990).
The proportions of oysters in each phase were calculated, and the MI determined (Smaoui-Damak et al. 2006, 2007) as follows:
Statistical analysis
The χ2 test was used to evaluate statistically significant deviations from a balanced sexual proportion of 1:1. We also used analysis of variance followed by Tukey’s post hoc test to identify the variations in the seasonal patterns of each variable. The findings were presented as the mean ± confidence interval (CI), with a significance level of P = 0.05. The statistical analysis was carried using SAS software (1998).
Results
Sex ratio
In all P. radiata specimens collected, 57 were male (M), 51 were female (F), nine were indeterminate and three were hermaphroditic. The general sex ratio (F:M 0.9:1) was not considerably different from equivalence (F:M = 1:1; χ2 test, P > 0.05). To depict variations in specimen size as a function of sex ratio, we grouped the data according to size classes (shell height [SH] classes of 5 mm SH) (Fig. 2). The smallest size classes of specimens (SH < 65 mm) consisted predominately of males; the intermediate size classes (SH 60–70 mm) statistically comprised males and females equally; the largest size groups (SH > 65 mm SH) consisted predominantly of females (χ2 test, P < 0.05).
Condition index
Seasonal variations in the CI between males and females were not statistically different; therefore the results from both sexes are presented together in Fig. 3. The statistical analyses showed that there were no significant changes in CI between March 2012 and March 2013 (P > 0.05). The minimum CI values were observed in August–September and December 2012 (P < 0.05). The CI rose considerably from March (M1) to May, then from September to November and finally from December to February.
Maturity index
The monthly divergences observed in the MI were not significant among the sexes; therefore, the results from both sexes are presented together in Fig. 4. The maturity indices showed a significant discrepancy between the months (χ2 test, P < 0.05). The highest MI value (4.20) was recorded in June, whereas, the lowest one (1.33) was observed in March 2013.
Distribution of gametogenesis stages
The gonadal development of P. radiata was categorized into five stages in males and females separately (Table 1; Fig. 5).
Gonad developmental stages for male (a) and female (a) P. radiata specimens collected at the Zarat site. I Indifferent stage, II developmental stage 1, III developmental stage 2, IV mature stage, V spawning stage. Scale 50 μm. Ct Connective tissues, Gt gonadal tubule, Spg spermatogonia, Spt spermatid, Spz spermatozoa, O oocyte, Og oogonia, Or residual oocyte
Males
The percentage of oyster males at each gonad stage is shown in Fig. 6a . Most of the gonads were in developmental state 1 in March (66.68%) and April (50%) 2012. Males were mature in June (60%). Two spawning periods were observed in the male oysters, with the first occurring in the summer (June [40%] and August [20%]) accompanied by 50% of males in the indifferent stage in September, and the second one observed from September (25%) to December (22.22%) 2012. The sexual rest period began for a few specimens (25%) in January 2013, increasing up to March 2013 (33.33%), with a high percentage (50%) in February 2013.
Gonad stages of male (a) and female (b) P. radiata specimens in each monthly sample, from March 2012 to March 2013. Percentages of oysters corresponding to each gonad stage are identified by different bar patterns. I–V See caption to Fig. 5
Females
The development of female gonads based on histological studies is shown in Fig. 6b. For females, gametogenesis began in few specimens in March 2012 (20%), increasing to a high level in April (83.33%). In May, 100% of females were mature. Two spawning periods were observed in females, with the first period occurring in the summer (June [20%] to August [20%]) and the second observed from September (28.58%) to November (50%) (with the exception of October). The spawning periods were separated, with 28.58% of females in the indifferent stage observed in September. In December, 100% of females were in early development. The sexual rest period began for few specimens (14.29%) in January and continued up to March 2013 (33.33%). In March 2012 all reproductive stages (I, II, III, IV, V; Table 1) were observed in females with equal proportions (20%), while in September the composition of individuals in terms of reproductive stage was heterogeneous (I 28.58%, II 14.28%, III 14.28%, IV 14.28%, V 28.58%).
A few hermaphroditic individuals were identified (Fig. 7) in May, September and November. In the hermaphroditic specimens observed in May (Fig. 7a), both sex gametes were present in the same gonadal tubule. However, in the hermaphroditic specimens observed in September and November (Fig. 7b), male and female gonadal tubules occurring at the same time, even though they were set apart.
Temperature and salinity
Water temperatures and salinity from March 2012 to March 2013 are shown in Fig. 8. A water temperature cycle was observed, with temperature varying between 17 and 21 °C in winter and between 29 and 32 °C in summer. The maximum and minimum recorded water temperature was 32 °C in August and 16 °C in January, respectively. Salinity varied slightly from 1 month to another, with the lowest (33.9‰) and highest (38.43‰) salinity value recorded in April and June, respectively.
Discussion
The pearl oyster Pinctada radiata is a protandric hermaphrodite species (Tlig-Zouari and Zaouali 1994; Derbali 2009), as confirmed in the present study. In most pearl oysters, hermaphroditism is a rare phenomenon. Hermaphroditic animals suffer an energetic cost because they maintain two reproductive systems (Heath 1976). In Tunisian waters, Derbali et al. (2009) found only 26 bisexual specimens among 2360 pearl oysters collected, whereas we found three hermaphrodite individuals among 120 pearl oysters during the maturity and spawning seasons. Protandry is commonly found in many other pearl oysters, including P. margaritifera (Linnaeus, 1758), P. mazatlanica (Hanley, 1855), P. albina (Lamarck, 1819), P. imbricata (Röding, 1798) and P. fucata (Gould, 1850) (Tranter 1958; Saucedo and Monteforte 1997; O’Connor 2002; Kimani et al. 2006; Hwang 2007). For example, among 3360 pearl oysters collected by Pouvreau et al. (2000a), only seven bisexual specimens; however, there was no proof that the two gonads were functional. Another study reported that under stressful conditions, natural (temperature or food) or non-natural (handling or cleaning), changes from male to female and from female to male occur (Thielley 1993). These changes are likely to be linked to food availability (Tranter 1958). Pouvreau et al. (2000) suggested that good conditions will favor female development, whereas stress conditions will suppress it.
In our study we found that the sex ratio was statistically balanced in relation to the SH size-classes. This last finding is consistent with the results of a similar study in P. radiata from Tunisian waters (Derbali et al. 2009) and P. imbricata from Australia waters (O’Connor and Lawler 2004). In our study, the majority of individuals with a SH of < 65 mm were male; sex inversion appeared in individuals in the SH size class of 65–70 mm. Above a SH of 65–70 mm, the proportion of females increased progressively, ultimately dominating the largest size classes. Similar results have also been found in a population of P. radiata from the waters off Kerkennah Island (Derbali et al. 2009). However, in Pinctada margaritifera, individuals having a SH size of < 90 mm were exclusively male; above this size the percentage of females grew gradually, ultimately becoming the more predominant sex (Pouvreau et al. 2000). Lucas (1965) and Tranter (1958) attributed these changes in the sex ratio as a function of size to different causes: either a difference in growth and survival between the sexes or a sexual inversion.
In this study, the presence of different developmental stages of gametogenesis in the two sexes almost throughout the entire year was one of the most obvious characteristics of the P. radiata gametogenic cycle. This result indicates that gametogenesis, maturation and spawning happen year-round in P. radiata. A small number of gonads were in the resting phase (January–February), implying that gametogenesis is almost continual. These results signify that oysters rarely experience a resting phase or that this phase is brief. They are in agreement with other studies that have reported gonad activity and spawning in tropical and subtropical pearl oysters throughout the year (P. mazatlantica, Garcia-Dominguez et al. 1996; Pouvreau et al. 2000; Urban 2000). The peak of P. radiata spawning varied with marine locality, with spawning occurring in June/July in Bahrain (Khamdan 1998), in December–January/March–May in Australia (O’Connor and Lawler 2004) and in July/November in Kerkennah Island (Tunisia) (Derbali 2011). In our study, P. radiata had two spawning periods a year, with the first occurring in the summer (June and August) for both sexes and the second one occurring from September to December for males and from September to November (except October) for females. These two periods were separated by a retardation phase in September that was characterized by the occurrence of undifferentiated individuals. These results show that the spawning season of males totally coincided with that of females in the first period but that it only partially coincided with that of females in the second period. In contrast, a study in the waters off Kerkennah Island (Gulf of Gabès) showed a total coincidence during both spawning periods (Derbali 2011). Lubet and Mann (1987) suggested that differences in reproductive behavior can be observed within a species in the same geographical area and that they could arise from changes in temperature, food availability and salinity. Environmental conditions are generally taken into consideration when identifying and monitoring gametogenesis. Temperature is considered to be an essential factor influencing reproduction. Indeed, in bivalves increases in temperature speed up gametogenesis and facilitate the use of reserve material (Lubet 1991). In our study, gametogenesis started in March when the water temperature reached 17 °C. The first spawning period was correlated with a rise in seawater temperature from 27 °C in May to 32 °C in August. The second spawning period took place when the water temperature dropped from 30 °C in September to 20 °C in November. This observation suggests that the high temperatures in the summer months would appear to influence the release of gametes. The decrease in temperature makes it possible for the gonads to quickly ripen within a short period, resulting in the second spawning in the late autumn. Our findings support previous results found by Derbali et al. (2009) in the waters off Kerkennah Island. Hwang (2007) also reported that the spawning of Pinctada fucata and P. margaritifera in the waters off Taiwan was influenced by changes in water temperature. The first spawning peak of P. fucata was associated with a rising water temperature from April to May and the second one occurred when the water temperature decreased from September to October. P. margaritifera has also been observed to spawn when the water temperature increases (Hwang 2007). Contrary to what was previously observed for Cerastoderma glaucum (Iglesias 2006; Machreki-Ajmi 2009), Ruditapes decussatus (Smaoui-Damak 2005) and P. radiata (Derbali et al. 2009), our study shows that the salinity was not involved in sex change. In other bivalves, such as the cockle C. glaucum (Bruguière 1789) a rapid decline in salinity may negatively affect reproduction, resulting in a postponement of gametogenesis and even spawning (Iglesias 2006; Machreki-Ajmi 2009). In the clam R. decussatus (Linnaeus, 1758) the spawning period corresponds to the period of the highest seawater salinity (Smaoui-Damak 2005).
The MI closely reflects the gametogenic process and demonstrates the degree of maturity of gametes. In the present study, the MI was considerably different between the months of the study but not between the sexes. Therefore, male and female P. radiata were in synchrony in terms of the maturation and spawning of gametes. According to Derbali (2011), the MI shows that the main periods of gametogenesis and spawning throughout the year were roughly synchronous between sexes.
The relationship between seasonal variations in body weight and reproductive activity has been demonstrated in many bivalves (Pouvreau et al. 2000; Hwang 2007). Generally, in bivalve seasonal reproduction, gonadal biomass gives an indication of gamete production; consequently, the CI can directly assess the activity of gametogenesis (Laruelle 1999), and it can also be an indicator of food availability for the given species in a particular zone or time (Mzighani 2005). Using the CI, we established the CI as a specific sign of highest gonad maturity and spawning periods, noting that the values were associated directly with the gonad state determined by histological methods. The CI values increased in line with the majority of individuals being at an advanced stage of gametogenesis, with the highest CI values occurring when the oyster was mature, and the sharp decline coinciding with a high percentage of specimens at the post spawning stage, as a result of weight loss ensuing from gamete release. This finding is consistent with that reported by Tlig-Zouari (1993) in the waters off Kerkennah Island, possibly implying that no changes in food availability occurred in that year and the present study period. Since the CI takes into account all meat tissue (somatic and gonadal tissues), the differences may be affected by other factors which are not linked to gonad growth directly, such as somatic development and storage of reserves typically occurring during the summer (Larretxea 1995). This difference can also be explained by the degree of adaptation that differs from one population to another. Some studies also found a higher CI in clams R. decussatus and Mya arenaria harvested from contaminated sites in comparison with the same species harvested from control sites (Gauthier-Clerc et al. 2002; Smaoui-Damak et al. 2006).
Conclusions
The results of this study confirmed that P. radiata exhibited a clearly defined annual reproductive cycle with two spawning seasons. Temperature may play an important role in oyster maturity and spawning. In fact, the explanation of the reproductive cycle of the P. radiata given in our article provides an important insight into the reproductive behavior of this species. In the future, this species may possible be used to forecast the most favorable reproductive time to introduce artificial spawning in aquaculture systems. Our findings will serve as a reference for future studies in aquatic ecotoxicology.
References
Ajimi-Macherki M (2009) Validation des biomarqueurs de pollution chez le mollusque Bivalve Cerastoderma glaucum issu du golfe de Gabès: Etude in situ et transplantation in vivo). PhD thesis. University of Sfax, Sfax, Tunisia
Al-Madfa H, Abdel-Moati MA, Al-Gimaly FH (1997) Pinctada radiata (Pearl Oyster): a bio indicator for metal pollution monitoring in the Qatari waters (Arabian Gulf). Bull Environ Contam Tox 60:245–251
Ben Brahim M(2005) Contribution à l’étude de la posidonie Posidonia oceaica sur les îles Kerkennah:çenologie et épithytisme. PhD thesis. University of Sfax, Sfax, Tunisia
Ben Othman S (1973) Le sud tunisien (golfe de Gabès), hydrologie, sédimentologie, flore et faune. PhD thesis. University of Tunisia, Tunis, Tunisia
Bouchon-Brandely M, Berthoule A (1891) Les pêches maritimes en Algérie et en Tunisie. Librairie Militaire de L. Baudoin, Paris
Commissariat Régional de Développement Agricole (CRDA) (2002) Réseau national de surveillance des zones de production et de commercialisation des mollusques bivalves. Bilan de la campagne 2001–2002
Derbali A (2011) Biologie, abondance et cartographie de deux espèces de Bivalves: l’huitre perlièrePinctada radiataet la coque glauque Cerastodermaglaucumdans le golfe de Gabès. PhD thesis. University of Sfax, Sfax, Tunisia
Derbali A, Jarboui O, Ghorbel M, Dhieb K (2009) Reproductive biology of the pearl oyster, Pinctada radiata (Mollusca: Pteriidae), in northern Kerkennah Island (Gulf of Gabès). Cah Biol Mar 50:215–222
Dkhili S (2008) Etude comparative des caractéristiques morphologiques de la coquille et de la structure de la nacre chez différentes populations de l’huître perlière Pinctada radiata (Leach, 1814) du littoral tunisien. Tunisia
Garcia-Dominguez F, Ceballos-Vazquez BP, Quezada AT (1996) Spawning cycle of the pearl oyster, Pinctada mazatlanica (Hanley, 1856), (Pteriidae) at Isla Espiritu Santo, Baja California Sur, Mexico. J Shellfish Res 15:297–303
Gauthier-Clerc S, Pellerin J, Blaise C, Gangé F (2002) Delayed gametogenesis of Mya arenaria in the Saguenay fjord (Canada): a consequence of endocrine disruptors? Comp Biochem Physiol Part C 131:457–467
Gervis MH, Sims NA (1992) The biology and culture of pearl oysters (Bivalvia: Pteriidae). ICLARM Stud. Rev: Manila, Philippines, p 49.
Guillou J, Bachelet G, Desprez M, Ducrotoy JP et al (1990) Les modalités de la reproduction de la coque Cerastoderma eduleser le littoral français de la Manche et de l’Atlantique. Aquat Living Resour 3:29–41
Heath DJ (1976) Simultaneous hermaphroditism; cost and benefit. J Theoret Biol 64:363–373
Hwang JJ (2007) Reproduction cycles of the pearl oysters, Pinctada fucata (Gould) and Pinctada margaritifera(Linnaeus) (Bivalvia: Pteriidae) in southwestern Taiwan waters. J Mar Sci Tech 15:67–75
Iglesias D (2006) Estudiopatológico de laspoblaciones de berberecho Cerastoderma edule (L.). PhD thesis. University of Vigo, Pontevedra, Spain
Irathni I, Tlig-Zouari S, Ben Hassine OK (2007) Etude de la faune associée à l’espèce invasive Pinctada radiata sur le littoral Nord. Rapp Comm Inter Mer Médit 38:503
Khamdan SAA (1998) Aspects of reproduction in the pearl oyster, Pinctada radiata (Leach). In: Otsuki A, Abdulraheem MY, Reynolds RM (eds) Offshore environment of the ROPME (Regional Organization for the Protection of the Marine Environment) sea area after the war-related oil spill. Results of the 1993–94 umitaka-Maru Cruises. Terra Scientific Publishing Company (TERRAPUB), Tokyo, pp 203–214
Kimani EN, Mavuti KM, Mukiama T (2006) The reproductive activity of the pearl oyster Pinctada imbricata Roding 1798 (Pteriidae) in Gazi Bay, Kenya. Trop Zool 19:159–174
Larretxea X(1995) Estudios de crecimiento en Cerastoderma edule L. (Bivalvia, Cardiidae): bases fisiológicas de la producciónindividual. PhD thesis. University of País Vasco, Vizcaya, Spain
Laruelle F (1999) Phénologie et déterminisme de la reproduction chez Ruditapes decussatus (L.) et Ruditapes philippinarum (Adams et Reeve) en Bretagne. PhD thesis. University of Western Brittany, Brest, France
Lubet P (1991) Reproduction des mollusques. Lavoisier 3:167–203
Lubet P, Mann R (1987) Les différentes modalités de la reproduction chez les mollusques bivalves. Haliotis 16:181–195
Lucas A (1965) Recherche sur la sexualité des mollusques bivalves. PhD thesis. University of Rennes, Rennes, France
Machreki-Ajmi M, Ketata I, Ladhar-Chaabouni R, Hamza-Chaffai A (2009) The effect of in situ cadmium contamination on some biomarkers in Cerastoderma glaucum. Ecotoxicology 17:1–11
Mzighani S (2005) Fecundity and population structure of cockles, Anadara antiquata L. 1758 (Bivalvia: Arcidae) from a sandy/muddy beach near Dar es Salaam Tanzania. West Indian Ocean Mar Sci 4:77–84
O’Connor WA (2002) Latitudinal variation in reproductive behavior in the pearl oyster, Pinctada albina sugillata. Aquacult 209:333–345
O’Connor WA, Lawler NF (2004) Reproductive condition of the pearl oyster, Pinctada imbricata (Roding), in Port Stephens, New South Wales (Australia). Aquacult Res 35:385–396
Pouvreau S, Gangnery A, Tiapari J, Lagarde F et al (2000) Gametogenic cycle and reproductive effort of the tropical blacklip pearl oyster, Pinctada margaritifera (Bivalvia: Pteriidae), cultivated in Takapoto atoll (French Polynesia). Aquatic Living Resour 13:37–48
Rabaoui L, Balti R, El Zrelli R, Tlig-Zouari S (2013) Assessment of heavy metal pollution in the gulf of Gabès (Tunisia) using four mollusc species. Medit Mar Sci 15:45–58
SAS Statistical Institute (1998) SAS/STAT user’s guide, version 8. SAS Institute, Cary
Saucedo P, Monteforte M (1997) Breeding cycle of pearl oysters Pinctada mazatlanica and Pteria sterna (Bivalvia: Pteriidae) at Bahía de La Paz, Baja California Sur, Mexico. J Shel Res 16:103–110
Smaoui-Damak W (2005) Effets de la contamination in situ (Golfe de Gabès) par le cadmium sur la synthèse des métallothioneines et sur le potentiel reproducteur de la palourde (Ruditapes decussatus). PhD thesis. University of Sfax, Sfax, Tunisia
Smaoui-Damak W, Rebai T, Berthet B, Hamza-Chaffai A (2006) Does cadmium pollution affect reproduction in the clam Ruditapes decussatus? A 1-year case study. Comp Biochem Phys C 143:252–261
Smaoui-Damak W, Mathieu M, Rebai T, Hamza-Chaffai A (2007) Histology of the reproductive tissue of the clam Ruditapes decussatus from the Gulf of Gabe`s (Tunisia). Invertebr Reprod Dev 50:117–126
Soufi-Kechaou E, Aloui-Bejaoui N (2004) Données récentes sur l’aire de répartition de l’espèce invasive Pinctada radiata dans les îles Kerkennah (Tunisie). Rapp Comm Int Expl Mer Méditerr Barcelone Espagne 37:444
Thielley M (1993) Etude cytologique de la gamétogenèse, de la sex-ratio et du cycle de reproduction chez l’huître perlière Pinctada margaritifera (L) var. cummingi (Jameson), (mollusques, bivalves). Comparaison avec le cycle de Pinctada maculata (Gould). PhD thesis. University of French Polynesia, Punaauia, Tahiti
Tlig-Zouari S (1993) Contribution à l’étude écobiologique de deux espèces de mollusques lamellibranches Pinctadaradiata (Leach, 1814) et Pinnanobilis (Linné, 1758) des îles Kerkennah. PhD thesis. Universty of Tunis, Tunis, Tunisia
Tlig-Zouari S, Zaouali J (1994) Reproduction de Pinctada radiata (Leach, 1814, Mollusques Bivalves) dans les îles Kerkennah (Tunisie). Mar Life 4:41–45
Tlig-Zouari S, Rabaoui L, Irathni I, Diawara K et al (2010) Comparative morphometric study of the invasive pearl oyster Pinctada radiata along the Tunisian coastline. Biologia 65:294–300
Tranter DJ (1958) Reproduction in Australian pearl oysters (Lamellibranchia). III. Pinctada albina (Lamarck): breeding season and sexuality. Aust J Mar Freshw Res 9:191–216
Tranter DJ (1959) Reproduction in Australian pearl oysters (Lamellibranchia). V. Pinctada fucata (Gould). Aust J Mar Freshw Res 10:45–66
Uemoto H (1958) Studies on the gonad of the pearl oyster Pinctada martensii (Dünker), II. Histological observation with regard to both the seasonal variation and the change during the course of the artificial spawning. Bull Natl Pearl Res Lab 4:287–307
Urban HJ (2000) Culture potential of the pearl oyster Pinctada imbricata from the Caribbean. I. Gametogenic activity, growth, mortality and production of a natural population. Aquacult 189:361–373
Zaouali J (1978) Les peuplements malacologiques de la mer de Boughrara. Bull Off Natl Pêch Tun 2:199–209
Acknowledgements
We would like to thank the fishermen in the region of Zaratfor their help in the collection of samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lassoued, M., Damak, W. & Chaffai, A. Reproductive cycle of the pearl oyster, Pinctada radiata (Mollusca: Pteridae), in the Zarat site (Gulf of Gabès, Tunisia). Euro-Mediterr J Environ Integr 3, 18 (2018). https://doi.org/10.1007/s41207-018-0056-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41207-018-0056-y