Abstract
The horizontal and vertical distribution and population structure of euphausiids in the Ross Sea and its adjacent waters were investigated during the summers of 2004/2005 using stratified towed samples. Nine species of euphausiids occurred in the survey area. Among them, Euphausia triacantha was dominant in biomass north of the southern boundary of the Antarctic circumpolar current (SB). Thysanoessa spp. was widely distributed north of the continental slope, while E. superba was distributed from the SB to the slope, where it showed the highest biomass. Juvenile E. superba was distributed offshore near the SB and remained at the surface, but gravid females were dominant in the slope and mainly occurred in the middle layers (400–600 m). Adult and juvenile E. crystallorophias were found at 200–300 m in the colder water of the continental shelf. In general, the peak biomass of euphausiids was found in the mid layers of the Ross Sea area. The life span and the number of spawns for major species are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seven species of euphausiids occur in the Southern Ocean, including five Euphausia and two Thysanoessa spp., which are commonly found in a circumpolar pattern around the continent and are generally well separated latitudinally (John 1936; Everson 2000). For the Euphausia species, E. crystallorophias is generally restricted to the continental shelf, while E. superba is generally distributed from the continental shelf to the Antarctic Polar Front, E. triacantha and E. frigida are distributed from north of the continental shelf break to the Antarctic Polar Front, and E. vallentini is distributed north of the Antarctic Polar Front (John 1936; Everson 2000). For the Thysanoessa spp., Thysanoessa macrura is broadly distributed south of the Antarctic Polar Front (Brinton 1985), and its congeneric T. vicina is dominant near the Antarctic Polar Front (Nemoto 1966).
Among the Euphausia spp., E. superba and E. crystallorophias are highest in biomass and are considered key species in marine ecosystems because many endemic and migrant predators depend on them for food (Everson 2000). In the Ross Sea, these two species are the primary euphausiids hunted by predatory fishes, whales, seals, penguins, and seabirds (Ainley 2004; Ainley et al. 1984, 2006; Ichii et al. 1998a; La Mesa et al. 2004; however, the Ross Sea (south of 71°S) is an area of relatively low food supply during the austral summer, as evidenced by the decreased stomach contents of Antarctic minke whales (Ichii et al. 1998a). In contrast, others have suggested that seasonal depletions of euphausiids result from intensive grazing on E. crystallorophias by predators such as Adélie penguins, Antarctic silverfish, and minke whales, which then switch their diets to small fishes during the early summer (Ainley 2004; Ainley et al. 2006).
Several studies describing the distribution and population structure of E. superba and E. crystallorophias in the Ross Sea and its adjacent waters (mainly south of 70°S) have been performed under the Italian National Project of Antarctic Research using plankton nets (Sala et al. 2002) and acoustics (Azzali et al. 2006). Sala et al. (2002) found that E. superba was primarily located in the open ocean near the shelf break, whereas E. crystallorophias was found on the continental shelf south of 74°S in the summer season of 2000. Sala et al. (2002) reported the complete absence of larval stages and very few juveniles of E. superba. Ichii et al. (1998a) suggested that the Ross Sea may be unsuitable as a nursery ground for E. superba based on the spatial distribution of body size and maturity of this species in the stomach contents of minke whales.
However, the net sampling surveys by Sala et al. (2002) mainly targeted populations within shallow waters (approximately the upper 200 m); therefore, the distribution and population structure may be poorly estimated if euphausiids occur in deeper layers. In addition, very little information is available regarding the vertical distribution pattern of euphausiid species in the Ross Sea and its adjacent waters, which is critical information for the analyses of interactions with several predators.
The objective of this study was to clarify the horizontal and vertical distribution and population structure of euphausiids in the Ross Sea and its adjacent waters during the summer seasons of 2004/2005 using stratified tows at depths of up to 1,000 m.
Materials and methods
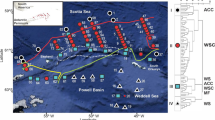
The surveys were conducted along three transect lines, 175°E and 60–77°33′S (15 stations), 180°E and 60–66°32′S (four stations), and 170°W and 73°07′–78°26′S (seven stations), from 26 December 2004 to 6 February 2005 (Fig. 1). The southernmost stations of the 175°E and 170°W transects were very close to the Ross Ice Shelf. The stations were identified by station number and sampling time [day (D) or night (N)]. For example, “Sta. 1N” refers to samples collected at night from Station 1. Because the nighttime duration decreased at southward stations, and stations south of 69°S experienced neither sunset nor nighttime (between Sta. 12D and 13D) during the survey period, sampling was mainly conducted during the day. A rectangular midwater trawl (RMT; Baker A de et al. 1973) with nominal mouth areas of 8 and 1 m2 (1 + 8) and mesh sizes of 4.5 and 0.33 mm, respectively, was towed obliquely at approximately two knots. The net was usually run down to 1,000 m or near the sea bottom at depths of <1,000 m, but trawls were only conducted at depths of 200 m at stations 1N–2N (60°00′–62°50′S, 180°E), 3D–4D (65°40′–66°32′S, 180°E), 9D (64°16′S, 175°E), 11D (67°05′S, 175°E), and 13D (69°55′S, 175°E). At stations 6 (60°00′S, 175°E) and 8 (62°50′S, 175°E), nets were run down to 1,000 m during the day and 200 m at night. The nighttime tows were restricted to shallow depth only due to the short nighttime during the summer season. At stations where nets were run down to 1,000 m, sampling was conducted at three depths (1,000–600, 600–200, and 200–0 m), and nets were sequentially opened and closed to sample three stratified layers for respective tows, resulting in a total of nine stratified layers (0–50, 50–100, 100–200, 200–300, 300–400, 400–500, 500–600, 600–800, and 800–1,000 m) at these stations. The stations at which the nets were run down to 1,000 m or near the sea bottom are hereafter designated “deeply towed stations.”
Locations of sampling stations and isobaths (m). Filled circle, offshore stations where the sea bottom depth was >3,000 m, cross, stations in the continental slope area where the sea bottom depth was 1,000–3,000 m, open circle, shallower stations on the continental shelf where the sea bottom depth was <500 m, open triangle, deeper stations on the continental shelf where the sea bottom depth was 500–1,000 m. SB area where the southern boundary of the Antarctic circumpolar current existed
To survey temperature and salinity profiles, conductivity–temperature–depth profilers (CTD, model SBE-9plus; Sea Bird Electronics Corp.) were used at each station. The salinity data obtained by the CTD sensors were calibrated based on Guildline AUTOSAL (8400B) salinity measurements.
In the laboratory, euphausiid individuals were selected under the microscope from the 8-m2 net samples. Species, developmental stage, sex, and the existence of spermatophores for females were determined in each individual. For E. superba and E. crystallorophias, reproductive maturity stages were further evaluated referring to Makarov and Denys (1981). Body length was measured from the anterior part of the eye to the tip of the telson (to the nearest 1 mm) for each individual. The relationship between body length and wet weight for T. macrura, T. vicina, E. frigida, E. crystallorophias, and E. triacantha were referred to Siegel (1992). In this case, the relation for T. vicina was regarded as that of its ally species, T. macrura. The relationship for E. superba was referred to Anon (1986), and the relationships for Stylocheiron maximum and Nematobrachion boopis were substituted for E. superba.
The filtered water volumes for each net were calculated from the calibrated flowmeter values using the formulas of Southampton Oceanography Centre (2004), assuming that the mouth angle during hauling remained constant at approximately two knots. For most analyses of horizontal distributions, the number of individuals per m2 integrated over the entire depth was calculated as follows:
where N s is the number of euphausiids per square meter at station S, b i is the number caught at depth interval i, v i is the volume filtered (m3) for depth interval i, and Z i is the thickness of the depth interval i (m).
The average contribution (%) of the jth layer to the total abundance of euphausiids in all of the layers at deeply towed stations (X j ) was calculated as follows:
where x ij is the abundance (standardized as individuals per square meter) in the jth layer at the ith station. Using this value, the layers in which the median depth and 80% range of abundance from the median depth occurred were identified, referring to Pennak (1943) for each stage of E. triacantha, Thysanoessa spp., E. superba, and E. crystallorophias at all deeply towed stations. The median depth was defined as the depth at which the cumulative percentage of abundance to the total population from the surface corresponded to 50%, with the assumption that an equal density of euphausiids was distributed in each sampling layer. The upper and lower limits of the 80% range of abundance, from the median depth, corresponded to the depth at which the cumulative percentage of abundance from the surface corresponded to 10 and 90%, respectively.
The length frequency data for E. triacantha, Thysanoessa spp., E. superba, and E. crystallorophias at all deeply towed stations were analyzed using statistical distribution mixture analysis (Macdonald and Green 1988) to examine the ages of the distinct size-groups. For each length class (mm) at each station, the number of individuals per square meter integrated over the entire depth samples was calculated using the equation for N s described above and was averaged among all deeply towed stations.
Variables of the age-class components were calculated by stepwise optimization (e.g., proportion, mean length, and standard deviation). If the observed and computed distributions were significant at the 5% level (P < 0.05), then the number of age groups was reduced, with the purpose of lowering the Chi-square value as a goodness-of-fit of the model to the data. This type of analysis assumes a normal distribution based on the limited recruitment time for Antarctic euphausiids (Siegel 1987; Pakhomov and Perissinotto 1996).
Results
Environment
The Ross Sea was almost completely ice-free during the survey period. Pack ice existed only near Sta. 11D (67°05′S, 175°E).
Along the 175°E transect, upper circumpolar deep water (UCDW), which is >2°C at the surface and composes the upper portion of the circumpolar deep water (Callahan 1972), was found north of Sta. 8 (62°50′S; Fig. 2). Because the potential temperature on the 27.6 σθ isopycnal changed from 2 to −1°C, the southern boundary of the Antarctic circumpolar current (SB) existed between stations 8 and 9 (64°16′S) (Orsi et al. 1995; Yabuki 2006; Figs. 1, 2). The lower circumpolar current water (LCDW), with temperatures around 0.5–2°C and salinities of 34.7 PSU below 100 m (Russo 2000), was found between Sta. 9 and Sta. 17 (72°13′S) in the continental slope area. Shelf water (SW), with temperatures <−1.5°C and salinities >34.4 PSU below 100 m, mainly existed on the continental shelf (stations 18–28; 72°45′–77°33′S), except near Sta. 18 where the modified circumpolar deep water (MCDW), with temperatures between −1.5 and 1°C and salinities between 34.4 and 34.6 PSU (Russo 2000), existed below the surface.
Temperature (°C; a) and salinity (PSU; b) profiles along three transect lines. UCDW area where the upper circumpolar deep water existed at the surface, LCDW area where the lower circumpolar current water occurred below the surface, MCDW area where the modified circumpolar deep water was found below the surface, SW area where the shelf water occurred below the surface. SB area where the southern boundary of the Antarctic circumpolar current existed. Italics indicate stations located within the continental slope area
On the 180° transect, the UCDW existed north of Sta. 2 (62°50′S) and SB existed between stations 2 and 3 (65°40′S). In contrast, the LCDW occurred below the surface at stations 3 and 4 (66°32′S).
The 170°W transect showed the lowest temperatures of the three transect lines, with water columns at temperatures of <0°C dominant over the surface layer. The LCDW occurred below the surface in the slope area (at stations 31–33; 73°03′–75°57′S), but the SW was dominant below the surface on the continental shelf (at stations 29 and 30; 77°00′–78°26′S).
Horizontal distribution of euphausiids
In total, nine species of euphausiids were identified in the survey area, including E. superba, E. crystallorophias, E. frigida, E. triacantha, E. vallentini, T. macrura, T. vicina, Stylocheiron maximum, and Nematobrachion boopis. Both juvenile and adult stages occurred in each species except for S. maximum (only juveniles were encountered), E. vallentini, and N. boopis (only adults were found in the latter two species). For Thysanoessa spp., only males of the species can be identified from the structure of petasmata. Because the ratio of male T. macrura to T. vicina was 89:11 and most (97%) of the Thysanoessa spp. females from the deeply towed stations had body lengths >16 mm, which is generally considered to be indicative of T. macrura (Mauchline 1980), most of the juvenile and female Thysanoessa spp. were probably T. macrura.
Euphausia triacantha was found in the UCDW and north of the northern LCDW and was dominant at the two northernmost stations (stations 6D and 8D) in the UCDW on the 175°E transect. Adults were dominant at each station (Fig. 4) and none of the females exhibited spermatophores.
Euphausia frigida was found in the UCDW and north of the northern LCDW but was lower in abundance than E. triacantha (Fig. 3). Because 4.5–20.7% of the females in the UCDW area contained spermatophores (not shown in Fig. 4), females were assumed to be spawning.
Abundance (top) and biomass (bottom) for each euphausiid species at each station. Sea bottom depths (line) were inserted in the bottom figure. Filled cirlce, offshore stations where the sea bottom depth was >3,000 m, cross, stations in the continental slope area where the sea bottom depth was 1,000–3,000 m, open circle, shallower stations on the continental shelf where the sea bottom depth was <500 m, open triangle, deeper stations on the continental shelf where sea bottom depth was 500–1,000 m. UCDW area where the upper circumpolar deep water existed at the surface, LCDW area where the lower circumpolar current water occurred below the surface, MCDW area where the modified circumpolar deep water was found below the surface, SW: area where the shelf water existed below the surface. SB area where the southern boundary of the Antarctic circumpolar current occurred. Star, shallowly towed stations (0–200 m). More detailed descriptions for each species as well as four other species not indicated are given in the literature
Abundance of euphausiids according to developmental stage and maturity. Sea bottom depths (line) were inserted in the bottom figure. Filled circle, offshore stations where the sea bottom depth was >3,000 m, cross, stations within the continental slope area where the sea bottom depth was 1,000–3,000 m, open circle, shallower stations on the continental shelf where the sea bottom depth was <500 m, open triangle, deeper stations on the continental shelf where the sea bottom depth was 500–1,000 m. UCDW area where the upper circumpolar deep water existed at the surface, LCDW area where the lower circumpolar current water was found below the surface, MCDW area where the modified circumpolar deep water occurred below the surface, SW area where the shelf water existed below the surface. SB area where the southern boundary of the Antarctic circumpolar current occurred. Star shallowly towed stations (0–200 m). More detailed descriptions for substages not indicated in this figure are given in the literature
Euphausia vallentini, S. maximum, and N. boopis occurred mainly in the UCDW area but contributed less than 2% to the total abundance (not shown in Fig. 3).
Thysanoessa spp. occurred in the widest range but did not occur on the continental shelf (Fig. 3). Females rarely showed spermatophores (only 1.6 and 4.7% of the total females at stations 9D and 3D). Low abundance of male T. vicina was observed at Stas. 1N, 2N, 6D, 8D, 11D, and 13D (not shown in Fig. 3).
Euphausia superba occurred mainly in the LCDW areas of the three transect lines. This species was abundant in the northern LCDW area (station 10D) and the continental slope area (at stations 14D–17D) on the 175°E transect (Fig. 3), and composed 81% of the total euphausiid abundance in the latter area. Very few individuals were captured on the 180° and 170°W transects (Fig. 3). An apparent segregation of the developmental and maturational stages in the horizontal distribution was observed (Fig. 4). Thus, only juveniles were found in the northern LCDW area (station 10D), but a lower abundance of subadults and adults (excluding gravid females, stages IIIC and D) were dominant in the central LCDW area at stations 11D–13D and the deeper slope area at Sta. 14D. In contrast, gravid females were dominant in the shallower slope area at stations 15D and 17D.
Euphausia crystallorophias was exclusively dominant on the continental shelf (Fig. 3) where MCDW and SW existed, and was abundant in the eastern basin area (>500 m, at stations 29D and 30D), but was not abundant in the western bank area (<500 m, at stations 18D, 24D, and 27D). Juveniles composed the largest portion at each station on the continental shelf, but no copulated or gravid females (IIIB-D) were found (Fig. 4).
Because E. superba, E. crystallorophias, and E. triacantha are larger, their biomass (wet weight) was relatively large compared to their abundance (Fig. 3). The highest biomass of total euphausiids (mean ± SD: 4.4 ± 3.9 g m−2) was observed in the slope area of the 175°E transect, 98% of which was composed of E. superba. The second highest biomass was observed on the continental shelf (mean ± SD: 2.8 ± 1.9 g m−2) on the 170°W transect, where nearly 100% consisted of E. crystallorophias.
Note that krill approximately <20 mm could be underestimated by the 4.5-mm mesh size trawl nets used in this study (Siegel 1989; Pakhomov 1995).
Vertical distribution
Both juvenile and adult stages of E. triacantha were found mainly at 100–200 m during nighttime, whereas they were found below 200 m during the day at stations 6D and 8D, the two northernmost stations in the UCDW area on the 175°E transect (Fig. 5). During the day, both juvenile and adult stages were primarily found at 300–400 m (Table 1).
Both juvenile and adult stages of Thysanoessa spp. occurred throughout the water column (Fig. 6). The median depth of abundance during the day, at the deeply towed stations, was shallower (100–200 m) for juveniles and deeper (400–500 m) for females (Table 1).
Juveniles of E. superba, occurred in the upper 50 m at Sta. 10D of the northern LCDW area on the 175°E transect (Fig. 7). Subadults occurred in the shallow layer (above 50 m) at Sta. 14D in the deeper slope area. Adults widely occurred above 600 m at Sta. 12D of the central LCDW area and at stations 14D, 15D and 17D of the slope area. Large abundances of gravid females occurred at 400–600 m in the shallower slope area (stations 15D and 17D). Thus, the layer in which median depth existed deepened with developmental stage (Table 1).
Both juvenile and adult stages of E. crystallorophias occurred throughout the water column on the continental shelf, but they were primarily found in the 200–300-m layer and rarely occurred above 100 m (Fig. 8; Table 1).
For other species, E. vallentini was found in the upper 200 m at night but below 300 m during the day at stations 6D and 8D. Only one or two juveniles of S. maximum were caught at the 300–400, 500–600, and 800–1000 m depths at Sta. 8D. Only one female of N. boopis was caught at the 500–600 m depth at Sta. 8D. Males of T. vicina usually occurred in the upper 200 m but were also found in deeper layers (i.e., 500–600 and 600–800 m at Sta. 6D and 800–1000 m at Sta. 8D). A previous study reported that this species only occurs at depths <350 m (Brinton et al. 1999).
Size composition
At the deeply towed stations, the size distribution of E. triacantha was bimodal at 24.1 ± 2.2 and 32.2 ± 2.1 mm (mean ± SD; Fig. 9; Table 2). The first component was made up of juvenile and adult stages, but the second consisted only of adults (Fig. 10). Both males and females showed two identically sized groups matching those of the entire population (Table 2). The first and second components for both males and females seemed to correspond to 1+ and 2+ year classes because the number of furcilia stages (<10 mm), which may be the 0+ year class, occurred simultaneously in the 1-m2 net samples (Taki unpublished data). A large portion of the 1+ year classes reached sexual maturity.
Size frequency distribution for all euphausiid individuals at deeply towed stations. Hypothetical distribution curves for each cohort were superimposed on the histogram using MIX software. The size groups for Euphausia superba are not rationally divided and the distribution curve is not superimposed
In Thysanoessa spp., the size distribution for all individuals at the deeply towed stations was divided into three modal groups (mean ± SD: 10.7 ± 1.5, 18.2 ± 2.5 and 24.2 ± 3.3 mm; Fig. 9; Table 2). The first, second, and third components included only juveniles, both juvenile and adult stages, and only adults, respectively (Fig. 10). Larval developmental stages between calyptopis I and furcilia VI in Thysanoessa spp. occurred simultaneously in the 1-m2 samples (Taki unpublished data). In January, these stages were regarded as the 0+ year class that had hatched in the previous austral spring following T. macrura in the Bransfield Strait region (Nordhausen 1992). In this region, T. macrura grow to a body length of approximately 7–8 mm by March of their first year (Nordhausen 1992). Thus, the first component of the distribution appeared to correspond to the 1+ year class. Males had a unit cohort of 17.9 ± 1.9 mm, which appeared to correspond to the 2+ year class, but females had two cohorts of 19.9 ± 2.2 and 25.3 ± 2.9 mm, which appeared to correspond to the 2+ and 3+ year classes, respectively (Table 2). A large portion of the 2+ year classes reached sexual maturity.
The size distribution for all E. superba individuals at the deeply towed stations was not divided into age groups. The smallest isolated sizes (15–25 mm), which mainly occurred at Sta. 10D in the northern LCDW area on the 175°E transect (Fig. 4), consisted only of juveniles (Fig. 10). These appeared to correspond to the 1+ year class because the number of calyptopis stages of this species, which may be the 0+ year class, occurred simultaneously in the slope area in the 1-m2 net samples (Taki unpublished data). Individuals with body lengths from 26 to 40 mm were conspicuously scarce, whereas individuals with body lengths of 40 to 51 mm were dominant. The largest size was 55 mm for males and 53 mm for females.
In E. crystallorophias, the size distribution for all individuals was divided into four modal groups (mean ± SD: 12.8 ± 1.4, 22.9 ± 2.4, 28.0 ± 1.7, and 32.6 ± 1.7 mm; Fig. 9; Table 2). The first component included only juveniles (Fig. 10), while the second component included juveniles, subadults, and adults. The third and fourth components consisted primarily of adult stages. Because the number of calyptopis and early furcilia stages, which may be the 0+ year class, occurred simultaneously in the 1-m2 net samples (Taki unpublished data), these four components appeared to correspond to the 1+ to 4+ year classes, respectively. Both males and females had size groups identical to the entire population, but males had no fourth group (Table 2). Some portion of the 2+ year class consisted of adults.
Discussion
Horizontal distribution of euphausiids
The horizontal distribution patterns of E. crystallorophias, E. superba, E. frigida, E. triacantha, E. vallentini, T. macrura, and T. vicina in the Ross Sea and its adjacent waters were reflected by the latitudinal zonation in distribution as generally described for the Southern Ocean (Everson 2000). The southern limit of distribution for S. maximum and N. boopis in the Southern Ocean is regarded to be 63° and 53°S, respectively (Brinton et al. 1999); thus, our observation, especially for very little catch of N. boopis, may have resulted from that our survey area was not north enough to cover their habitat.
Euphausia superba was mainly distributed in the LCDW north of the shelf break. In contrast, E. crystallorophias was dominant in the SW on the continental shelf. The horizontal distribution of E. superba and E. crystallorophias in midsummer rarely overlapped, and this distributional pattern was consistent with the results of the western Ross Sea survey in the same season (January to February 2000; Sala et al. 2002). Euphausia crystallorophias was abundant in the deeper basin area (stations 21D, 24D, 29D, and 30D). This observation is consistent with that of Sala et al. (2002), even though they sampled at much shallower depths.
The scarcity of juveniles in the slope area was consistent with whale stomach content data collected from 1991/1992, 1992/1993, and 1994/1995 (Ichii et al. 1998a) and with the results of a net sampling survey in 2000 (Sala et al. 2002). In contrast, the dominance of gravid females in the slope area was consistent with the results from the net sampling data in 2000 but inconsistent with 1992/1993 and 1994/1995 stomach-content data in which few individuals were found to be reproductively mature. This suggests that the reproductive and spawning intensities of E. superba vary conspicuously between years in the Ross Sea area as well as west of the Antarctic Peninsula (Siegel and Loeb 1995; Siegel and Harm 1996; Quetin and Ross 2001), where the proportion of females in reproductively advanced maturation stages may be related to the intensity of the sea ice extension.
For several regions of the Atlantic and Indian oceans in the summer, juveniles are primarily distributed inshore, whereas gravid females are found offshore of the shelf break (Siegel and Harm 1996; Ichii et al. 1998b; Lascara et al. 1999; Nicol et al. 2000; Pakhomov 2000). This distribution pattern is probably caused by the offshore migration of gravid females (Siegel 1988; Nicol 2006); however, in the Ross Sea area, juveniles occurred in the offshore area at Sta. 10D, while gravid females were found in the slope area. The distribution patterns, spawning, and development of E. superba in these areas are poorly understood and await further study.
Relationship between the vertical distribution and abundance of euphausiids and their predators
Previous studies have reported that the vertical migration of subadult and adult E. superba during the summer generally occurs within the upper 150 m of the water column off the Antarctic Peninsula (Miller and Hampton 1989; Demer and Hewitt 1995; Lascara et al. 1999); however, some were sampled with nets and echosounders at depths <250 m. Lascara et al. (1999) suggested that the decrease in krill biomass in winter was due to the migration of krill to greater depths beyond the sampling range. However, the vertical distribution tended to be restricted to the top 250 m in fall and winter, when the sampling range was extended to greater depths (Ashjian et al. 2004; Lancraft et al. 2004; Zhou and Dorland 2004; Lawson et al. 2008). Therefore, the occurrence of a peak in abundance for gravid females in deeper layers (400–600 m) may be characteristic of the Ross Sea area.
In E. crystallorophias, apparent diurnal vertical migrations have been reported to be within the upper 200 m west of the Antarctic Peninsula in the fall (Everson 1987), off Enderby Land in the spring (O’Brien 1987), and in the shelf region in the Weddell Sea in the summer (Pakhomov et al. 1998). However, E. crystallorophias has been found distributed off the west coast of the Antarctic Peninsula at depths of 50–290 m during the winter with no obvious peak in the depth distribution (Nordhausen 1994). The present study found that E. crystallorophias generally occurred during the day in summer at depths down to 400–500 m in the Ross Sea.
For E. superba, the overall mean abundance of 0.72 g m−2 at all deeply towed stations was comparable to the mean biomass in the eastern Indian Ocean (0.375 g m−2; Nicol et al. 2000), the northern Weddell Sea (0.03–2.06 g m−2; Siegel et al. 1992), and the Bellinghausen Sea (0.03–0.30 g m−2; Siegel and Harm 1996); it was somewhat lower than the mean biomass in the western Indian Ocean (0.4–8.8 g m−2; Pakhomov 2000), but much lower than the abundance in the Scotia sea area (18.7 g m−2, Siegel et al. 2004; 0.8−75.2 g m−2, Siegel 2005). However, these comparisons may be confounded due to differences in gear size and the extent of vertical sampling among these studies, which could bias the interpretation of the overall kill biomass in the Ross Sea area in this study.
“A poor prey area” for several predators in the Ross Sea area (Hopkins 1987; Ichii et al. 1998a) may be partly attributable to the lower abundance of euphausiids as mentioned above. Alternatively, we found E. superba mainly in the 400–600-m layers of the slope area, and E. crystallorophias in the 200–300-m layer on the continental shelf. Several mammalian and bird species could conceivably dive to 500 m to forage for euphausiids (Kooyman and Kooyman 1995; Panigada et al. 1999), but this would be highly inefficient (Kooyman and Kooyman 1995). Thus, deep distribution may be advantageous to euphausiids because of the substantial metabolic cost imposed upon their visual predators, which must then seek alternative prey. This may also explain the relative scarcity of euphausiids in the stomach contents of minke whales in the Ross Sea area (Ichii et al. 1998a).
In contrast, several studies have shown that diurnal migration is closely related to the predation pressure (Zhou and Dorland 2004; Ichii et al. 2007). In the Ross Sea, the biomass of mammals and birds during the summer is comparable to the richest top-trophic marine communities found elsewhere (Ainley 1985). Therefore, increased predation pressure may greatly impact the ecological behavior of euphausiids, especially gravid females of E. superba, which have higher caloric values per unit wet weight (Ichii et al. 2007) and are thus more likely to be targeted by predators. Although how the high-calorie, gravid females would be targeted by predators is unclear, the possibility exists that extensive predation pressure drives the observed change in vertical distribution in euphausiids.
Further studies are required to survey seasonal changes in the abundance and vertical distribution of various euphausiid species in relation to seasonal changes in predation pressure and feeding behaviors of predators.
Age composition of euphausiids
Based on the relationship between their size at sexual maturity and the year classes collected, we suspect that E. triacantha spawn approximately twice during their 3-year life span, and Thysanoessa spp. spawn approximately twice during their 4-year life span. Thus, both age composition and the number of spawns during their life span are similar to those observed off the Antarctic Peninsula (Siegel 1987), except that the number of spawnings for Thysanoessa spp. was one time less than that of the population off the Antarctic Peninsula.
The scarcity of E. superba with body lengths of 26–40 mm, the dominance of those with body lengths of 40–51 mm, and a maximum size of 53–55 mm were consistent with the size ranges observed in the slope area of the Ross Sea in 2000, except that very few juveniles were found in the samples of 2000 (Sala et al. 2002). According to Sala et al. (2002), the former and latter sizes correspond to the 2+ and 3 + to 4+ year classes, respectively. Given the segregation in the horizontal distribution of developmental stages, the offshore occurrence of juveniles, and the results of the survey in 2000, we suspect that this probable 2+ year class was distributed separately from other year classes north of the slope area and could not be captured because of our coarse sampling. The probable life span of 5 years and our observed maximum size in the Ross Sea area are similar to those reported for the southeastern Weddell Sea (Siegel 1987; Pakhomov 1995). However, off the Antarctic Peninsula and in the Indian Ocean, E. superba has a life span of 6 years and a maximum size of >61 mm (Siegel 1987; Pakhomov 1995), suggesting that the life span of E. superba may be shorter in the higher latitude areas.
Based on the finding that some portion of the 2+ year class consisted of adults, E. crystallorophias is suspected to spawn approximately three times during their 5-year life span in the Ross Sea. The age composition and the number of spawns as well as the longer life span of females compared to males agrees with previous observations for the western Ross Sea (Sala et al. 2002), off the Antarctic Peninsula (Siegel 1987), and in the western Indian Ocean (Pakhomov and Perissinotto 1996). Pakhomov and Perissinotto (1996) suggested that no major differences existed among populations in the Southern Ocean despite their restricted neritic distribution, which may be due to genetic homogeneity and/or similar environmental conditions found around the Antarctic continent. The present results support this hypothesis.
References
Ainley DG (1985) The biomass of birds and mammals in the Ross Sea, Antarctica. In: Siegfried WR, Condy PR, Laws RM (eds) Antarctic nutrient cycles and food webs. Springer, Heidelberg , pp 498–515
Ainley DG (2004) Acquiring a “base datum of normality” for a marine ecosystem: the Ross Sea, Antarctica. SC-CCAMLR WG-EMM-04/20: 1–19
Ainley DG, O’Connor EF, Boekelheide RJ (1984) The marine ecology of birds in the Ross Sea, Antarctica. Ornithol Monogr 32:1–97
Ainley DG, Ballard G, Dugger KM (2006) Competition among penguins and cetaceans reveals trophic cascades in the Ross Sea, Antarctica. Ecology 87:2080–2093
Anon (1986) Post FIBEX acoustic workshop. BIOMASS Report Series, No 40, Scientific Committee Antarctic Research, Cambridge, England, 1–106
Ashjian CJ, Rosenwaks GA, Wiebe PH, Davis CS, Gallager SM, Copley NJ, Lawson GL, Alatalo P (2004) Distribution of zooplankton of the continental shelf off Marguerite Bay, Antarctic Peninsula, during Austral fall and winter, 2001. Deep-Sea Res II 51:2073–2098
Azzali M, Leonori I, Felice ADE, Russo A (2006) Spatial-temporal relationships between two euphausiid species in the Ross Sea. Chem Ecol 22:S219–S233
Baker A de C, Clarke MR, Harris MJ (1973) The N.I.O combination net (RMT 1 + 8) and further developments of Rectangular Midwater Trawls. J Mar Biol Assoc UK. 53: 176–184
Brinton E (1985) The oceanographic structure of the eastern Scotia Sea-III. Distributions of euphausiid species and their developmental stages in 1981 in relation to hydrography. Deep Sea Res 32:1153–1180
Brinton E, Ohman MD, Townsend AW, Knight MD, Bridgeman AL (1999) Euphausiids of the World Ocean. World Biodiversity Database CD-ROM series. ETI Expert Center for Taxonomic Identification
Callahan JE (1972) The structure and circulation of deep water in the Antarctic. Deep-Sea Res 19:563–575
Southampton Oceanography Centre (2004) RMT 1 + 8 Multinet Coaxial Cable Version, Operations Manual Issue 4, September 2004. In: White D (ed). Southampton Oceanography Centre, Southampton, 1–31
Demer DA, Hewitt RP (1995) Bias in acoustic biomass estimates of Euphausia superba due to diel vertical migration. Deep Sea Res I 42:455–475
Everson I (1987) Some aspects of the small scale distribution of Euphausia crystallorophias. Polar Biol 8:9–15
Everson I (2000) Role of krill in marine food webs 7.3 The Southern Ocean. In: Everson I (ed) Krill: biology, ecology and fisheries. Blackwell , Oxford, pp 194–201
Hopkins TL (1987) Midwater food web in McMurdo Sound, Ross Sea, Antarctica. Mar Biol 96:93–106
Ichii T, Shinohara N, Fujise Y, Nishiwaki S, Matsuoka K (1998a) Interannual changes in body fat condition index of minke whales in the Antarctic. Mar Ecol Prog Ser 175:1–12
Ichii T, Katayama K, Obitsu N, Ishii H, Naganobu M (1998b) Occurrence of Antarctic krill (Euphausia superba) concentrations in the vicinity of the South Shetland Islands: relationship to environmental parameters. Deep-Sea Res I 45:1235–1262
Ichii T, Bengtson JL, Boveng PL, Takao Y, Jansen JK, Hiruki-Raring LM, Cameron MF, Okamura H, Hayashi T, Naganobu M (2007) Provisioning strategies of Antarctic fur seals and chinstrap penguins produce different responses to distribution of common prey and habitat. Mar Ecol Prog Ser 344:277–297
John DD (1936) The southern species of the genus Euphausia. Discov Rep 14:193–324
Kooyman GL, Kooyman TG (1995) Diving behavior of emperor penguins nurturing chicks at Coulman Island, Antarctica. Condor 97:536–549
La Mesa M, Eastman JT, Vacchi M (2004) The role of notothenioid fish in the food web of the Ross Sea shelf waters: a review. Polar Biol 27:321–338
Lancraft TM, Reisenbichler KR, Robinson BH, Hopkins TL, Torres JJ (2004) A krill-dominated micronekton and macrozooplankton community in Croker Passage, Antarctica with an estimate of fish predation. Deep-Sea Res II 51:2247–2260
Lascara CM, Hofmann EE, Ross RM, Quetin LB (1999) Seasonal variability in the distribution of Antarctic krill, Euphausia superba, west of the Antarctic Peninsula. Deep Sea Res 46:951–984
Lawson GL, Wiebe PH, Ashjian CJ, Stanton TK (2008) Euphausiid distribution along the Western Antarctic Peninsula-Part B: distribution of euphausiid aggregations and biomass, and associations with environmental features. Deep-Sea Res II 55:432–454
Macdonald PDM, Green PEJ (1988) User’s Guide to Program MIX: an interactive program for fitting mixtures of distributions. Release 2.3, January 1988. Ichthus Data Systems, Hamilton, Ontario, iv + 60 pp
Makarov PR, Denys CJ (1981) Stages of sexual maturity of Euphausia superba, Dana. Biomass Handb 11:1–13
Mauchline J (1980) Key for the identification of Antarctic euphausiids. Biomass Handb 5:1–4
Miller DGM, Hampton I (1989) Biology and ecology of Antarctic krill (Euphausia superba Dana): a review. BIOMASS Sci Ser 9:1–166
Nemoto T (1966) Thysanoessa euphausiids, comparative morphology, allomorphosis and ecology. Sci Rep Whales Res Inst 20:109–155
Nicol S (2006) Krill, currents, and sea ice: Euphausia superba and its changing environment. BioScience 56:111–120
Nicol S, Kitchener J, King R, Hosie G, de la Mare WK (2000) Population structure and condition of Antarctic krill (Euphausia superba) off East Antarctica (80–150°E) during the Austral summer of 1995/1996. Deep-Sea Res II 47:2489–2517
Nordhausen W (1992) Distribution and growth of larval and adult Thysanoessa macrura (Euphausiacea) in the Bransfield Strait Region, Antarctica. Mar Ecol Prog Ser 83:185–196
Nordhausen W (1994) Winter abundance and distribution of Euphausia superba, E. crystallorophias, and Thysanoessa macrura in Gerlache Strait and Crystal Sound, Antarctica. Mar Ecol Prog Ser 109:131–142
O’Brien DP (1987) Direct observations of the behavior of Euphausia superba and Euphausia crystallorophias (crustacean: Euphausiacea) under pack ice during the Antarctic spring of 1985. J Crust Biol 7:437–448
Orsi AH, Whitworth III, Nowlin WD Jr (1995) On the meridional extent and fronts of the Antarctic Circumpolar Current. Deep-Sea Res I 42:641–673
Pakhomov EA (1995) Demographic studies of Antarctic Euphausia superba in the Cooperation and Cosmonaut Seas (Indian sector of the Southern Ocean). Mar Ecol Prog Ser 119:45–61
Pakhomov EA (2000) Demography and life cycle of Antarctic krill, Euphausia superba, in the Indian sector of the Southern Ocean: long-term comparison between coastal and open-ocean regions. Can J Fish Aquat Sci 57(Suppl 3):68–90
Pakhomov EA, Perissinotto R (1996) Antarctic neritic krill Euphausia crystallorophias: spatio-temporaldistribution, growth and grazing rates. Deep-Sea Res 43:59–87
Pakhomov EA, Perissinotto R, Froneman PW (1998) Abundance and trophodynamics of Euphausia crystallorophias in the shelf region of the Lazarev Sea during austral spring and summer. J Mar Syst 17:313–324
Panigada S, Zanardelli M, Canese S, Jahoda M (1999) How deep can baleen whales dive? Mar Ecol Prog Ser 187:309–311
Pennak RW (1943) An effective method of diagramming diurnal movements of zooplankton organisms. Ecology 24:405–407
Quetin L, Ross R (2001) Environmental variability and its impact on the reproductive cycle of Antarctic krill. Am Zool 41:74–89
Russo A (2000) Water masses characteristic during the ROSSMIZE cruise (western sector of the Ross Sea, November-December, 1994). In: Faranda FM, Guglielmo L, Ianora A (eds) Ross sea ecology. Springer, Berlin, pp 83–93
Sala A, Azzali M, Russo A (2002) Krill of the Ross Sea: distribution, abundance and demography of Euphausia superba and Euphausia crystallorophias during the Italian Antarctic Expedition (January–February 2000). Sci Mar 66:123–133
Siegel V (1987) Age and growth of Antarctic Euphausiacea (Crustacea) under natural conditions. Mar Biol 96:483–495
Siegel V (1988) A concept of seasonal variation of krill (Euphausia superba) distribution and abundance west of the Antarctic Peninsula. In: Sahrhage D (ed) Antarctic Ocean and resources variability. Springer, Heidelberg, pp 219–230
Siegel V (1989) Winter and spring distribution and status of the krill stock in Antarctic Peninsula waters. Arch FischWiss 39:45–72
Siegel V (1992) Review of length-weight relationships for Antarctic krill. CCAMLR Sel Sci Pap 9:145–155
Siegel V (2005) Distribution and population dynamics of Euphausia superba: summary of recent findings. Polar Biol 29:1–22
Siegel V, Harm U (1996) The composition, abundance, biomass and diversity of the epipelagic zooplankton communities of the southern Bellingshausen Sea (Antarctic) with special reference to krill and salps. Arch Fish Mar Res 44:115–139
Siegel V, Loeb V (1995) Recruitment of Antarctic krill Euphausia superba and possible causes for its variability. Mar Ecol Prog Ser 123:45–56
Siegel V, Skibowski A, Harm U (1992) Community structure of the epipelagic zooplankton community under ther sea-ice of the northern Weddell Sea. Polar Biol 12:15–24
Siegel V, Kawaguchi S, Ward P, Litvinov F, Sushin V, Loeb V, Watkins J (2004) Krill demography and large-scale distribution in the southwest Atlantic during January/February 2000. Deep-Sea Res II 51:1253–1273
Yabuki T (2006) Hydrography of the Antarctic Ocean and its variability based on JARPA and R/V Kaiyo Maru survey. Dr Thesis, Tohoku University, Sendai, 1–107
Zhou M, Dorland RD (2004) Aggregation and vertical migration behavior of Euphausia superba. Deep-Sea Res II 51:2119–2137
Acknowledgments
We would like to express our sincere gratitude to Taro Ichii, Hiroto Murase, Shigeyuki Kawahara and Tsutomu Tamura for their kindness in critically reading the manuscript and invariable comments for the manuscript. We are grateful to officers and crew of the R.V. Kaiyo-maru, Fisheries Agency, for their devoted assistance with the collection of samples. Thanks are also due to Yuko Watanabe and Kazuyuki Korenaga, for their kind help in collecting and sorting samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taki, K., Yabuki, T., Noiri, Y. et al. Horizontal and vertical distribution and demography of euphausiids in the Ross Sea and its adjacent waters in 2004/2005. Polar Biol 31, 1343–1356 (2008). https://doi.org/10.1007/s00300-008-0472-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-008-0472-6