Abstract
Members of the HSP70 gene family comprising the inducible (HSP70) genes and GRP78 (glucose-regulated protein 78 kDa) were identified in an Antarctic sea star (Odontaster validus) and an Antarctic gammarid (Paraceradocus gibber). These genes were surveyed for expression levels via Q-PCR after an acute 2-hour heat shock experiment in both animals and a time course assay in O. validus. No significant up-regulation was detected for any of the genes in either of the animals during the acute heat shock. The time course experiment in O. validus produced slightly different results with an initial down regulation in these genes at 2°C, but no significant up-regulation of the genes either at 2 or 6°C. Therefore, the classical heat shock response is absent in both species. The data is discussed in the context of the organisms’ thermal tolerance and the applicability of HSP70 to monitor thermal stress in Antarctic marine organisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The production of heat shock proteins is one of the classical cellular responses of all organisms to environmental insult. These highly conserved proteins act as chaperones to stabilise and refold denatured proteins, preventing the formation of cytotoxic aggregates, maintaining the integrity and functioning of the cell until conditions improve (Parsell and Lindquist 1993; Hartl 1996; Fink 1999). The most studied of these chaperone molecules are the 70 kDa heat shock protein family (HSP70s) comprising constitutive forms (HSC70: heat shock cognate 70) and stress inducible forms (HSP70s: heat shock protein 70) (Ritossa 1962; Morimoto et al. 1998). Their action has been described in response to a wide variety of stresses, however, the classical activation of this family is in response to elevated environmental temperatures (cf. Lund et al. 2002; Tomanek and Sanford 2003). Indeed they are often suggested as primary ecological biomarkers (Feder and Hofmann 1999; Sorensen et al. 2003).
One of the regions of most rapid climate change on Earth is the Antarctic Peninsula, where shallow seawater temperatures along the west Antarctic Peninsula have risen in excess of 1°C over the last 50 years (Meredith and King 2005). This marine environment is also the habitat of some of the Worlds most stenothermal species described to date (Peck and Conway 2000). Although Antarctic invertebrates have survivable temperature envelopes between 5 and 12°C above the minimum sea temperature of −2°C (Peck 2002), they start to lose critical biological functions at temperatures 2–3°C higher than current summer maxima (Peck et al. 2004). The question posed is how do we monitor the effects of heat stress in such organisms and are heat shock proteins the most appropriate biomarker for environmental stress in the Antarctic?
The latter is particularly pertinent, given that with the exception of Hydra oligactis (Bosch et al. 1988), the only species that have been shown to lack the classical heat shock response are an Antarctic ciliate (La Terza et al. 2001, 2004) and two Antarctic Notothenioid fish (Carpenter and Hofmann 2000; Hofmann et al. 2000; Place and Hofmann 2005; Clark et al. 2007). In addition two Antarctic molluscs Laternula elliptica (bivalve) and Nacella concinna (gastropod) have a demonstrable heat shock response, but at threshold levels (10–15°C) that are incompatible with moderating any deleterious effects from elevated Polar sea water temperatures (Clark et al. 2008a). The example of N. concinna is slightly more complex, in that it occurs inter-tidally and HSP70 genes are induced in response to emersion, a situation with a more complex suite of stressors than purely temperature (Clark et al. 2008b). In a continuing survey into the HSP70 response and thermal stress in Antarctic marine species, we expanded the phylogenetic range of species to include a gamarid crustacean, Paraceradocus gibber and an echinoderm, Odontaster validus, two sub-tidal species that seasonally experience a temperature window restricted to between −1.86 and +1°C (from the Rothera Time Series long term data set, provided by Professor A. Clarke).
P. gibber is the largest Antarctic amphipod. It lives under stones in burrows in the sediment and is predominantly a detrital feeder (Coleman 1989). O. validus is ubiquitous and abundant in Antarctic waters with a circum-polar distribution (McClintock et al. 1988). Although it is known as a predatory scavenger, it also gets much of its energy from detrital feeding (Pearse 1965). So these are both species that are positioned towards the top of the Antarctic marine food web and have different lifestyles to those Antarctic species already studied for their HSP response.
In this study members of the HSP70 gene family comprising inducible (HSP70) members and GRP78, a related HSP70 family member were cloned using degenerate PCR from P. gibber and O. validus. The expression of these genes was surveyed via Q-PCR after an acute heat shock experiment in both organisms, and an additional time course experiment in O. validus. The data is discussed in the context of the organisms’ evolutionary adaptation to life in an extreme environment, thermal tolerance and the applicability of HSP70 to monitor thermal stress in Antarctic marine organisms.
Methods
Animal sampling and experimental work
All Odontaster validus (sea star) and Paracerodocus gibber (amphipod) used in experimental work were collected at Rothera Research Station, Adelaide Island, Antarctic Peninsula (67°07″34′S, 68°30″07′W) by SCUBA divers during the austral summer and returned to the British Antarctic Survey’s aquarium in Cambridge, UK. The animals were maintained throughout in a re-circulating seawater aquarium at a mean water temperature of −0.7°C and a 12:12 light:dark cycle.
O. validus and P. gibber were exposed to 10°C (9.96 ± 0.05°C) and 15°C (14.85 ± 0.05°C) for 2 h (n = 5/species). Animals were directly transferred from −0.7°C to the elevated temperatures without an acclimation period. Separate control groups (n = 5/species) of O. validus and P. gibber were also sampled from the aquarium. O. validus was also subjected to two time course experiments at 20°C (2.0 ± 0.05°C) and 60°C (6.0 ± 0.02°C) for periods of 2, 6, 12, 24, 48 and 168 h each (n = 4 at each time period).
Sample analysis
RNA extraction and isolation of heat shock protein (HSP) genes
Total RNA was extracted from whole body (P. gibber) and gonad (O. validus) using TRI Reagent (Sigma) according to the manufacturer’s instructions. One microgram of total RNA was DNAse treated using 0.4 U DNase I (Ambion) in 10 mM DTT/100 mM MgCl2 buffer and reverse transcribed using a first strand synthesis kit (Promega). Degenerate primers for HSP70 were designed from a protein alignment of HSP70 genes from a variety of species as described in Clark et al. (2008a) (Table 1). PCR cycling conditions were as follows: 95°C 5 min, 35 cycles of 95°C 20 s, 45°C 20 s and 72°C for 40 s with a final elongation step of 72°C for 5 min with 40 cycles. Products were subcloned into p-GEMT-easy (Promega), transformed into E. coli strain XL-2 Blue MRF’ (Stratagene) and a minimum of 48 clones sequenced from each species. Sequence data was assembled using the phred, Phrap and consed packages (Ewing et al. 1998; Gordon et al. 1998). Consensus sequences were database searched using WU-blast2 (WU-blastx) (Altschul et al. 1997) against Uniprot (Boeckmann et al. 2003; Wu et al. 2006) to assign their HSP identity. The nucleotide sequences were aligned using Clustal W (Thompson et al. 1994) and specific primers designed to each different member of the HSP family for each species (Table 1), all with an annealing temperature of 60°C. Amplified fragment sizes varied between 86 and 146 bp. The specificity of each of the primers was checked, by amplification and sequencing of the products.
Isolation of β actin genes
For comparative analysis to be made between the different HSP genes, a housekeeping sequence (β actin) was isolated from both species. Degenerate primers were designed from a ClustalW alignment of a number of β actin genes as described in Clark et al. (2008a) (Table 1). The PCR conditions were as follows: 95°C 5 min, 40 cycles of 95°C 20 s, 45°C 20 s and 72°C for 40 s with a final elongation step of 72°C for 5 min. PCR products were sequenced, assembled and checked as described above for the HSP genes. Where multiple β actin fragments were amplified from the same organism, primers were designed to regions of identity between the different family members. Primers were designed to anneal at 60°C. Expression levels of β actin between different tissues and different treatment states were checked to ensure constant expression and reproducibility.
The HSP sequences described in this paper have been submitted to the EMBL database with accession numbers AM408048 (O. validus, HSP70), AM408049 (O. validus, GRP78), AM408050 (P. gibber, HSP70A), AM408051 (P. gibber, HSP70B), AM408052 (P. gibber, HSP70C) and AM408053 (P. gibber, HSC70).
Q PCR
HSP and actin sequences were amplified from each organism under each treatment condition using specific primers, Brilliant SYBR® Green QPCR Master Mix (Stratagene) and an MX3000P Q-PCR machine (Stratagene). PCR conditions were as follows: 95°C 10 min, 40 cycles of 95°C 30 s, 60°C 1 min and 72°C for 1 min with a final dissociation curve step as per manufacturers recommendations. The plate set-up for each Q-PCR experiment consisted of five control individuals and five experimental (“treated”) individuals, both sets were amplified with a specific HSP primer pair and an actin control primer set. All amplifications were reproduced in triplicate. Each primer set was checked to ensure that no primer dimers were produced during the course of the amplification reaction. RSq values and PCR efficiencies were checked over a four fold 10× dilution series and the values calculated using the MxPro-MX3000P v 3.00 Build 311 Schema 74 software (Table 2). Primers producing low RSq values were discarded and new primers designed. Amplifications were analysed using the MxPro-MX3000P v 3.00 Build 311 Schema 74 software and Ct (dR) values exported into Excel.
Relative expression ratios of the HSP genes compared to the actin housekeeping genes between the control and treated samples were derived using the relative expression software tool (REST) (http://www.gene-quantification.info/) (Pfaffl 2001; Pfaffl et al. 2002). This is an excel macro that incorporates both a mathematical model to calculate relative expression ratios on the basis of the PCR efficiency and crossing point derivation of the investigated samples and a two sided pair wise fixed reallocation randomisation test. This test makes no assumptions about distribution (such as normality of distribution) and assumes that treatments were randomly allocated. The randomisation test repeatedly and randomly reallocates the observed values to the two groups and notes the apparent effect (expression ratio). The proportion of these effects, which are as great as that actually observed in the experiment provides the P value of the test. Two thousand randomisations were used in the test (Pfaffl 2001; Pfaffl et al. 2002). These results were then followed by further statistical analysis (MINITAB v 14) using Fisher’s test for combining probabilities (Fisher 1954) and two-way ANOVA tests were run on the time course dataset to identify any effect of either gene, temperature or time.
Results
Four different HSP gene family members were cloned from both P. gibber and O. validus. These were defined according to their sequence similarity scores after searching the sequence databases using WU-blastx and comprised three inducible HSP70s and HSC70 (P. gibber) and one inducible HSP70 and GRP78 (O. validus) (Table 1). Specific primers for Q-PCR were successfully designed to all clones (Table 2). A further two clones were isolated from O. validus for another inducible form of HSP70 and also HSC70, however, in spite of repeatedly testing different primers for these clones (30 potential primer combinations for each) none worked effectively either in terms of amplification, reproducibility or producing a linear relationship in a dilution series and they were excluded from the analysis. To obtain a crude estimate of the relative expression levels of each of the HSP genes in each organism the genes were assayed using PCR and gel electrophoresis with ethidium bromide staining in a set of control animals (data not shown). Actin expression, as expected, produced a strong signal in each species, but there was no discernable expression of the HSP family members either in P. gibber or O. validus.
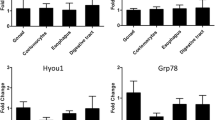
The acute heat shock experiments at 10 and 15°C showed no significant up regulation of HSP genes in either species (Fig. 1). Application of Fisher’s method for combining probabilities on each of the two species datasets is consistent with no detectable effect of temperature on HSP gene expression (for values see figure legend).
Table shows Q-PCR results for P. gibber and O. validus after an acute 10 and 15°C temperature heat shock. Absolute fold change in gene expression level is given, alongside REST calculated change in gene expression levels. Graph depicts tabulated results. Fisher’s method for combining probabilities: chi squared test on P. gibber dataset: F = 9.727, df = 16, P = 0.119; chi squared test on O. validus dataset: F = 4.458, df = 8, P = 0.186
In the O. validus time course experiment, at 2°C both GRP78 and HSP70 were down regulated. With both genes there was a trend showing an initial decrease in relative expression levels (−40-fold with GRP78 and −6.9-fold with HSP70), which over the period of the time course gradually returned to levels approaching those of the controls (Fig. 2). The 6°C time course experiment showed a different pattern with a non-significant level of up-regulation (cf. individual P values) in relative gene expression level for both genes. Two-way ANOVA testing on the combined 2 and 6°C dataset identified an effect of both temperature and time (temperature: df = 1, F = 63.84, P = 0.000; time: df = 5, F = 4.34, P = 0.017), but no effect of the gene when tested against the variables of time and temperature (data not shown).
Discussion
Neither P. gibber nor O. validus showed any significant up-regulation of heat shock protein activity in the acute temperature challenges above the level of individual variation/experimental noise for any of the family members (Fig. 1). Whilst these are temperatures far in excess of those the animals would experience in the Antarctic marine environment, previous laboratory based experiments on L. elliptica and sub-tidal N. concinna have demonstrated the classical heat shock response at 10°C (L. elliptica) and 15°C (N. concinna) (Clark et al. 2008a). Hence there are Antarctic marine organisms that still possess the ability to up-regulate their HSP genes. Whether these genes are utilised in their natural environment, or the threshold level for induction is a remnant of a temperate ancestor remains to be determined and may become more apparent as a greater number of species are surveyed. One such example of related work on inter-tidal N. concinna has demonstrated the up-regulation of inducible forms of HSP70 (albeit to much lower levels than the laboratory experiments) in response to emersion (Clark et al. 2008b). Clearly the stresses imposed on inter-tidal animals are more complex, but this data does show that HSP70 genes, at least in N. concinna are induced by stresses in the natural environment and not merely a vestigial attribute from a phylogenetic history. That may be the situation with P. gibber and O. validus in that they have maintained a suite of HSP70 genes, but they may be induced by different cellular perturbations. Although only one inducible form of HSP70 was surveyed in O. validus [sequence data indicated that other forms were present (Clark, unpublished)], results from the bivalve, L. elliptica and N. concinna, limpets, show that heat stress reproducibly induced all forms of HSP70 in the latter species (Clark et al. 2008a). Therefore, results from a single inducible HSP70, in this experimental context, are potentially indicative of the generalised expression of multiple isoforms, where they exist. However, validation will always required to define subtle differences in control between paralogous genes.
In the other main example of lack of a classical heat shock response, the Antarctic Notothenioids, have maintained HSP70 genes, but their expression mode has been altered from inducible to constitutive (Carpenter and Hofmann 2000; Hofmann et al. 2000; Place et al. 2004; Place and Hofmann 2005; Clark et al. 2007), unless like the limpets, these chaperones are induced by other environmental stresses. A PCR-based survey of the HSP70 genes in control animals of P. gibber and O. validus did not show constitutive expression of any of the genes under study. This is in contrast to the work on the Antarctic Notothenioids and the molluscs, where there is considerable constitutive expression of HSP70, HSC70 and GRP78 (Place and Hofmann 2005; Clark et al. 2007, 2008a). This is thought to be due to an enhanced requirement for chaperone proteins to help with the problems of protein folding at low temperatures (Place et al. 2004). This requirement would not necessarily appear to be present in all species, but again requires further investigation.
The results of the O. validus time course experiment are more complex (Fig. 2). Again, the individual P values show no significance at the 95% confidence interval. Although there would appear to be a trend in the 2°C experiment for an initial down-regulation of both genes, followed by a gradual return to “normal” levels over the period of the week. This trend is validated by the ANOVA analysis, showing a combined effect of time and temperature. The shape of the 2°C graphs mirror the results of a 6°C 48 h time course experiment in Harpagifer antarcticus for both HSP70 gene family members and the warm acclimated protein (WAP65) (Clark et al. 2007; Clark and Burns 2007). It has been proposed that initial down-regulation of certain genes is due to the initial “shock” response followed by a return to equilibrium as these animals acclimate. Certainly Antarctic fish have been acclimated to +4°C (Carpenter and Hofmann 2000; Lowe and Davison 2005; Jin and deVries 2006; Podrabsky and Somero 2006) and H. antarcticus has spent several months at 3°C in the Rothera aquarium (Clark, unpublished). O. validus has also been successfully acclimated to +6°C (Peck et al. 2008) and experiments on larvae indicate a certain level of temperature tolerance (Stanwell-Smith and Peck 1998; Peck and Prothero-Thomas 2002), indicating that the fish and the sea star are some of the more robust Antarctic stenotherms. Why the shape of the 2°C graphs are not mirrored in the 6°C experiments (with a lack of initial down regulation) is unknown. Even with this experiment, the longer timescale and higher temperature does not invoke significant HSP expression, but given the data of where oxygen consumption and feeding in O. validus are not affected by 25 days at 6°C, this is perhaps not surprising.
These two species (P. gibber and O. validus) expand the previous surveys into the heat shock response of Antarctic marine organisms. The more species surveyed, the more complex the picture. There is now data on several fish species (Place and Hofmann 2005; Clark et al. 2007), a filter feeding bivalve mollusc and a grazing gastropod mollusc (Clark et al. 2008a). We have now added to this with data for a detritivore and a carnivore/detritivore.
This encompasses a wide phylogenetic range, different feeding guilds and variety in lifestyle. Not all species show permanent expression of inducible HSPs, not all species show the classical heat shock response and in at least one case, multiple stressors can invoke HSPs at temperatures lower than that tested in the laboratory. This combined data shows that it will not be a simple task to derive generalisations about the ability of Antarctic marine organisms to invoke the classic cellular first aid kit of HSP70 genes in response to elevated sea water temperatures or indeed the utility of HSPs as a biomarker for stress in Antarctic waters. The results of the gene expression level surveys also demonstrate that for a molecular biomarker (Antarctic or otherwise) to be effective, the change in gene expression has to be large. The one-off sampling regime for “before” and “after” effects and the high inter-individual variability of non-interbred, non-model organisms produces wide 95% confidence intervals with Q-PCR experiments (discussed in more detail in Clark et al. 2007). Therefore, biomarker gene expression levels must show a strong signal above background variation in order for valid conclusions to be drawn about perturbation effects. This provides problems when evaluating field populations of non-model organisms when gene expression changes are moderate or small. In conclusion, the data presented here show that the HSP response in the Antarctic has to be investigated on a species-specific basis.
Wider surveys are required to elucidate the most common response patterns, along with investigation of the potential for different types of environmental perturbation to invoke HSP expression both in the laboratory and wild populations. Also given these limitations, a wider range of genes should be investigated and EST libraries are currently in production within our laboratory for a number of Antarctic invertebrates with the aim of targeting genes involved in the reaction to heat stress.
References
Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Boeckmann B, Bairoch A, Apweiler R, Blatter MC, Estreicher A, Gasteiger E, Martin MJ, Michoud K, O’Donovan C, Phan I, Pilbout S, Schneider M (2003) The SWISS-PROT protein knowledgebase and its supplement TrEMBL in 2003. Nucleic Acids Res 31:365–370
Bosch TCG, Krylow SM, Bode HR, Steele RE (1988) Thermotolerance and synthesis of heat-shock proteins—these responses are present in hydra-attenuata but absent in hydra-oligactis. Proc Natl Acad Sci USA 85:7927–7931
Carpenter CM, Hofmann GE (2000) Expression of 70 kDa heat shock proteins in Antarctic and New Zealand Notothenioid fish. Comp Biochem Physiol A 125:229–238
Clark MS, Burns G (2007) Characterisation of the warm acclimated protein gene (wap65) in the Antarctic plunderfish (Harpagifer antarcticus). DNA Seq. doi:10.1080/10425170701388586
Clark MS, Fraser KPPF, Burns G, Peck LS (2007) The HSP70 heat shock response in the Antarctic fish Harpagifer antarcticus. Polar Biol 31:171–180. doi:10.1007/s00300-007-0344-5
Clark MS, Fraser KPPF, Peck LS (2008a) The HSP70 heat shock response in Antarctic marine molluscs. Cell Stress Chaperones 13. doi:10.1007/s12192-008-0014-8
Clark MS, Geissler P, Waller C, Fraser KPPF, Barnes DKA, Peck LS (2008b) The environmental HSP70 heat shock response in the Antarctic limpet: Nacella concinna. Cell Stress Chaperones 13. doi:10.1007/s12192-008-0015-7
Coleman CO (1989) Burrowing, grooming and feeding behaviour of Paraceradocus, an Antarctic amphipod genus (Crustacea). Polar Biol 10:43–48
Ewing B, Hillier L, Wendl MC, Green P (1998) Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 8:175–185
Feder ME, Hofmann GE (1999) Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Ann Rev Physiol 61:243–282
Fink AL (1999) Chaperone-mediated protein folding. Physiol Rev 79:425–449
Fisher RA (1954) Statistical methods for research workers. Pub. Oliver and Boyd, Edinburgh
Gordon D, Abajian C, Green P (1998) Consed: a graphical tool for sequence finishing. Genome Res 8:195–202
Hartl FU (1996) Molecular chaperones in cellular protein folding. Nature 381:571–580
Hofmann GE, Buckley BA, Airaksinen S, Keen JE, Somero GN (2000) The Antarctic fish Trematomus bernachii lacks heat inducible heat shock protein synthesis. J Exp Biol 203:2331–2339
Jin Y, deVries AL (2006) Antifreeze glycoprotein levels in Antarctic Notothenioid fishes inhabiting different thermal environments and the effect of warm acclimation. Comp Biochem Physiol B 144:290–300
LaTerza AL, Miceli C, Luporine P (2001) Divergenec between two Antarctic species of the ciliate Euplotes, E. focardii and E. nobilii, in the expression of heat-shock protein 70 genes. Mol Ecol 10:1061–1067
LaTerza AL, Miceli C, Luporine P (2004) The gene for the heat-shock protein 70 of Euplotes focardii, an Antarctic psychrophilic ciliate. Antarct Sci 16:23–28
Lowe CJ, Davison W (2005) Plasma osmolarity, glucose concentration and erythrocyte responses of two Antarctic Notothenioid fishes to acute and chronic thermal change. J Fish Biol 67:752–766
Lund SG, Caissie D, Cunjak RA, Vijayan MM, Tufts BL (2002) The effects of environmental heat stress on heat-shock mRNA and protein expression in Miramichi Atlantic salmon (Salmo salar) parr. Can J Fish Aquat Sci 59:1553–1562
McClintock JB, Pearse JS, Bosch I (1988) Population structures and energetics of the shallow water Antarctic sea star Odontaster validus in contrasting habitats. Mar Biol 99:235–246
Meredith MP, King JC (2005) Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys Lett 32:L19604–L19609
Morimoto RI (1998) Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev 12:3788–3796
Parsell DA, Lindquist S (1993) The function of heat-shock proteins in stress tolerance—degradation and reactivation of damaged proteins. Ann Rev Genet 27:437–496
Pearse J (1965) Reproductive periodicities in several contrasting populations of Odontaster validus Koehler, a common Antarctic asteroid. Antarct Res Ser 5:39–85
Peck LS (2002) Ecophysiology of Antarctic marine ectotherms: limits to life. Polar Biol 25:31–40
Peck LS, Conway LZ (2000) The myth of metabolic cold adaptation: oxygen consumption in stenothermal Antarctic bivalve molluscs. In: Harper E, Crame AJ (eds) Evolutionary biology of the bivalvia. Geological Society of London Special Publication 177. Cambridge University Press, Cambridge, pp 441–450
Peck LS, Prothero-Thomas E (2002) Temperature effects on the metabolism of larvae of the Antarctic starfish Odontaster validus, using a novel microrespirometry method. Mar Biol 141:271–276
Peck LS, Webb KE, Bailey DM (2004) Extreme sensitivity of biological function to temperature in Antarctic marine species. Funct Ecol 18:625–630
Peck LS, Webb KE, Miller A, Clark MS, Hill T (2008) Temperature limits to activity, feeding and metabolism in the Antarctic starfish Odontaster validus. MEPS (in press)
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2002–2007
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:1–10
Place SP, Hofmann GE (2005) Constitutive expression of a stress-inducible heat shock protein gene, hsp70, in a phylogenetically distant Antarctic fish. Polar Biol 28:261–267
Place SP, Zippay ML, Hofmann GE (2004) Constitutive roles for inducible genes: evidence for the alteration in expression of the inducible hsp70 gene in Antarctic Notothenioid fishes. Am J Physiol Regul Integr Comp Physiol 287:R429–R436
Podrabsky JE, Somero GN (2006) Inducible heat tolerance in Antarctic Nothothenioid fishes. Polar Biol 30:39–43
Ritossa F (1962) A new puffing pattern induced by temperature shock and DNP in Drosophila. Experimentia 18:571–573
Sorensen JG, Kristensen TN, Loeschcke V (2003) The evolutionary and ecological role of heat shock proteins. Ecol Lett 6:1025–1037
Stanwell-Smith D, Peck LS (1998) Temperature and embryonic development in relation to spawning and field occurance of larvae of three Antarctic echinoderms. Biol Bull 194:44–52
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL-W—improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tomanek L, Sanford E (2003) Heat-shock protein 70 (Hsp70) as a biochemical stress indicator: an experimental field test in two congeneric intertidal gastropods (Genus: Tegula). Biol Bull 205:276–284
Wu CH, Apweiler R, Bairoch A, Natale DA, Barker WC, Boeckmann B, Ferro S, Gasteiger E, Huang H, Lopez R, Magrane M, Martin MJ, Mazumder R, O’Donovan C, Redaschi N, Suzek B (2006) The universal protein resource (UniProt): an expanding universe of protein information. Nucleic Acids Res 34:D187–D191
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clark, M.S., Fraser, K.P.P. & Peck, L.S. Lack of an HSP70 heat shock response in two Antarctic marine invertebrates. Polar Biol 31, 1059–1065 (2008). https://doi.org/10.1007/s00300-008-0447-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-008-0447-7