Abstract
Members of the HSP70 gene family comprising the constitutive (HSC70) and inducible (HSP70) genes, plus GRP78 (Glucose-regulated protein 78 kDa) were surveyed for expression levels via Q-PCR after both an acute 2-h heat shock experiment and a time course assay in the Antarctic plunderfish Harpagifer antarcticus. In general, down regulation of all genes was observed during the course of the heat shock experiments. This thermally induced down regulation was particularly acute for the GRP78 gene, which at one time point was more than 100-fold down regulated. These results demonstrate the loss of the heat shock response in H. antarcticus, a basal member of the Notothenioidei. This finding is discussed with reference to the survival of Notothenioids during observed ocean warming and also the reorganisation of cellular protein mechanisms of species living in extreme environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability of an organism to survive environmental perturbation is reliant upon a whole series of species-specific mechanisms. These may be physical barriers, such as impermeable exoskeletons, through physiological adaptation to molecular and biochemical responses. All have evolved in tandem with the niche adaptation of the species and enable survival within constrained environmental limits. So far, the best-characterised transcriptional stress response is that of elevated production of heat shock proteins (Gross 2004). These are a family of highly conserved proteins, which act as chaperones to stabilise and refold denatured proteins, preventing the formation of cytotoxic aggregates (Parsell and Lindquist 1993; Hartl 1996; Fink 1999). Numerous families of heat shock proteins have been identified, the naming of which is related to their weight in kiloDaltons. The most studied of these family members is the 70 kDa heat shock proteins (HSP70s) (Ritossa 1962), comprising constitutive forms (HSC70: heat shock cognate 70) and stress inducible family members (HSP70s: heat shock protein 70) (reviewed in Morimoto 1998). Whilst their action has been described in response to a wide variety of stresses, the classical activation of the HSP70 genes is in response to elevated environmental temperatures.
The induction of these genes does come with a caveat, in that HSP70 gene expression is highly plastic. Levels of induction are influenced by thermal history such as seasonal temperature cycling, vertical zonation and biogeography (Somero 2002; Hofmann 2005). This heat shock plasticity phenomenon is well characterised in eurythermal organisms, but has also lead to great interest in the response of Antarctic marine organisms, which have more restricted thermal limits. These animals are highly stenothermal having survivable temperature envelopes between 5 and 12°C above the minimum sea temperature of −1.86°C (Somero and DeVries 1967; Peck and Conway 2000; Peck 2002). This range of upper lethal/critical temperatures was determined experimentally, as the organisms live almost permanently within a 4°C temperature envelope. Data from the RaTS (Rothera Time course Series) Long Term Monitoring Programme shows that the waters around the Antarctic Peninsula vary between a minimum of −1.8°C in the winter to a maximum of +1.7°C in the height of the austral summer (data provided by Professor Andrew Clarke). Shallow seawater temperatures along the west Antarctic Peninsula have risen in excess of 1°C over the last 50 years (Meredith and King 2005). While the IPCC Third Assessment climate model predicts a further 2°C increase in global seawater temperatures over the next 100 years, albeit with large regional variations and confidence intervals. In light of current and predicted seawater temperature increases Antarctic stenotherms are therefore at considerable future risk of seasonal exposure to ambient water temperatures that exceed those known to result in the loss of critical biological functions (Peck et al. 2004).
Given what was known about heat shock protein induction in eurythermal fish, the initial prediction for the heat shock induction temperature of Antarctic marine organisms was close to the maximum environmental temperature. Surprisingly, it was found that members of the Antarctic Nototheniidae family completely lacked the classic heat shock response (up-regulation of HSP70 expression) (Place and Hofmann 2005). Although two other animal species to date have been shown to lack this response Hydra oligactis (Bosch et al. 1988) and an Antarctic ciliate Euplotes focardii (LaTerza et al. 2001, 2004), the fish situation is complex. The inducible form of HSP70 is permanently expressed (Place et al. 2004). It is thought that permanent expression of chaperone proteins is an adaptation to survival in the ice-laden Southern Ocean and the consequential biological problems of inefficient protein folding at such low temperatures (−1.86°C) (Place et al. 2004). This observation is substantiated to a certain extent by the constitutive expression of HSP70 in the distantly related Antarctic fish Lycodichthys dearborni (Fig. 1). However, this zoarcid can still up-regulate HSP70 expression when exposed to 4°C (Place and Hofmann 2005).
Schematic diagram showing relatedness of H. antarcticus and other Antarctic Perciformes, which have been studied for their heat shock response (adapted from Near et al. 2004), previous data taken from Place and Hofmann (2005). The New Zealand notothenioids investigated in Hofmann et al. (2005) have also been annotated. The plus and minus after the fish names indicates whether they exhibit a heat shock response or not, respectively
Recent data (Clark et al. 2007) on the heat shock response of Antarctic marine molluscs has shown that the classical heat shock response is significantly and reproducibly initiated in both Nacella concinna and Laternula elliptica at 15°C. This is a temperature far in excess of that which could be experienced by either of these molluscs or indeed other Antarctic marine organisms even under the most extreme predictions of elevated seawater temperatures as a result of global climate change predictions. Previous investigations of Antarctic fish species’ heat shock response have only taken the animals to a maximum of 10°C (Hofmann et al. 2000).
The questions then arise as to whether up-regulation of HSP70 can occur in Antarctic fish at an elevated temperature of 15°C? Whether constitutive expression of HSP70 is a general phenomenon across all Antarctic fish species? Is the inability to up-regulate HSP70 in response to heat specific only to the Nototheniidae family or is this phenomenon present more widely in the infraorder Notothenioidei?
In this study three members of the HSP70 gene family comprising the constitutive (HSC70) and inducible (HSP70) members, plus GRP78 (Glucose-regulated protein, 78 kDa) a related HSP70 family member were cloned using degenerate PCR from the Antarctic plunderfish Harpagifer antarcticus (family: Harpagiferidae). The expression of the HSP70 family members was surveyed via Q-PCR after an acute 2-h heat shock experiment at both 10 and 15°C and also a time course experiment at 6°C. The data are discussed in the context of general cellular metabolic constraints under stressful conditions.
Methods
Animal sampling and experimental work
All H. antarcticus used in the experimental work were collected at Rothera Research Station, Adelaide Island, Antarctic Peninsula (67°07′′34′S, 68°07′′30′W) by SCUBA divers during the austral summer. The fish were collected and immediately returned to the laboratory where they were maintained in a through-flow aquarium under a simulated natural light:dark cycle. Predicted sunrise and sunset times (POLTIPS 3, Proudman Oceanographic Laboratory) were used in conjunction with a mechanical timer to control the lighting regime. During the time the animals were held in the aquarium the water temperature was 0.75 ± 0.00°C. H. antarcticus were fed chopped white fish muscle (Notothenia coriiceps) twice per week to satiation.
Acute heat shock experiments
Animals were maintained for 5–7 days in the aquarium prior to experimental work. Groups of five animals were transferred to the experimental tanks. The fish were exposed to a thermal shock, by immediate transfer to seawater maintained at a range of temperatures −0.5°C (±0.07°C) (control), 10.0°C (10.16 ± 0.07°C) and 15°C (±0.1°C) for 2 h. After the 2 h thermal shock the animals were killed according to approved Home Office protocols, weighed (±0.1 g), measured (±0.1 mm) and tissues collected and placed in RNA-Later (Ambion, USA) for subsequent analysis. About 200 mg samples of white muscle, liver and digestive tract were collected from each fish.
Time course heat shock experiments
H. antarcticus were returned to the UK in a refrigerated transport aquarium and maintained in a recirculating aquarium at close to 0°C until required for experimental work. Animals were fed twice per week on chopped white fish or krill to satiation and maintained under a light:dark period of 12:12 h. Thirty-five fish were transferred to the experimental tank at time zero and maintained at 6.0 ± 0.08°C. Five animals were killed according to approved Home Office protocols at zero, 2, 4, 8, 12, 24 and 48 h and 200 mg of liver removed and placed in RNALater for subsequent analysis. Animals were measured and weighed as described previously.
Sample analysis
RNA extraction and isolation of heat shock protein (HSP) genes
Total RNA was extracted from liver, white muscle and digestive tract using TRI Reagent (Sigma) according to the manufacturer’s instructions. One microgram of total RNA was DNase treated using 0.4 U DNase I (Ambion) in 10 mM DTT/100 mM MgCl2 buffer and reverse transcribed using a first strand synthesis kit (Promega). Degenerate primers for HSP70 were designed from a protein alignment of HSP70 genes from a variety of species (H. sapiens to molluscs) and amplified a 500 bp fragment comprising amino acids 30–125 (motifs used for primers: IIANDQGD and TVPAYFNN) (Table 1). PCR cycling conditions were as follows: 95°C 5 min, 35 cycles of 95°C 20 s, 60°C 20 s and 72°C for 40 s with a final elongation step of 72°C for 5 min. Products were subcloned into p-GEMT-easy (Promega), transformed into E. coli strain XL-2 Blue MRF’ (Stratagene) and a minimum of 48 clones sequenced. Sequence data was assembled using the phred, Phrap and consed packages (Ewing et al. 1998; Gordon et al. 1998). Consensus sequences were database searched using WU-blast2 (WU-blastx) (Altschul et al. 1997) against Uniprot (Boeckmann et al. 2003; Wu et al. 2006) to assign their HSP identity. The nucleotide sequences were aligned using Clustal W (Thompson et al. 1994) and specific primers designed for each different member of the HSP family, all with an annealing temperature of 60°C. Amplified fragment sizes varied between 143 and 145 bp. The specificity of each of the primers was checked, by amplification and sequencing of the products.
Isolation of β actin genes
To allow for a comparative analysis to be made between the different HSP genes, a housekeeping sequence β actin was isolated from H. antarcticus. Primers previously designed to the Takifugu rubripes β actin gene family (Venkatesh et al. 1996) (Table 1) were used to amplify 500 bp heterologous sequences from H. antarcticus using the following PCR conditions: 95°C 5 min, 35 cycles of 95°C 20 s, 60°C 20 s and 72°C for 40 s with a final elongation step of 72°C for 5 min. PCR products were sequenced, assembled and checked as described above for the HSP genes. Fragments of two members of the β actin gene family were cloned, but primers were designed to the predominating clone (86% of clones). Primers were designed to anneal at 60°C. Expression levels of β actin between different tissues and different treatment states were checked to ensure constant expression and reproducibility. Sequences of all primers are listed in Table 1. All HSP sequence fragments have been submitted to the EMBL database with the accession numbers AM293602–AM293604 inclusive.
Sequence analysis
Sequence manipulation and analysis was performed using the EMBOSS suite of open source software (Rice et al. 2000) http://www.emboss.sourceforge.net.
Q-PCR
HSP and actin sequences were amplified under each treatment condition using specific primers, Brilliant SYBR® Green QPCR Master Mix (Stratagene) and an MX3000P (Stratagene). PCR conditions were as follows: 95°C 10 min, 40 cycles of 95°C 30 s, 60°C 1 min and 72°C for 1 min with a final dissociation curve step as per manufacturers recommendations. The plate set-up for each Q-PCR experiment consisted of five control individuals and five experimental (“treated”) individuals amplified with a specific HSP primer set (designated Expt 1) and an actin control primer set (designated Normaliser). Actin was used as the housekeeping reference sequence as it had previously been shown not to change under the experimental conditions used (data unpublished). Each HSP and actin amplification was performed in triplicate. Each primer set was checked to ensure that no primer dimers were produced during the course of the amplification reaction. RSq values and PCR efficiencies were checked over a fourfold 10× dilution series and the values calculated using the MxPro-MX3000P v 3.00 Build 311 Schema 74 software (Table 1). Primers producing low RSq values were discarded and new primers designed. Amplifications were analysed using the MxPro-MX3000P v 3.00 Build 311 Schema 74 software and Ct (dR) values (threshold cycle of the baseline subtracted fluorescent reading) exported into Excel. The differences in Ct (dR) scores for the control actin and HSP amplifications and also for the treated actin and HSP amplifications were derived and recalculated to include the PCR efficiency. These results were subjected to a two-sample t test using MINITAB v14 to determine the 95% confidence range. Relative expression ratios of the HSP and actin genes between the control and treated samples were derived using the Relative Expression Software tool (REST) (http://www.gene-quantification.info/), which incorporates the Pfaffl method and also uses a Pair Wise Fixed Reallocation Randomisation Test (Pfaffl 2001; Pfaffl et al. 2002). This Excel macro was also used to calculate the significance level of the fold changes. The data was then subjected to Fisher’s method for combining probabilities (Fisher 1954).
Results
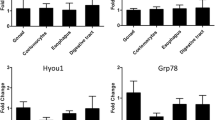
Three members of the HSP70 gene family were cloned and identified from H. antarcticus; the inducible form (HSP70), the constitutive form (HSC70) and GRP78 (Glucose-regulated protein, 78 kDa). These were initially defined according to their sequence similarity scores after searching the sequence databases using WU-BLASTx (Table 2). The designation of the inducible form was narrowed further to HSP70-2 by comparison to the two duplicated platyfish HSP70 genes (accession numbers: Uniprot: Q8UWN0 and Uniprot: Q8UMM9). The H. antarcticus sequence fragment showed 86.2% identity to the platyfish HSP70-2 gene, with a lower level of identity (80.7%) to the platyfish HSP70-1 gene. Only one member of the inducible form of HSP70 was cloned from H. antarcticus in spite of performing two additional sets of PCR amplifications from 10°C treated animals and several amplifications from genomic DNA (data not shown). To obtain a crude estimate of the relative expression levels of each of the HSP genes in H. antarcticus, the genes were assayed using standard PCR and gel electrophoresis in a set of control animals (data not shown). All three genes were permanently expressed in liver, white muscle and digestive tract with no real quantifiable tissue-specific expression. In the 10°C acute 2-h heat shock experiments, five out of the nine tissue sample/primer combinations showed down regulation of gene expression, these included both liver and digestive tract amplified using HSC70 primers and all of the GRP78 amplifications (Fig. 2). This result was mirrored in the 15°C acute heat shock experiments (Fig. 3). Up-regulation of gene expression was minimal (maximum 3.4-fold at 10°C and 5.97-fold at 15°C for HSP70) and well within the boundaries of experimental variation, when considering the 95% confidence interval ranges. Application of Fisher’s method for combining probabilities on each of the two datasets is consistent with no detectable effect of temperature on HSP gene expression (see figure legends for probability data). Over the period of the 6°C time course experiment all sample/primer combinations showed effective down regulation of gene expression. The general trend in the gene expression pattern was of a gradual decrease to a maximum down regulation level, followed by recovery. However, even after 48 h, expression levels of all genes under study had not returned to pre-experimental levels. The time point at which maximal down regulation occurred varied according to the gene: 8 h for HSC70, 12 h for HSP70 and 24 h for GRP78 (Fig. 4).
Table shows Q-PCR results for H. antarcticus with an acute 10°C temperature heat shock on three different tissues (liver, white muscle and digestive gland). Absolute fold change in gene expression level is given, alongside REST calculated updown regulation of gene expression level. P values calculated using a pair wise fixed reallocation randomisation test (Pfaffl 2001; Pfaffl et al. 2002) via the Relative Expression Software tool (REST) (http://www.gene-quantification.info/). Graph depicts tabulated results. Fisher’s method for combining probabilities: Chi-squared test on whole dataset: F = 0.449, df = 18, P = 1.00
Table shows Q-PCR results for H. antarcticus with an acute 15°C temperature heat shock on three different tissues (liver, white muscle and digestive gland). Change in gene expression levels calculated as defined in Fig. 2. Graph depicts tabulated results. Fisher’s method for combining probabilities: Chi-squared test on whole dataset: F = 21.576, df = 18, P = 0.25
Table shows Q-PCR results for H. antarcticus heat shock experiment at 6°C at 2, 6, 8, 12, 24 and 48 h time points. Data are presented only for liver. Column headings are as for Fig. 2. Graph depicts tabulated results. Fisher’s method for combining probabilities: Chi-squared test on whole dataset: F = 44.89, df = 36, P = 0.15
Discussion
This study details for the first time, the cloning and expression profiles of HSP70 gene family members from an Antarctic fish over both acute heat shock experiments and an elevated temperature time course. Two types of heat shock were used, the first of which was acute at both 10 and 15°C for 2 h. This experiment was carried out to mirror previous Antarctic fish heat shock experiments and provide a point of reference for the time course assay. Previously Antarctic fish have only been heat shocked to 10°C (Hofmann et al. 2000). Although the temperatures used are 8 and 13°C above their natural environmental temperature, data from Antarctic invertebrates (Clark et al. 2007) shows that these latter organisms possess a significant and reproducible heat shock response at 15°C. Whilst the heat shock response is an acute phenomenon that occurs within minutes of stress (Linquist 1986), it has also been previously documented that Antarctic fish may have a depressed/slowed stress response in terms of catecholamine synthesis levels and that cortisol may not be an important stress hormone in these species (Davison et al. 1995; Whitely and Egginton 1999). Hence they may have a slower stress response compared to that measured in temperate animals. Therefore a longer lower temperature heat shock (6°C) was carried out as a time course series of 2, 4, 8, 12, 24 and 48 h to ensure that a longer mRNA activation time may be detected. This temperature was chosen with reference to previous work on the upper lethal temperatures of Notothenioids (Somero and DeVries 1967).
Three HSP70 genes were isolated and nomenclature assigned using database sequence similarity searching. Only one inducible form of HSP70 was characterised from H. antarcticus. This is very interesting as evidence from other organisms suggests that there are multiple copies of this family member in most genomes surveyed to date (Voellmy et al. 1985; Hunt and Morimoto 1985; Ali et al. 1996; Milner and Campbell 1990). Studies in fish indicate that at least two HSP70 paralogues are present in each species (Yamishita et al. 2004), probably the result of a fish-specific gene whole genome duplication event (Amores et al. 1998), as the duplicated genes are present across four orders of fishes within the Euteleostei. The H. antarcticus gene corresponds to the HSP70-2 isoform. Previous work on Antarctic fish HSP70 gene expression has only indicated the presence of a single gene, albeit detection was via probe hybridization onto Northern blots and this technique would not necessarily be able to discriminate between very closely related paralogues (Place et al. 2004; Place and Hofmann 2005; Hofmann et al. 2005). Possession of additional members of the inducible HSP70 gene in H. antarcticus cannot be discounted, as they may be present in a very restricted tissue or developmental range. However, The identification of only a single H. antarcticus HSP70 gene so far, is not surprising given previous examples of gene loss in Antarctic fish (c.f. haemoglobin and functional erythrocytes in icefish (Cocca et al. 1995; di Prisco et al. 2002; Hureau et al. 1977; Barber et al. 1981) and the modification of expression patterns from temperate fish species (discussed below). But it does suggest a restriction in overall functional capacity, the reasons for which remain to be elucidated.
H. antarcticus displays both the constitutive expression of HSP70 and the absence of a reproducible classical heat shock response via up-regulation of the HSP70 genes in response to increased environmental temperatures as demonstrated previously in several other Antarctic fish species (Hofmann et al. 2000; Place and Hofmann 2005). To date the lack of up-regulation of HSP70 has only been demonstrated in the Antarctic Nototheniidae. The data presented here add another data point for Antarctic fish species extending this finding across three members of the Antarctic Notothenioidei (Fig. 1). This potentially indicates that the mutation resulting in the lack of HSP70 induction in the Antarctic Notothenioids occurred before their diversification, but after the separation of the Bovichtidae (which have the classical heat shock response) and the migration of the New Zealand Notothenioids from the Southern Ocean (Carpenter and Hofmann 2000).
In addition to the constitutive expression of the inducible form of HSP70, GRP78 is also strongly constitutively expressed (a situation identified in the Antarctic molluscs: Nacella concinna and Laternula elliptica; Clark et al. 2007). GRP78 has been shown to be constitutively expressed in rainbow trout cell lines, but at a relatively low level, compared that identified in our experiments (Ojima et al. 2005). This provides an increasing body of evidence to support the theory that constitutive expression of heat shock proteins may be a compensatory mechanism for coping with elevated protein damage at low temperatures. There is some evidence that protein degradation rates appear comparatively higher and protein synthesis rates lower in invertebrates at polar water temperatures than in species living at warmer temperatures (Fraser et al. 2002; Fraser and Rogers 2007). Other studies have also shown elevated levels of ubiquitin-conjugated proteins in polar fishes, a likely indication of increased levels of denatured proteins at polar water temperatures (Place et al. 2004). Taken together this evidence suggests that transcribing, translating and folding proteins at polar water temperatures may be problematic. Indeed, cold denaturation of proteins is well known (Privalov 1990) and exposure of endotherm cells and ectotherms to cold shock can induce HSP70 expression (Ali et al. 2003; Laios et al. 1997).
H. antarcticus does not show any significant up-regulation of heat shock protein activity above the level of individual variation/experimental noise for any of the family members surveyed at either the acute temperatures or over the time course. There are wide 95% confidence intervals in all the data and some results which show large fold increases in gene expression are still not significant at the 95% level using the pair wise fixed reallocation randomisation test in the REST software (Pfaffl 2001; Pfaffl et al. 2002) or Fisher’s method for combining probabilities. The wide confidence intervals are a result of enforced experimental design and animal sampling: repeat sampling of animals before and after treatment was not possible. Thus analysis of five “paired” results was in fact analysis of ten different individuals. The work was carried out on non-interbred, non-model organisms and implicit in this is the problem of high inter-individual variability (also noted in Place and Hofmann 2005). So care has to be taken in interpreting Q-PCR results from these animals, with general trends being more important indicators rather than absolute figures.
The overall trend for all members of the HSP70 gene family examined in this study is down regulation. This is particularly the case with the time course results, which are a more accurate reflection of thermal stress compared to the very quick (in some ways, artificial) acute heat shock experiments at elevated temperatures. In the time course, this down regulation is followed by indications of recovery to “normal” levels. There is clearly a reaction by H. antarcticus to thermal stress, but there is also evidence that at least some Antarctic fish can acclimate successfully to higher temperatures such as 4°C (Carpenter and Hofmann 2000; Lowe and Davidson 2005; Jin and deVries 2006, Podrabsky and Somero 2006).
The reason for this general down-regulation of HSP genes cannot be determined given this set of experiments and the limited gene set. However, there are three potential reasons:
-
There is a general down-regulation of all genes under these conditions. Although tests on β actin as a housekeeping sequence for Q-PCR show no change in expression levels with treatment.
-
Small temperature increases above 0°C may improve protein stability, reducing the requirement for HSPs until a higher threshold is released.
-
A more selective process is occurring with down regulation of “non-essential” genes, whilst other genes more vital to cell survival are up regulated. Certainly experiments in invertebrates indicate that oxygen limitation is a significant factor in physiological responses to temperature (Frederich and Pőrtner 2000) and it may well be that hypoxic genes are up regulated at the expense of most others.
This down regulation trend is particularly acute for GRP78. During the time course experiment GRP78 expression falls by over 100-fold and this was confirmed by duplicate experiments on both the same samples and newly isolated cDNAs. To date, most of the work on this gene has been carried out on mammals and the results of work on fish cell lines after heat shocking have been contradictory so far. Ojima et al. (2005) reported no change in the expression of this gene in rainbow trout cell lines, whilst Yamashita et al. (2004) showed up-regulation of GRP78 protein in heat shocked platyfish cell lines. Comparing results from cell line studies and whole animals is problematic and should be interpreted with caution. Our results on whole animals present a further alternative with constitutive expression at “normal” temperatures and down regulation of this gene in immediate response to heat shock.
GRP78 is located in the endoplasmic reticulum and is a classic marker of the unfolded protein response with an HSP-like chaperone function (Sommer and Jarosch 2002). It is up regulated under conditions of glucose starvation (Hendershot et al. 1994). Therefore, in addition to the three potential reasons for gene down-regulation outlined above, there are two other possible reasons, which may contribute to the massive down regulation of GRP78 compared to the other genes. GRP78 is not classically induced under heat stress conditions and in theory would not be expected to alter in expression level with thermal stress. However, in marine organisms, any increase in water temperature is associated with a decrease in oxygen content of the water (an approximate 2% decrease per degree) and therefore hypoxia is also potentially an additional stressor (Hochachka et al. 1996). Up-regulation of carbohydrate metabolism and production of glucose has been shown in hypoxia experiments with Gillichthys mirabilis (Gracey et al. 2001) and daily temperature fluctuation in Austrofundulus limnaeus (Podrabsky and Somero 2004). Up-regulation of glucose would inhibit GRP78 expression. Also preliminary microarray sequence screening provides a further antagonist for GRP78 expression with identification of the Diablo gene, a protein which activates caspases and is involved in the apoptosis pathway. Gene Ontology data (Gene Ontology Consortium 2000) (http://www.geneontology.org) for GRP78 includes not only chaperone activity, but also caspase regulator activity (GO:0043028), more specifically caspase inhibitor activity (GO:0043027). Potentially these two genes (GRP78 and Diablo) are interacting in the activation/inhibition of apoptosis under stress. If Diablo is up-regulated, GRP78 would consequently be down regulated. More comprehensive microarray analysis will be needed to define this potential relationship between these two genes.
In summary, this work describes for the first time, the cloning and expression profiles of HSP70 gene family members from an Antarctic fish, H. antarcticus.
The metabolic complexity of any organism is balanced within a confined biochemical envelope constrained by cellular energetics. In the case of HSPs this is a cost-benefit trade-off between successful protein folding (and re-folding) compared to other essential cellular metabolic activities. Heat shock proteins help protect against the unfolded protein response in extreme environmental temperatures, but there are potential negative effects, such as impacts on growth, development rate and fertility (Krebs and Loeschcke 1994; Silbermann and Tatar 2000; Sorensen et al. 2003). In these Antarctic fish, HSP70 proteins are produced continuously whilst in other temperate species their production is tightly regulated and they are only produced when the organism is under stress. This suggests that Antarctic fish have extra housekeeping costs compared to temperate species associated with the expression of the inducible HSP70s. The ability to further up-regulate HSPs under stressful conditions could potentially have deleterious effects in terms of cellular energetic costs if the net benefits of protein stability are outweighed by the energetic costs (Sorensen et al. 2003). Experiments are on-going in our laboratory to further dissect the intricate nature of the heat shock response in this fish species. A DNA microarray containing HSPs has been produced in our laboratory and will be used to provide a more detailed description of the complex biochemical reactions underlying this process.
References
Ali A, Salter-Cid L, Flajnik MF, Heikkila JJ (1996) Isolation and characterisation of a cDNA encoding a Xenopus 70-kDa heat shock cognate gene. Comp Biochem Physiol 113B:681–687
Ali KS, Dorgai L, Abraham M, Hermesz E (2003) Tissue- and stressor-specific differential expression of two hsc70 genes in carp. Biochem Biophys Res Commun 307:503–509
Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Amores A, Force A, Yan Y-L, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang Y-L, Westerfield M, Ekker M, Postlethwait JH (1998) Zebrafish hox clusters and vertebrate genome evolution. Science 282:1711–1714
Barber DL, Mills JE, Westermann JE, White MG (1981) The blood cells of the Antarctic icefish Chaenocephalus aceratus Lonnberg: light and electron microscopic observations. J Fish Biol 19:11–28
Boeckmann B, Bairoch A, Apweiler R, Blatter MC, Estreicher A, Gasteiger E, Martin MJ, Michoud K, O’Donovan C, Phan I, Pilbout S, Schneider M (2003) The SWISS-PROT protein knowledgebase and its supplement TrEMBL in 2003. Nucleic Acids Res 31:365–370
Bosch TCG, Krylow SM, Bode HR, Steele RE (1988) Thermotolerance and synthesis of heat-shock proteins—these responses are present in hydra-attenuata but absent in hydra-oligactis. Proc Natl Acad Sci USA 85:7927–7931
Carpenter CM, Hofmann GE (2000) Expression of 70 kDa heat shock proteins in Antarctic and New Zealand Notothenioid fish. Comp Biochem Physiol A 125:229–238
Clark MS, Fraser KPP, Peck LS (2007) Antarctic marine molluscs do have an HSP70 heat shock response. Cell Stress Chaperones (in press)
Cocca E, Ratnayake-Lecamwasam M, Parker SK, Camardella L, Ciaramella M, di Prisco G, Detrich HW III (1995) Genomic remnants of α-globin genes in the hemoglobinless Antarctic icefishes. Proc Natl Acad Sci USA 92:1817–1821
Davison W, Axelsson M, Forster ME, Nilsson S (1995) Cardiovascular responses to acute handling stress in the Antarctic fish Trematomous bernachii are not mediated by circulatory catecholamines. Fish Physiol Biochem 14:253–257
di Prisco G, Cocca E, Parker S, Detrich HW III (2002) Tracking the evolutionary loss of hemoglobin expression by the white blooded Antarctic icefishes. Gene 295:185–191
Ewing B, Hillier L, Wendl MC, Green P (1998) Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 8:175–185
Fink AL (1999) Chaperone-mediated protein folding. Physiol Rev 79:425–449
Fisher RA (1954) Statistical methods for research workers. Pub Oliver and Boyd, Edinburgh
Fraser KPP, Rogers AD (2007) Protein metabolism in marine animals: the underlying mechanism of growth. Adv Mar Biol 52:267–362
Fraser KPP, Clarke A, Peck LS (2002) Feast and famine in Antarctica: seasonal physiology in the limpet Nacella concinna. Mar Ecol Prog Ser 242:169–177
Frederich M, Pőrtner HO (2000) Oxygen limitation of thermal tolerance defined by cardiac and ventilatory performance in the spider crab, Maja squinado. Am J Physiol 279:R1531–R1538
Gene Ontology Consortium (2000) Gene ontology: tool for the unification of biology. Nat Genet 25:25–29
Gordon D, Abajian C, Green P (1998) Consed: a graphical tool for sequence finishing. Genome Res 8:195–202
Gracey AY, Troll JV, Somero GN (2001) Hypoxia induced gene expression profiling in the euryoxic fish Gillichthys mirabilis. Proc Natl Acad Sci USA 98:1993–1998
Gross M (2004) Emergency services: a bird’s eye perspective on the many different functions of stress proteins. Curr Protein Peptide Sci 5:213–223
Hartl FU (1996) Molecular chaperones in cellular protein folding. Nature 381:571–580
Hendershot LM, Valentine VA, Lee AS, Morris SW, Shapiro DN (1994) Localization of the gene encoding human bip/grp78, the endoplasmic-reticulum cognate of the hsp70 family, to chromosome-9q34. Genomics 20:281–284
Hochachka PW, Buck LT, Doll CJ, Land SC (1996) Unifying theory of hypoxia tolerance: molecular metabolic defense and rescue mechanisms for surviving oxygen lack. Proc Natl Acad Sci USA 93:9493–9498
Hofmann GE (2005) Patterns of gene expression in ectothermic marine organisms on small to large-scale biogeographical patterns. Intergr Comp Biol 45:247–255
Hofmann GE, Buckley BA, Airaksinen S, Keen JE, Somero GN (2000) The Antarctic fish Trematomus bernachii lacks heat inducible heat shock protein synthesis. J Exp Biol 203:2331–2339
Hofmann GE, Lund SG, Place SP, Whitmer AC (2005) Some like it hot, some like it cold: the heat shock response is found in New Zealand but not Antarctic notothenioid fishes. J Exp Mar Biol Ecol 316:79–89
Hunt C, Morimoto RI (1985) Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci USA 82:6455–6459
Hureau JC, Petit D, Fine JM, Marneax M (1977) New cytological, biochemical and physiological data on the colorless blood of the Channichthyidae (Pisces, Teleosteans, Perciformes). In: Llano GA (ed) Adaptations within Antarctic ecosystems. Smithsonian Institution, Washington DC, pp 459–477
Jin Y, deVries AL (2006) Antifreeze glycoprotein levels in Antarctic notthenioid fishes inhabiting different thermal environments and the effect of warm acclimation. Comp Biochem Physiol B 144:290–300
Krebs RA, Loeschcke V (1994) Costs and benefits of activation of the heat-shock response in Drosophila melanogaster. Funct Ecol 8:730–737
Laios E, Rebeyka IM, Prody CA (1997) Charcterization of cold-induced heat shock protein expression in neonatal rat cardiomyocytes. Mol Cell Biochem 173:153–159
LaTerza AL, Miceli C, Luporine P (2001) Divergenec between two Antarctic species of the ciliate Euplotes, E. focardii and E. nobilii, in the expression of heat-shock protein 70 genes. Mol Ecol 10:1061–1067
LaTerza AL, Miceli C, Luporine P (2004) The gene for the heat-shock protein 70 of Euplotes focardii, an Antarctic psychrophilic ciliate. Antarct Sci 16:23–28
Lindquist S (1986) The heat shock response. Annu Rev Biochem 55:1151–1159
Lowe CJ, Davison W (2005) Plasma osmolarity, glucose concentration and erythrocyte responses of two Antarctic notothenioid fishes to acute and chronic thermal change. J Fish Biol 67:752–766
Meredith MP, King JC (2005) Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys Lett 32:L19604–L19609
Milner CM, Campbell RD (1990) Structure and expression of the three MHC-linked HSP70 genes. Immunogenet 32:242–251
Morimoto RI (1998) Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev 12:3788–3796
Near TJ, Pesavento JJ, Cheng CC-H (2004) Phylogenetic investigations of Antarctic notothenioid fishes (Perciformes: Notothenioidei) using complete gene sequences of the mitochondrial encoded 16s rRNA. Mol Phylogenet Evol 32:881–891
Ojima N, Yamashita M, Watabe S (2005) Quantitative mRNA expression profiling of heat shock protein families in rainbow trout cells. Biochem Biophys Res Commun 329:51–57
Parsell DA, Lindquist S (1993) The function of heat-shock proteins in stress tolerance—degradation and reactivation of damaged proteins. Annu Rev Genet 27:437–496
Peck LS (2002) Ecophysiology of Antarctic marine ectotherms: limits to life. Polar Biol 25:31–40
Peck LS, Conway LZ (2000) The myth of metabolic cold adaptation: oxygen consumption in stenothermal Antarctic bivalve molluscs. In: Harper E, Crame AJ (eds) Evolutionary biology of the bivalvia. Geol Soc Lond Specl Publ, vol 177, pp 441–450, Cambridge University Press, Cambridge
Peck LS, Webb KE, Bailey DM (2004) Extreme sensitivity of biological function to temperature in Antarctic marine species. Funct Ecol 18:625–630
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2002–2007
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:1–10
Place SP, Hofmann GE (2005) Constitutive expression of a stress-inducible heat shock protein gene, hsp70, in a phylogenetically distant Antarctic fish. Polar Biol 28:261–267
Place SP, Zippay ML, Hofmann GE (2004) Constitutive roles for inducible genes: evidence for the alteration in expression of the inducible hsp70 gene in Antarctic notothenioid fishes. Am J Physiol Regul Integr Comp Physiol 287:R429–R436
Podrabsky JE, Somero GN (2004) Changes in gene expression associated with acclimation to constant temperatures and fluctuating daily temperatures in an annual killifish Austrofundulus limnaeus. J Exp Biol 207:2237–2254
Podrabsky JE, Somero GN (2006) Inducible heat tolerance in Antarctic nothothenioid fishes. Polar Biol 30:39–43
Privalov PL (1990) Cold denaturation of proteins. Crit Rev Biochem Mol Biol 25:281–305
Rice P, Longden I, Bleasby A (2000) EMBOSS: the European molecular biology open software suite. Trends Genet 16:276–277
Ritossa F (1962) A new puffing pattern induced by temperature shock and DNP in Drosphila. Experimentia 18:571–573
Silbermann R, Tatar M (2000) Reproductive costs of heat shock protein in transgenic Drosophila melanogaster. Evol Int J Org Evolution 54:2038–2045
Somero GN (2002) Thermal physiology and vertical zonation of intertidal animals: optima, limits and costs of living. Integr Comp Biol 42:780–789
Somero GN, DeVries AL (1967) Temperature tolerance of some Antarctic fishes. Science 156:257–258
Sommer T, Jarosch E (2002) BiP binding keeps ATF6 at bay. Dev Cell 3:1–2
Sorensen JG, Kristensen TN, Loeschcke V (2003) The evolutionary and ecological role of heat shock proteins. Ecol Lett 6:1025–1037
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL-W—improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Venkatesh B, Tay BH, Elgar G, Brenner S (1996) Isolation, characterization and evolution of nine pufferfish (Fugu rubripes) actin genes. J Mol Biol 259:655–665
Voellmy R, Ahmed A, Schiller P, Bromley P, Rungger D (1985) Isolation and functional analysis of a human 70,000-dalton heat shock protein gene fragment. Proc Natl Acad Sci USA 82:4949–4953
Whiteley NM, Egginton S (1999) Antarctic fishes have a limited capacity for catecholamine synthesis. J Exp Biol 202:3623–3629
Wu CH, Apweiler R, Bairoch A, Natale DA, Barker WC, Boeckmann B, Ferro S, Gasteiger E, Huang H, Lopez R, Magrane M, Martin MJ, Mazumder R, O’Donovan C, Redaschi N, Suzek B (2006) The Universal Protein Resource (UniProt) an expanding universe of protein information. Nucleic Acids Res 34:D187–D191
Yamashita M, Hirayoshi K, Nagata K (2004) Characterisation of multiple members of the HSP70 family in platyfish culture cells: molecular evolution of stress protein HSP70 in vertebrates. Gene 336:207–218
Acknowledgments
This paper was produced within the BAS Q3 LATEST and BAS Q4 BIOREACH/BIOFLAME core programmes. The authors would like to thank all members of the Rothera Dive Team for providing samples and to Pete Rothery for statistics advice. Overall diving support was provided by the NERC National Facility for Scientific Diving at Oban.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clark, M.S., Fraser, K.P.P., Burns, G. et al. The HSP70 heat shock response in the Antarctic fish Harpagifer antarcticus . Polar Biol 31, 171–180 (2008). https://doi.org/10.1007/s00300-007-0344-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-007-0344-5