Abstract
Seabirds that form large colonies often act as biovectors that transport and concentrate large amounts of nutrients, metals, and contaminants from marine feeding areas to inland breeding grounds. This enrichment can potentially transform and structure primary productivity, vegetation communities, and species richness. In a previous paleolimnological study, we examined approximately 1700 years of population change in the world’s largest colony of Leach’s Storm-petrel (Hydrobates leucorhous) on Baccalieu Island (Newfoundland and Labrador, Canada) and, using a variety of proxies, we identified two peaks in colony around 500 and 1980 CE. Here, we analyzed the same sediment cores for fossil pollen assemblages to explore the effects of changing seabird populations on terrestrial vegetation. Aerial imagery revealed the island’s vegetation cover that increased from about 23% to about 58% between 1940 and 2017, in part coinciding with the rapid colony growth until around 1980. Palynological analyses indicated shifts from tree and shrub habitat to storm-petrels’ preferred habitat of fern, grass, and moss during peak seabird abundances around 500 and 1980 CE. Also, during peaks in colony size, nitrogen-fixing alder (Alnus spp.) decreased in relative abundance likely due to poorer competitive potential because of guano-derived nitrogen fertilization. Furthermore, we observed increases in fungal hyphae concurrent with the inferred size of the storm-petrel colony, providing the potential for a novel proxy to track burrowing seabirds in sediment records. Collectively, our data show that storm-petrels acted as ecosystem engineers by markedly modifying the island’s vegetation cover and composition. If global seabird colonies continue to decline at current rates, there may be considerable bottom-up ramifications to terrestrial island ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
Storm-petrel colonies have markedly affected terrestrial and aquatic ecosystems.
-
Vegetation cover expanded in response to seabird-derived nutrients and behavior.
-
Island vegetation shifted from trees and shrubs to ferns, grasses, and mosses.
Introduction
Birds play a critical role in the health and functioning of ecosystems via the movement of nutrients from their feeding grounds to breeding territories in the forms of nutrient-rich guano, carcasses of chicks and adults, feathers, eggshells, and other wastes (for example, Sánchez-Piñero and Polis 2000). Organisms that transfer and concentrate significant amounts of resources to receptor sites are termed ‘biovectors’ and provide numerous ecological services, including the cycling of nutrients and contaminants (Post and others 1998; Croll and others 2005; Ellis and others 2006; Blais and others 2007), contributing to soil formation (Heine and Speir 1989; Wait and others 2005), pollination (Paton and Ford 1977; Kelly and others 2010), and seed dispersal (Sekercioglu 2006). Aquatic birds are particularly potent biovectors as they often occupy high-trophic levels and are gregarious in nature, thus forming dense colonies that can alter the ecosystem via the introduction of marine-derived nutrients (Keatley and others 2009; Otero and others 2018), trace metals (Liu and others 2006; Brimble and others 2009), and contaminants (Blais and others 2005, 2007; Evenset and others 2007).
Due to the increased availability of biovector-derived nutrients to the terrestrial ecosystem, birds have the potential to act as ecosystem engineers by causing considerable biotic and abiotic changes to the environment. However, the impacts of biovectors on the environment vary depending on the receiving ecosystem. In some cases, nutrient subsidies increase vegetative biomass and primary productivity (Polis and others 1997; Anderson and Polis 1999; González-Bergonzoni and others 2017). For example, Croll and others (2005) described an ecosystem shift from grassland to tundra due to a diminished supply of guano-derived subsidies as seabird density declined caused by the introduction of Arctic foxes (Vulpes lagopus). Conversely, an increase in bird-derived nutrients may truncate food webs and reduce species richness (Ishida 1996; Wait and others 2005). Boutin and others (2011) described a dramatic reduction in vegetation and seed bank richness in Lake Erie in response to rapidly increasing numbers of Double-crested Cormorants (Phalacrocorax auritus), whose ammonia-rich guano toxified soil and killed native vegetation. Regardless of the direct impacts of bird-derived nutrient deposition on vegetation communities, changes in colony size can indirectly structure many facets of the ecosystem, and their disappearance can have considerable bottom-up effects on the environment (Sánchez-Piñero and Polis 2000; Graham and others 2018).
Leach’s Storm-petrels (Hydrobates leucorhous), which are the smallest but most abundant seabird nesting in eastern Canada (Hedd and others 2006), are of considerable interest as biovectors because they form large, dense breeding colonies that introduce large quantities of acidic (pH ≈ 5.86; Duda and others 2020) and nutrient-rich (in the forms of nitrogen (N) and phosphorus (P)) guano to the terrestrial environment. Otero and others (2018, supplementary material) estimated that the Leach’s Storm-petrel is the eighth-most abundant seabird on the planet and excrete 0.351 Gg N and 0.058 Gg P annually, which is in the top 30% of global nutrient deposition of the 320 seabirds studied.
Using a variety of paleolimnological proxies that are responsive to guano inputs (that is, sub-fossil diatom and chironomid biological indicators, as well as sedimentary geochemistry including δ15N, chlorophyll-a, and fossil cholesterol), Duda and others (2020) reconstructed population dynamics of the world’s largest colony of Leach's Storm-petrels over the past approximately 1700 years on Baccalieu Island, Newfoundland and Labrador. Only two complete colony surveys (in 1984 and 2013) had been conducted on Baccalieu Island, which indicated that the colony had declined by about 40% in only 29 years from 3.36 (Sklepkovych and Montevecchi 1989) to 1.95 million breeding pairs (Wilhelm and others 2019). The paleolimnological data not only corroborated this decline, but more importantly determined that the storm-petrel colony experienced dramatic changes in size over the past approximately 1700 years. Specifically, Duda and others (2020) recorded two peaks in colony size: a larger, modern colony, which grew rapidly starting in the 1800s and peaked in the 1980s, and an earlier, smaller colony from about 270 CE to about 610 CE, which peaked at about 500 CE. The two peaks in storm-petrel colony size on Baccalieu Island provide a rare opportunity to study how marine animals shape terrestrial vegetation over long time scales.
As a parallel study to our paleolimnological work (Duda and others 2020), here we investigate how the changing storm-petrel colony size on Baccalieu Island altered the structure and diversity of terrestrial vegetation over the past 1700 years. We used the earliest available aerial photography of Baccalieu Island from 1940, as well as subsequent aerial photographs and satellite images from 1978 to 2017 to visually examine overall changes in the greening of the island. This period encompasses the storm-petrel colony’s recent growth to its maximum size, as well as its most recent decline since the 1980s. Using the same sediment records used in Duda and others (2020), we also examined genus-specific changes in vegetation using palynological analyses of pollen and spores. Finally, we corroborated our inferred changes in seabird-driven island greening using temporal changes in sedimentary sterols and stanols, which are natural, lipophilic biomarkers associated with various biological groups (Hargan and others 2019) to directly track changes in the colony size and terrestrial vegetation.
Materials and Methods
Study Sites
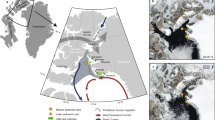
Baccalieu Island (48° 08′ N, 52° 48′ W) is located 64 km north of St. John’s, NL, Canada (Figure 1). In 1995, the island was designated as a provincial ecological reserve to maintain important breeding seabird populations. Baccalieu Island currently supports the world’s largest colony of Leach’s Storm-petrels (48–59% of the global population), with the most recent survey in 2013 estimating about 1.95 million pairs (Wilhelm and others 2019). There are also smaller colonies of Atlantic Puffin (Fratercula arctica), Common Murre (Uria aalge), Northern Gannet (Morus bassanus), and others along the island’s perimeter (Montevecchi and Tuck 1987).
Map of the study region. Baccalieu Island is shown as a red star on the inset map. The two sampling locations are only about 6 km apart, but differences in vegetation cover are apparent. A Lunin Pond on Baccalieu Island, a pond with a high concentration of storm-petrels within its catchment. B Mainland reference pond without nesting storm-petrels or seabirds.
Importantly, storm-petrels are the only seabird on Baccalieu Island that both build burrows for their chicks and also form large persistent colonies in the central parts of the island (Duda and others 2020). Therefore, the majority of seabird-derived inputs tracked in the interior ponds of Baccalieu Island are a result of Leach’s Storm-petrel wastes (that is, feces, eggshells, feathers, carcasses) that were washed into the ponds. The influence of other seabirds (for example, Black-legged Kittiwakes (Rissa tridactyla)) using the ponds during the breeding season and other species briefly stopping over on the island is likely minimal.
Baccalieu Island and the surrounding area are classified as part of the Eastern Hyper-Oceanic Barrens ecoregion and is dominated by softwood coniferous forest species (for example, Black Spruce (Picea mariana), White Spruce (P. glauca), Balsam Fir (Abies balsamea)), shrubs (for example, Paper Birch (Betula papyrifera), alder (Alnus crispa, occasionally A. incana)), ferns, and mixed grasses (Damman 1983; Sklepkovych and Montevecchi 1989; Wilhelm and others 2019). Much of the tree growth on the island is stunted, resulting in krummholz, locally referred to as ‘tuckamore’ or simply ‘tuck’.
Based on nesting density estimates by Sklepkovych and Montevecchi (1989) and Wilhelm and others (2019), low-lying soft vegetation such as ferns, grasses, and mosses bear the highest burrow density despite scarce availability, suggesting that this vegetation is the storm-petrels’ preferred nesting habitat. Low-lying vegetation is thought to be preferred because it provides a soft substrate in which to burrow with minimal encumbering root structures (Sklepkovych 1986). Forest habitat, including both trees and shrubs, is also suitable habitat for storm-petrel burrows, but generally has lower burrow density and likely acts as overflow once low-lying habitat is saturated (Wilhelm and others 2019). For this reason, we termed tree and shrub habitat as ‘secondary habitat’ on Baccalieu. Storm-petrels rarely nest on hard ground heath.
In this study, we focused on Lunin Pond (48° 08′ 27.2″ N, 52° 48′ 01.7″ W) on Baccalieu Island, which has a high density of nesting storm-petrels in its catchment (Duda and others 2020). We then compared these results to a nearby (~ 6 km) mainland reference pond (48° 06′ 01.2″ N, 52° 51′ 44.5″ W), with no nesting seabirds (Duda and others 2020). Storm-petrels are very unlikely to have ever nested near the mainland reference pond because they are highly susceptible to mammalian predation on mainlands (Pollet and others 2019). As detailed in Duda and others (2020), the ponds have similar physical features and underlying geology, primarily consisting of red conglomerate and sandstone (King 1988). In this study, a single impact pond was deemed adequate to describe island-wide trends for two reasons. First, in Duda and others (2020), all storm-petrel influenced ponds had synchronous trends in all six measured proxies, and therefore any trends in sterols and stanols in Lunin Pond should be representative of the other ponds. Second, pollen grains are often aerially transported long distances (for example, Hjelmroos 1991; Rosseau and others 2008), and therefore changes in pollen assemblage should be uniform across the study island.
Climate
Climate data for the study region were derived from weather stations in St. John’s, NL, approximately 60 km south of Baccalieu Island. Data from weather station #8403500 (47° 34′ N, 52° 42′ W) were available from January 1874 until 1942, after which regular monitoring is available from station #8403506 (47° 37′ N, 52° 44′ W) until 2018. Environment and Climate Change Canada guidelines recommend annual datapoints be omitted under certain conditions, such as if at least 1 month is unavailable, or if more than 15 days of the year are missing. Given these guidelines, a continuous record is available from 1874 until 2018, omitting: 1882, 1883, 1889, 1895, 1897, 1906, 1921–1931, 1933, 1934 (Figure 2). For these 144 years, the mean annual temperature was 4.9 °C ± 0.7 SD, and mean annual precipitation was 1460 mm ± 199 SD. Trends in the data were identified using a generalized additive model, and significant periods of change were identified using a first derivative of the fitted trend (Simpson 2018). Analyses were carried out in R using the package mgcv v.1.8-28 (Wood 2017), and supplemented with gratia v.0.2-8 (Simpson 2019).
Mean annual air temperature and precipitation in St. John’s, NL, from 1874 to 2018. Data from weather station #8403500 are in circles, and data from weather station #8403506 are in diamonds. An annual datapoint is omitted if at least 1 month is unavailable or if more than 15 days of the year are missing. Trends are estimated using a generalized additive model (GAM) using a black line, with 95% pointwise confidence intervals in gray. Significant periods of increase are estimated by GAM first derivatives and expressed in red.
Paleolimnological Techniques
-
(i)
Site Selection and Sediment Collection
Techniques used to select study sites and collect sediment records are detailed in Duda and others (2020). Briefly, ponds were selected a priori reflecting a gradient of known storm-petrel influence (available from ornithological surveys on the island), while having minimal differences in physical parameters. We selected two ponds that provided the best comparison of storm-petrel influence to minimal-to-no seabird influence. We compare Lunin Pond, which receives large amounts of inputs from storm-petrels to a mainland reference pond, which has no nearby nesting seabirds (Figure 1). Inputs from other parameters that may confound our results, such as underlying geology or climate, had minimal impact on our sediment proxies due to the proximity of the two sampling locations (~ 6 km apart). Sediment cores (76 mm or 3″ in diameter) were collected September 13 and 15, 2017 from the deepest point of each pond as determined using a handheld depth sounder, using a high-resolution push corer (Glew and Smol 2016). This push corer is similar to gravity corers, but is driven by a push rod for more precise sediment collection in shallow systems. Sediment cores were then sectioned onsite at 0.5 cm intervals using a Glew (1988) extruder. The Lunin Pond sediment core was 39.5 cm in length, and the mainland reference core was 10.5 cm.
-
(ii)
Sediment Core Dating
Core chronology for recent sediments (~ past 150 years) was established using a constant-rate-of-supply (CRS) model applied to excess 210Pb inventories, using the ScienTissiME (Barry’s Bay, ON, Canada) software created for Matlab, and corroborated by a 137Cs peak denoting 1963 (Duda and others 2020, supplementary material). All cores were counted, following standard procedures (Schelske and others 1994), on a digital, high purity germanium γ spectrometer (DSPec, Ortec) located at the Paleoecological Environmental Assessment and Research Laboratory (PEARL), Queen’s University, Kingston Ontario. In Lunin Pond, a basal age was determined using accelerator mass spectrometry 14C on a well-preserved terrestrial macrophyte from the 37.5–38 cm interval. An age-depth model using 210Pb and the basal 14C age was established using CLAM v.2.3.2 (Blaauw and Christen 2011). The absence of a basal 14C date from the reference pond necessitated extrapolation using a second-order polynomial regression, and therefore should be viewed with caution.
-
(iii)
Palynology
We chose to examine changes in the palynological record (encompassing both pollen and non-pollen palynomorphs (NPP)) as it provided an effective and well-established proxy to analyze the changes in vegetation in response to changes in the terrestrial environmental, such as a varying seabird colony size (Bennett and Willis 2001). To isolate pollen and NPP, our palynological procedures followed Johnson and Fredlund (1985). Briefly, 0.01 g dry sediment per sample was repeatedly boiled for 20 min in 10 mL of 10% KOH until the supernatant ran clear to dissolve humic acids and organic components. The pellets were then sieved through a 120-µm mesh with deionized water to remove large inorganic material. Next, samples were digested with acetolysis to remove polysaccharides. Aliquots of each sample were dehydrated with tert-butyl alcohol and mounted using silicon oil. Conventionally, treatment with HCl is required to remove carbonate material; however, since the water column was highly acidic (pH ~ 4.0), this step was omitted. A minimum of 400 pollen grains and NPP were counted per sample using a LEICA DMRB microscope at 1000× magnification. Taxonomic identification to genus generally followed Kapp (1969) and Bassett and others (1978). Palynology profiles were created in C2 v.1.7.7.
Fungal hyphae were present in our palynology slides, but because hyphal strands can be broken into numerous segments, hyphae were counted separately to provide a ratio of fungal hyphae to total pollen. Each unbroken hyphal segment was considered a single ‘hypha’, and broken ends of hyphae were counted as halves. Hyphae are considered rare in sediments (van Geel 2001) due to the necessity of a constant supply of nutrients (organic debris) and a high concentration of oxygen (Bärlocher and Boddy 2016). Because such conditions would occur in storm-petrel burrows during periods of high seabird activity, we surmised that the accumulation of fungal hyphae in sediments were likely an additional proxy associated with the storm-petrel’s burrowing behavior (and therefore increased shoreline erosion) introducing an influx of allochthonous material. As an estimate of changing diversity patterns, we also calculated Hill’s N2 (Hill 1973) of pollen and spores at each depth to determine if storm-petrels changed the island’s vegetation diversity, and compare between seabird-influenced and reference sites. This measure is the reciprocal of Simpson’s index and gives more weight to common taxa while downweighting rare taxa (Heip and others 1998), and is, therefore, the most appropriate diversity measure for pollen (Felde and others 2016).
Island Vegetation
Recent changes to Baccalieu Island’s vegetation cover were directly assessed using historical aerial photography from the National Air Photo Library in Ottawa, ON. The photos available for this site were taken October 10, 1940 (Roll #A6820, print 14) and August 21, 1978 (Roll #25052, print 49). Meanwhile, our 2017 figure was made using a Google Earth© satellite photo taken June 2017. To minimize bias due to increased resolution and to standardize between historical photography and modern satellite, all used images were printed, equally scaled, and gray-scaled. Percent vegetation cover was estimated using a dot grid system (U.S. EPA 2002). Each map was binarily simplified to (1) areas of rocky habitat, not suitable for nesting storm-petrels; and (2) any vegetated area suitable for nesting storm-petrels. Visual inspection of historical photos of the terrestrial environment surrounding the mainland reference pond indicated minimal-to-no change in vegetation, and were therefore omitted from further analysis.
Sterols and Stanols
Sterols are natural lipophilic biomarkers that are a key component of biological function and membranes, and stanols are the reduction product of sterols (Cheng and others 2016). The analytical and quality assurance methods utilized for sterol and stanol analyses are detailed in Hargan and others (2019) and Duda and others (2020). Here, to track seabird-derived inputs, we focused on cholesterol (cholest-5-en-3β-ol), which is commonly found in vertebrate tissues and fecal material (Volkman 1986; Cheng and others 2016), and its aerobic microbial reduction product, cholestanol (5α-cholestan-3β-ol) (Bull and others 2002; Cheng and others 2016). To track changes in vegetation (termed plant-derived), we used sitosterol (β-sitosterol), which is commonly derived in plant-material (Peng and others 2002), and its aerobic microbe reduction product, stigmastanol (5α-stigmastan-3β-ol) (Bull and others 2002; Cheng and others 2016). All values are presented as amount per dry weight (DW).
Results
Increasing Vegetation Cover and Climate
There was a 35% increase in Baccalieu Island’s vegetation cover from the earliest available aerial photograph from 1940 until the most recent satellite image available from 2017 (Figure 3). Greening continued despite the recently declining storm-petrel colony size starting in the 1980s (Wilhelm and others 2019; Duda and others 2020).
Increasing vegetation and available nesting habitat on Baccalieu Island from 1940 to 2017 determined from aerial photography. Vegetation, and thus available nesting area for storm-petrels, is shown in green, and hard heath, thus unsuitable nesting habitat, is shown in gray. The estimated percent vegetation cover is shown below each illustration.
Precipitation generally increased in the region throughout the available record, with no prolonged shifts (Figure 2). The temperature was stable until 1987, at which point there was a statistically significant warming period (Figure 2). Due to the proximity of the reference site and Baccalieu Island (only ~ 6 km apart), we assumed both locations experience similar climate.
Palynological Changes
During the documented growth periods of the storm-petrel colony, there are distinct changes in the relative abundances of tree and shrub habitat and low-lying vegetation. During the growth of the modern, larger colony from around 1800 to its peak in around 1980, there is a distinct decline in tree and shrub pollen from 45 to 34% relative abundance, predominately in taxa including Abies spp., Pinus spp., Betula spp., and Alnus spp. (Figure 4A). Concurrently, we observed a modest increase in pollen grains from preferred habitat taxa from 38 to 42% relative abundance, particularly fern (for example, Dryopteris spp., Pteridium spp.), grasses (for example, Poaceae), and Bryophyta spores. A similar trend of vegetation change was observed during the earlier storm-petrel colony from about 270 CE to its peak in about 500 CE, with a decline in tree and shrub habitat from 62 to 50% relative abundance, and an increase from 33 to 43% in preferred habitat (Figure 4A). Shifts in vegetation from tree and shrub habitat to preferred habitat are simplified as a ratio of preferred to secondary habitat (Figure 4).
Comparison of palynological and sterol and stanol changes. A Pollen and spore relative abundances, ratios of fungal hyphae to pollen and non-pollen palynomorphs (NPP), Alnus spp., preferred to secondary habitat, Hill’s N2, and sterols and stanols from the storm-petrel influenced Lunin Pond. The storm-petrel colony trends in bold are a summary of six storm-petrel proxies from Duda and others (2020). Regions of high storm-petrel numbers are highlighted in green. The larger, modern colony is in darker shading, and the smaller, earlier colony is in a lighter shade. B Palynology, ratios, and sterols and stanols from the mainland reference pond. Preferred habitat is the sum of fern, grass, wildflower, and bryophyte pollen and spores, and secondary habitat the sum of tree and shrub pollen. Each measure includes the average of the profile in a gray dashed line. Seabird-derived (cholesterol and cholestanol) and plant-derived (sitosterol and stigmastanol) sterol and stanol data (details in Duda and others 2020) are presented as dry weight (DW). Italicized dates are extrapolated from 210Pb, and therefore must be interpreted with caution.
Alder peaked at about 20% relative abundance around 1950 and then declined to a minimum of about 9% relative abundance around 2008, following the peak in colony size in the 1980s. Similarly, alder was at a maximum 21% relative abundance about 270 CE, however after the storm-petrel colony began to increase it declined to about 16% relative abundance around 790 CE (Figure 4). Notably, the decline in alder appears to be delayed after the peak in storm-petrel colony size (Figure 4A), though a longer record before the colony’s growth would be required to confirm this trend.
We also observed a high concentration of fungal spores and hyphae throughout the seabird-influenced sediment core. In Lunin Pond, fungal spores were abundant (15% ± 5 SD), without any distinct peaks (Figure 4A). Hyphae, however, were abundant throughout the seabird-influenced sediment record (24% ± 9 SD), with increases in abundance generally when the storm-petrel colony size was at its highest in both the approximately 500 CE and 1980 colony peak (Figure 4A). Hyphae are predominantly terrestrial in origin, and therefore generally considered rare in aquatic sediments (van Geel 2001). However, because hyphae were found in abundance in our sediment samples, we correlated their presence to the burrowing behavior of the storm-petrels introducing high amounts of allochthonous material (that is, fungal hyphae) to the ponds.
Sterols and stanols tracked the growth in storm-petrel colony size as detailed in our previous paleolimnological study (Duda and others 2020). During the larger, modern storm-petrel colony growth period from the early-1800s to the 1980s, there was a distinct increase in seabird-derived sterols and stanols (sum of cholesterol and cholestanol) from 14.8 µg g−1 DW to 37.8 µg g−1 DW, mirrored by a twofold increase in plant-derived sterols and stanols (sum of sitosterol and stigmastanol) from 26.4 µg g−1 DW to 52.7 µg g−1 DW (Figure 4A). Interestingly, seabird-derived sterols and stanols did not track the decline in storm-petrel numbers from the 1980s to the present, nor the earlier colony peak at ca. 500 CE (Figure 4A).
Compared to Lunin Pond, there was little change throughout the palynological record of the mainland reference site (Figure 4B). Both habitat types remain stable throughout, with preferred habitat at 29 ± 3% SD and secondary habitat at 68 ± 2% SD. The relative abundance of alder was lower and unchanging throughout the record, at 6 ± 1% SD. Additionally, relative to Lunin Pond, there were very few fungal spores (3 ± 1% SD), and hyphae were almost absent (Figure 4B).
Both seabird-derived cholesterol and cholestanol (4.5 ± 2.7 SD µg g−1 DW) and plant-derived sitosterol and stigmastanol (3.9 ± 1.9 SD µg g−1 DW) concentrations were low and complacent throughout the entire mainland reference record (Figure 4B). Hill’s N2, a measure of effective species diversity, was higher in the storm-petrel influenced sediment record, at 6.5 ± 0.5 SD, compared to 4.5 ± 0.6 SD in the mainland reference (Figure 4). There were no notable changes in Hill’s N2 through either the seabird-influenced or mainland reference record.
Discussion
Our weight-of-evidence approach indicates that storm-petrels acted as ‘ecosystem engineers’ on Baccalieu Island, and were an important driving factor in changes of both the aquatic (Duda and others 2020) and terrestrial (this study) environment via the deposition of acidic and nutrient-rich guano. We previously demonstrated how varying storm-petrel colony size had dramatic effects on the pond’s water chemistry via the introduction of storm-petrel refuse (Duda and others 2020). Here, we show widespread modification of the terrestrial ecosystem by nutrient deposition. Aerial photographs of Baccalieu Island from 1940 to 2017 illustrate a continued greening from 23 to 58% vegetation cover (Figure 3), coincident with the increasing storm-petrel colony size until about 1980. These data indicate that greening has continued on Baccalieu Island despite the declining storm-petrel colony since the 1980s (Wilhelm and others 2019; Duda and others 2020). The continued greening is likely a result of inertia in plant communities, in which existing plant communities on fertilized soils can continue to survive for prolonged periods despite reduced nutrient inputs (Milchunas and Lauenroth 1995), combined with climate warming and associated longer growing seasons driving regional greening (Notaro and others 2006). When examining the 2017 satellite image that encompasses both Baccalieu Island and the mainland (only ~ 6 km apart), the island landscape is clearly denser and more vegetated (Figure 1), despite both areas being classified as part of the Eastern Hyper-Oceanic Barrens ecoregion. Guano-derived nutrients (as biologically available nitrogen and phosphorus) are among the most important variables in changing the diversity of plant communities, particularly in nutrient-poor ecosystems (Zwolicki and others 2016) as is typical of Eastern Hyper-Ocean Barrens (Damman 1983).
Using the available long-term climate record, changes in Baccalieu Island’s terrestrial ecosystem seem less linked to climatic changes than to the storm-petrel colony sizes, as variances in precipitation or temperature do not correspond to the measured timing of island greening. For example, in the St. John’s region, precipitation was subtly increasing linearly throughout the last 144 years (Figure 2). Temperature, however, had a significant warming period beginning in 1987 (Figure 2) and is projected to continue increasing in the Newfoundland and Labrador region into the future (Han and others 2019). Although warming temperatures may be currently affecting vegetation growth, the measured greening on Baccalieu Island at about 1940 coincides closely with the timing of increasing storm-petrel numbers, as documented in Duda and others (2020), and climatic influences may act synergistically with those of the storm-petrels.
Due to Baccalieu Island’s greening, vegetation is steadily replacing the uninhabitable rocky heath, thus increasing available habitat for the storm-petrels (Figure 3). Furthermore, we observed a shift from secondary tree and shrub habitat to preferred low-lying habitat suitable for the storm-petrel’s burrowing behavior (Figure 4), indicating the potential formation of a positive feedback loop between enhanced nesting grounds and colony size. The increase in the relative abundance of preferred vegetation is likely due to a combination of a reduction in viability of tree and shrub taxa and increasing abundance of fern and grassland due to seabird-derived fertilization. A decline in tree and shrub habitat in response to guano deposition has also been observed in the North American Great Lakes, where dense colonies of cormorants increase the soil nutrients (that is, nitrogen, phosphorus), pH, salinity, and moisture diminished tree canopies and understory vegetation (Hebert and others 2005; Boutin and others 2011; Stewart and others 2015). On Baccalieu Island, as the secondary habitat declined in relative abundance, it was in part replaced with low-lying vegetation in the forms of ferns, grasses, and mosses. Our reconstructions also match the observations of Zwolicki and others (2016), who demonstrated that vascular plants and mosses responded positively to ornithogenic nutrient inputs. Increased nutrient availability, as was observed on Baccalieu Island, competitively favors plants with high growth rates (like grasses and ferns) compared to slow-growing shrubs and trees (Tilman 1986; Zwolicki and others 2016). The decrease in relative abundance of tree and shrub habitat, and its replacement with low-lying vegetation, is an indirect, but desirable outcome for the nesting storm-petrels as they prefer the soft and thin-rooted low-lying vegetation to build their burrows (Sklepkovych and Montevecchi 1989; Wilhelm and others 2019). The vegetation changes documented in our palynological record were also corroborated in a previous study by Wilhelm and others (2019), who used a combination of geographic information system (GIS) and on-the-ground plot surveying to measure Baccalieu Island’s changes in vegetation from 1984 to 2013. Similar to our study, the researchers documented a 25% decrease in trees, and replacement by ferns, providing ground-truth evidence for our findings.
Our palynological data indicate substantial genera-specific changes linked to guano inputs. For example, we observed a decrease in the percentage of pollen grains from N2-fixing alder species (Alnus crispa and A. incana) following both peaks in the storm-petrel population (Figure 4). A similar trend was recorded by Havik and others (2014), who documented that marine-derived seabird nitrogen increased the density of non-N2 fixing Capparis scabrida, compared to the competing N2-fixing plant Prosopis pallida. The authors proposed two hypotheses to explain this trend, which may also be occurring on Baccalieu Island. First, seabird nutrient subsidies may affect the competitive ability of N2-fixing plants by promoting the growth and/or survival of the non-fixing plants, and second that N2-fixing plants growth is suppressed due to guano toxicity (that is, soil ammonification). Modern shifts in alder are inevitably also linked to warming climate.
Digging and burrow-building behavior, as displayed by Leach’s Storm-petrels, has multifaceted interactions with soil development and quality, and thereby plant diversity. Burrowing has the potential to decrease plant diversity through physical trampling, uprooting, and disruption of plant growth (Ellis 2005). Furthermore, burrow building can make soil drier and increase soil density (Bancroft and others 2005). However, burrowing can also increase soil fertility via nutrient deposition (Fukami and others 2006). By examining the effects on soil by burrowing mammals, Davies and others (2019) observed that digging and re-excavation of burrows increases soil fertility because the burrows can act as traps for organic matter, resulting in lower soil hardness compared to undisturbed soils. Given that Newfoundland is one of the wettest regions of Canada (mean annual rainfall = 1460 mm ± 199 SD; Figure 2), a reduction in soil moisture is unlikely to have a notable impact on the Baccalieu Island vegetation. Further, Leach’s Storm-petrels annually return to and re-excavate their burrows at the beginning of the breeding season in May and June in preparation for egg laying (Pollet and others 2019). High precipitation, coupled with annual burrow re-excavation and high nutrient deposition, provide the conditions required for the soil development and vegetation cover expansion observed on Baccalieu Island (Figure 3).
Despite over 920 bird species contributing to the seed dispersal and pollination of terrestrial environments (Whelan and others 2015), we do not correlate the observed changes in vegetation on Baccalieu Island to seed dispersal or pollination by Leach’s Storm-petrel. Seabirds are generally considered poor seed dispersers due to their predominantly piscivorous and planktonic diet (as opposed to pollen, fruit, nectar) and highly aquatic foraging behavior (generally preventing seed and pollen adhesion to feathers). Seed dispersal associated with seabirds has been documented by omnivorous gull species, which have opportunistic and diverse diets (for example, Nogales and others 2001; Calvino-Cancela 2011). However, seed dispersal by non-omnivorous seabirds appears to be rare in nature and requires specialized epizoochory (seed dispersal via adhesion to fur or feathers), such as with the extremely sticky resin exhibited by Grand Devil’s-claw (Pisonia grandis) (Burger 2005) or mucilage layer development by Cook’s Scurvy Grass (Lepidium oleraceum) (Dale and others 2017).
Fungal hyphae in the Lunin Pond sediment record potentially provide an independent proxy for the presence of storm-petrels, matching trends in several paleolimnological proxies presented in Duda and others (2020) (Figure 4). Fungal hyphae would be expected to be present in high abundance in the burrows of storm-petrels as fungi require high concentrations of nutrients and oxygen (Bärlocher and Boddy 2016), conditions that are present in seabird burrows. However, the storm-petrel’s burrowing behavior disrupts the catchment area and introduces the hyphae and other allochthonous material into the water column, which then accumulate in the sediment, providing an additional proxy for the storm-petrels. In our study region, the continuous presence of hyphae in the seabird-influenced Lunin Pond and absence in the mainland reference pond suggests storm-petrels always inhabited Baccalieu in some abundance, and continually introduced the hyphae. The moderate correlation between fungal hypha peaks and storm-petrel colony peaks suggest that the relationship between hyphae in sediments and seabird abundance is not one-to-one, and may only be effective as a presence-absence measure. Regardless, fungal hyphae have the potential to be used as a sedimentary proxy for other biovectors that disrupt watersheds and introduce large amounts of allochthonous material, such as other burrow-building seabirds like puffins or den-building mammals, however more research is required.
Hill’s N2 is a diversity index that can be used to compare pollen assemblages between sites (Felde and others 2016), despite admittedly simplifying the complex relationship between the vegetation producing pollen and the pollen deposited in sediments (Birks and others 2016). Hill’s N2 corroborated qualitative observations that Baccalieu Island is more diverse floristically than the mainland throughout the entire records, suggesting that storm-petrels increase the diversity of terrestrial vegetation through nutrient inputs and burrowing as has been suggested by previous work (Fukami and others 2006; Davies and others 2019). Interestingly, however, complacency in Hill’s N2 throughout the sediment record suggests that floristic diversity was changed and replaced, but not lost or gained.
Increases in both seabird-derived and plant-derived sterols and stanols in Lunin Pond occurred concurrently with the palynological changes reported here during the modern colony from the early-1800s to the present, further supporting that the storm-petrel colony is an important driver of the terrestrial ecosystem’s greening and community (Figure 4A). The complacency in sterol and stanol data during the earlier colony from around 270 CE to around 610 CE is likely due to a smaller storm-petrel colony size during the earlier peak (Duda and others 2020).
Conclusions
Our study documents how Leach’s Storm-petrels, with their acidic and nutrient-rich guano subsidies, act as ecosystem engineers and provide numerous ecological services to their habitat by altering the overall vegetation of the island affecting community structure shifts. However, as shown by both monitoring data (Wilhelm and others 2019) and our earlier paleolimnological study (Duda and others 2020), the Baccalieu storm-petrel colony is rapidly declining, which may result in bottom-up trophic effects, as seabird-derived nutrients not only enhance their environmental vegetation but also entire nutrient pathways (Sánchez-Piñero and Polis 2000; Croll and others 2005; Graham and others 2018). Due to differences in trophic status, colony size, and phenology, seabirds have diverse effects on ecosystems which cannot be easily generalized or predicted. Therefore, as more populations begin to decline globally due to increased human perturbation (for example, habitat degradation, overexploitation, pollution, climate change), long-term data, such as those provided by paleoecological studies, are vital to better understand the potential ecosystem-wide ramifications of changes in key biovectors.
References
Anderson WB, Polis GA. 1999. Nutrient fluxes from water to land: seabirds affect plant nutrient status on Gulf of California islands. Oecologia 118:324–32.
Bancroft WJ, Garkaklis MJ, Roberts JD. 2005. Burrow building in seabird colonies: a soil-forming process in island ecosystems. Pedobiologia 49:149–65.
Bärlocher F, Boddy L. 2016. Aquatic fungal ecology—How does it differ from terrestrial? Fungal Ecology 19:5–13.
Bassett IJ, Crompton CW, Parmelee JA. 1978. An atlas of airborne pollen grains and common fungus spores of Canada. Hull, Québec: Thorn Press Limited. p 321p.
Bennett KD, Willis KJ. 2001. Pollen. In: Smol JP, Birks HJB, Last WM, Eds. Tracking environmental change using lake sediments, volume 3: Terrestrial, algal, and siliceous indicators. Dordrecht: Kluwer Academic Publishers. pp 5–32.
Birks HJB, Felde VA, Bjune AE, Grytnes J-A, Seppä H, Giesecke T. 2016. Does pollen-assemblage richness reflect floristic richness? A review of recent developments and future challenges. Review of Palaeobotany and Palynology 228:1–25.
Blais JM, Kimpe LE, McMahon D, Keatley BE, Mallory ML, Douglas MSV, Smol JP. 2005. Arctic seabirds transport marine-derived contaminants. Science 309:445.
Blais JM, Macdonald RW, Mackay D, Webster E, Harvey C, Smol JP. 2007. Biologically mediated transport of contaminants in aquatic systems. Environmental Science & Technology 41:1075–84.
Blaauw M, Christen JA. 2011. Flexible paleoclimate age-depth models using an autoregressive gamma process. Bayesian Analysis 6:457–74.
Boutin C, Dobbie T, Carpenter D, Hebert CE. 2011. Effects of Double-crested Cormorants (Phalacrocorax auritus Less.) on island vegetation, seedbank, and soil chemistry: evaluating island restoration potential. Restoration Ecology 19:720–7.
Brimble SK, Foster KL, Mallory ML, MacDonald RW, Smol JP, Blais JM. 2009. High Arctic ponds receiving biotransported nutrients from a nearby seabird colony are also subject to potentially toxic loadings of arsenic, cadmium, and zinc. Environmental Toxicology and Chemistry 28:2426–33.
Bull ID, Lockheart MJ, Elhmmali MM, Roberts DJ, Evershed RP. 2002. The origin of faeces by means of biomarker detection. Environment International 27:647–54.
Burger AE. 2005. Dispersal and germination of seeds of Pisonia grandis, an Indo-Pacific tropical tree associated with insular seabird colonies. Journal of Tropical Ecology 21:263–71.
Calvino-Cancela M. 2011. Gulls (Laridae) as frugivores and seed dispersers. Plant Ecology 212:1149–57.
Cheng W, Sun L, Kimpe LE, Mallory ML, Smol JP, Gallant LR, Li J, Blais JM. 2016. Sterols and stanols preserved in pond sediments track seabird biovectors in a high Arctic environment. Environmental Science & Technology 50:9351–60.
Croll DA, Maron JL, Estes JA, Danner EM, Byrd GV. 2005. Introduced predators transform subarctic islands from grassland to tundra. Science 307:1959–61.
Dale E, de Lange P, Burns B. 2017. Seed dispersal but not seed germination facilitated by seabirds: seed ecology of Cook’s scurvy grass. New Zealand Journal of Ecology 41:226–33.
Damman AWH. 1983. An ecological subdivision of the island of Newfoundland. In: South GR, Ed. Biogeography and ecology of the island of Newfoundland. The Hague: Dr W. Junk Publishers. p 163–206.
Davies GTO, Kirkpatrick JB, Cameron EZ, Carver S, Johnson CN. 2019. Ecosystem engineering by digging mammals: effects on soil fertility and condition in Tasmanian temperate woodland. Royal Society Open Science 6:180621.
Duda MP, Robertson GJ, Lim JE, Kissinger JA, Eickmeyer DC, Grooms C, Kimpe LE, Montevecchi WA, Michelutti N, Blais JM, Smol JP. 2020. Striking centennial-scale changes in the population size of a threatened seabird. Proceedings of the Royal Society B 287:20192234.
Ellis JC. 2005. Marine birds on land: a review of plant biomass, species richness, and community composition in seabird colonies. Plant Ecology 181:227–41.
Ellis JC, Fariña JM, Witman JD. 2006. Nutrient transfer from sea to land: the case of gulls and cormorants in the Gulf of Maine. Journal of Animal Ecology 75:565–74.
Evenset A, Carroll J, Christensen GN, Kallenborn R, Gregor D, Gabrielsen GW. 2007. Seabird guano is an efficient conveyer of persistent organic pollutants (POPs) to Arctic lake ecosystems. Environmental Science & Technology 41:1173–9.
Felde VA, Peglar SM, Bjune AE, Grytnes J-A, Birks HJB. 2016. Modern pollen-plant richness and diversity relationships exist along a vegetational gradient in southern Norway. The Holocene 26:163–75.
Fukami T, Wardle DA, Bellingham PJ, Mulder CPH, Towns DR, Yeates GW, Bonner KI, Durrett MS, Grant-Hoffman MN, Williamson WM. 2006. Above- and below-ground impacts of introduced predators in seabird-dominated island ecosystems. Ecology Letters 9:1299–307.
Glew JR. 1988. A portable extruding device for close interval sectioning of unconsolidated core samples. Journal of Paleolimnology 1:235–9.
Glew JR, Smol JP. 2016. A push corer developed for retrieving high-resolution sediment cores from shallow waters. Journal of Paleolimnology 56:67–71.
González-Bergonzoni I, Johansen KL, Mosbech A, Landkildehus F, Jeppesen E, Davidson TA. 2017. Small birds, big effects: the little auk (Alle alle) transforms high Arctic ecosystems. Proceedings of the Royal Society B: Biological Sciences 284:20162572.
Graham NAJ, Wilson SK, Carr P, Hoey AS, Jennings S, MacNeil MA. 2018. Seabirds enhance coral reef productivity and functioning in the absence of invasive rats. Nature 559:250–3.
Han G, Ma Z, Long Z, Perrie W, Chassé J. 2019. Climate change on Newfoundland and Labrador shelves: results from a regional downscaled ocean and sea-ice model under an A1B forcing scenario 2011-2069. Atmosphere-Ocean 57:3–17.
Hargan KE, Gilchrist HG, Clyde NMT, Iverson SA, Forbes MR, Kimpe LE, Mallory ML, Michelutti N, Smol JP, Blais JM. 2019. Multicentury perspective assessing the sustainability of the historical harvest of seaducks. Proceedings in the National Academy of Sciences of the United States of America 116:8425–30.
Havik G, Catenazzi A, Holmgren M. 2014. Seabird nutrient subsidies benefit non-nitrogen fixing trees and alter species composition in South American coastal dry forests. PLoS ONE 9:e86381.
Hebert CE, Duffe J, Weseloh DVC, Senese EMT, Haffner GD. 2005. Unique island habitats may be threatened by Double-crested Cormorants. Journal of Wildlife Management 69:68–76.
Hedd A, Montevecchi WA, Davoren GK, Fifield DA. 2006. Diets and distributions of Leach’s storm-petrel (Oceanodroma leucorhoa) before and after an ecosystem shift in the Northwest Atlantic. Canadian Journal of Zoology 87:787–801.
Heine JC, Speir TW. 1989. Ornithogenic soils of the Cape Bird Adelie Penguin rookeries, Antarctica. Polar Biology 10:89–99.
Heip CHR, Herman PMJ, Soetaert K. 1998. Indices of diversity and evenness. Océanis 24:61–87.
Hill MO. 1973. Diversity and evenness: a unifying notation and its consequences. Ecology 54:427–32.
Hjelmroos M. 1991. Evidence of long-distance transport of Betula pollen. Grana 30:215–28.
Ishida A. 1996. Effects of the common cormorant, Phalacrocorax carbo, on evergreen forests in two nest sites at Lake Biwa, Japan. Ecological Research 11:193–200.
Johnson WC, Fredlund GG. 1985. A procedure for extracting palynomorphs (pollen and spores) from clastic sediments. Transactions of the Kansas Academy of Science 88:51–8.
Kapp RO. 1969. How to know pollen and spores. Dubuque, IA: Wm. C. Brown Company Publishers. p 249p.
Keatley BE, Douglas MSV, Blais JM, Mallory ML, Smol JP. 2009. Impacts of seabird-derived nutrients on water quality and diatom assemblages from Cape Vera, Devon Island, Canadian High Arctic. Hydrobiologia 621:191–205.
Kelly D, Ladley JJ, Robertson AW, Anderson SH, Wotton DM, Wiser SK. 2010. Mutualisms with the wreckage of an avifauna: the status of bird pollination and fruit-dispersal in New Zealand. New Zealand Journal of Ecology 34:66–85.
King AF. 1988. Geology of the Avalon Peninsula, Newfoundland (parts of 1 K, 1L, 1 M, 1 N and 2C), Map 88-01. Newfoundland: Department of Mines and Energy.
Liu X, Zhao S, Sun L, Yin X, Xie Z, Honghao L, Wang Y. 2006. P and trace metal contents in biomaterials, soils, sediments and plants in colony of red-footed booby (Sula sula) in the Dongdao Island of South China Sea. Chemosphere 65:707–15.
Milchunas DG, Lauenroth WK. 1995. Inertia in plant community structure: state changes after cessation of nutrient-enrichment stress. Ecological Applications 5:452–8.
Montevecchi WA, Tuck LM. 1987. Newfound birds: exploitation, study, conservation. Cambridge, MA: Nuttall Ornithological Club. p 273p.
Nogales M, Medina FM, Quilis V, González-Rodríguez M. 2001. Ecological and biogeographical implications of Yellow-Legged Gulls (Larus cachinnans Pallas) as seed dispersers of Rubia fruticosa Ait. (Rubiaceae) in the Canary Islands. Journal of Biogeography 28:1137–45.
Notaro M, Vavrus S, Liu Z. 2006. Global vegetation and climate change due to future increases in CO2 as projected by a fully coupled model with dynamic vegetation. Journal of Climate 20:70–90.
Otero XL, De La Peña-Lastra S, Pérez-Alberti A, Ferreira TO, Huerta-Diaz MA. 2018. Seabird colonies as important global drivers in the nitrogen and phosphorus cycles. Nature Communications 9:246.
Paton DC, Ford HA. 1977. Pollination by birds of native plants in South Australia. Emu 77:73–85.
Peng L, Kawagoe Y, Hogan P, Delmer D. 2002. Sitosterol-β-glucoside as primer for cellulose synthesis in plants. Science 295:147–50.
Polis GA, Anderson WB, Holt RD. 1997. Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annual Review of Ecology and Systematics 28:289–316.
Pollet IL, Bond AL, Hedd A, Huntington CE, Butler RG, Mauck R. 2019. Leach’s Storm-Petrel (Oceanodroma leucorhoa), version 2.0. In: Rodeward PG, Ed. The birds of North America. Cornell Lab of Ornithology: Ithaca.
Post DM, Taylor JP, Kitchell JF, Olson MH, Schindler DE, Herwig BR. 1998. The role of migratory waterfowl as nutrient vectors in a managed wetland. Conservation Biology 12:910–20.
Rosseau D-D, Schevin P, Ferrier J, Jolly D, Andreasen T, Ascanius SE, Hendriksen S-E, Poulsen U. 2008. Long-distance pollen transport from North America to Greenland in Spring. Journal of Geophysical Research 113:G02013.
Sánchez-Piñero F, Polis GA. 2000. Bottom-up dynamics of allochthonous input: direct and indirect effects of seabirds on islands. Ecology 81:3117–32.
Schelske CL, Peplow A, Brenner M, Spencer CN. 1994. Low-background gamma counting: applications for 210Pb dating of sediments. Journal of Paleolimnology 10:115–28.
Sekercioglu CH. 2006. Increasing awareness of avian ecological function. Trends in Ecology and Evolution 21:464–71.
Simpson GL. 2018. Modelling palaeoecological time series using generalised additive models. Frontiers in Ecology and Evolution 6:149.
Simpson GL. 2019. Package ‘gratia’ v.0.2-8. (https://cran.r-project.org/web/packages/gratia/gratia.pdf).
Sklepkovych BO. 1986. The predatory behaviour and impact of Red Foxes (Vulpes vulpes) on the seabird colonies of Baccalieu Island, Newfoundland. Masters thesis, Memorial University, St. John’s, NL, Canada.
Sklepkovych BO, Montevecchi WA. 1989. The world’s largest known nesting colony of Leach’s Storm-Petrels on Baccalieu Island, Newfoundland. American Birds 43:38–42.
Stewart EM, Michelutti N, Shenstone-Harris S, Grooms C, Weseloh C, Kimpe LE, Blais JM, Smol JP. 2015. Tracking the history and ecological changes of rising Double-crested Cormorant populations using pond sediments from islands in Eastern Lake Ontario. PLoS ONE 10:e0134167.
Tilman D. 1986. Resources, competition and the dynamics of plant communities. In: Crawley M, Ed. Plant Ecology. Oxford UK: Blackwell Scientific Publications. p 51–75.
U.S. EPA. 2002. Methods for evaluating wetland condition: land-use characterization for nutrient and sediment risk assessment. Office of water, U.S. environmental protection agency, Washington, DC. EPA-822-R-02-025.
van Geel B. 2001. Non-pollen palynomorphs. In: Smol JP, Birks HJB, Last WM, Eds. Tracking environmental change using lake sediments, volume 3: Terrestrial, algal, and siliceous indicators. Dordrecht: Kluwer Academic Publishers. p 99–120.
Volkman JK. 1986. A review of sterol markers for marine and terrigenous organic matter. Organic Geochemistry 9:83–99.
Wait DA, Aubrey DP, Anderson WB. 2005. Seabird guano influences on desert islands: soil chemistry and herbaceous species richness and productivity. Journal of Arid Environments 60:681–95.
Whelan CJ, Şekercioğlu ÇH, Wenny DG. 2015. Why birds matter: from economic ornithology to ecosystem services. Journal of Ornithology 156:227–38.
Wilhelm SI, Hedd A, Robertson GJ, Mailhiot J, Regular PM, Ryan PC, Elliot RD. 2019. The world’s largest breeding colony of Leach’s Storm-petrel Hydrobates leucorhous has declined. Bird Conservation International: 1–12.
Wood SN. 2017. Generalized additive models: an introduction with R. Boca Raton, FL: CRC Press. p 496p.
Zwolicki A, Zmudczyńska-Skarbek K, Richard P, Stempniewicz L. 2016. Importance of marine-derived nutrients supplied by planktivorous seabirds to high Arctic tundra plant communities. PLoS ONE 11:e0154950.
Acknowledgements
Thank you to D. Fifield, A. Hedd, P. Ryan and S. Wilhelm at Environment and Climate Change Canada (ECCC) in St. John’s, Newfoundland for their logistical assistance preparing for fieldwork, and thank you to C. Grooms for assistance with fieldwork. We thank A. Telka at Paleotec Services, Ottawa, for macrofossil extraction and identification, and the Keck Carbon Cycle AMS Laboratory at the University of California for handling 14C dating. Funding was provided by the Natural Sciences and Engineering Research Council (NSERC) of Canada (Grant #RGPIN-2017-04548, awarded to J.P.S. and J.M.B.). We acknowledge the Land Management Division, Newfoundland and Labrador Department of Fisheries and Land Resources as the managing agency for the Baccalieu Island Ecological Reserve.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
MPD, GJR, and JPS designed the study. MPD collected the sediment cores. JRG delineated vegetation from the aerial and satellite photographs, which were digitized by MPD. MPD completed palynological analyses. DCE and JAK completed sterol and stanol analysis. MPD, NM, JMB, and JPS completed data analysis. All authors contributed to writing and editing the manuscript.
Rights and permissions
About this article
Cite this article
Duda, M.P., Glew, J.R., Michelutti, N. et al. Long-Term Changes in Terrestrial Vegetation Linked to Shifts in a Colonial Seabird Population. Ecosystems 23, 1643–1656 (2020). https://doi.org/10.1007/s10021-020-00494-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-020-00494-8