Abstract
Environmental conditions influence the breeding and migratory patterns of many avian species and may have particularly dramatic effects on long-distance migrants that breed at northern latitudes. Environment, however, is only one of the ecological variables affecting avian phenology, and recent work shows that migration tactics may be strongly affected by changes in predator populations. We used long-term data from 1978 to 2000 to examine the interactions between snowmelt in western Alaska in relation to the breeding or migration phenologies of small shorebirds and their raptor predators. Although the sandpipers’ time of arrival at Alaskan breeding sites corresponded with mean snowmelt, late snowmelts did delay breeding. These delays, however, did not persist to southward migration through British Columbia, likely due to the birds’ ability to compensate for variance in the length of the breeding season. Raptor phenology at an early stopover site in British Columbia was strongly related to snowmelt, so that in years of early snowmelt falcons appeared earlier during the sandpipers’ southbound migration. These differential effects indicate that earlier snowmelt due to climate change may alter the ecological dynamics of the predator–prey system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An annual migration to distant breeding sites is a common strategy among many taxonomic groups, as it allows animals to raise their offspring in highly-seasonal areas that may not support life year-round. Many ecological factors influence migratory behaviors (Alerstam et al. 2003), including abiotic factors such as climate (Crick 2004) and photoperiod (Quinn and Adams 1996; Coppack and Pulido 2004), as well as biotic factors such as perceived predation risk (Lank et al. 2003) and prey abundance (Walter 1979; Schneider and Harrington 1981). In particular, those animals migrating long distances face great challenges in fine-tuning the timing of their annual cycles, as they have little knowledge of the environmental conditions that lie ahead and any adjustments to their schedule must be made from afar (Both and Visser 2001).
During the boreal spring, many birds of different species migrate thousands of kilometres to high latitude ecosystems to breed, taking advantage of the seasonally-rich food resources for the raising of the young (Alerstam et al. 2003). High latitude breeders may be especially vulnerable to ecological changes, as climate becomes more extreme and variable with latitude (Martin and Wiebe 2004), making assessment of conditions from distant non-breeding areas correspondingly less accurate. Understanding the proximate factors influencing the breeding and migratory behaviors of birds at high latitudes is essential in understanding the likely influence of changing environmental conditions on their ecological dynamics.
In many northern regions, migrating birds appear to be able to adapt to long-term changes in the abiotic environment. Plasticity in migratory and breeding behaviors has allowed some migrating birds to lay their eggs earlier in response to decreased severity of winters and earlier springs (e.g., Crick et al. 1997; Jenni and Kéry 2003; Pearce-Higgins 2005), potentially increasing reproductive output (Lack 1968). Certainly, rising temperatures during winter and spring are likely to affect snow cover at tundra breeding sites, but the timing of snowmelt in these areas may be a more direct correlate for the timing of breeding. Late snow limits both nest sites and the food resources of migrant birds and consequently delay breeding (Holmes 1972; Young et al. 1995; Babcock et al. 2002). Thus, annual variation in snowmelt could be an important factor in setting the timing of the spring migration to breeding sites and the timing of egg-laying. Delays in breeding caused by the late snowmelt could then have long-term effects on the timing of southward migration.
Avian breeding and migration strategies may also be strongly shaped by the biotic environment. Some long-distance migrants appear unable to change their timing to correspond with environmental changes at breeding sites (Both and Visser 2001). For these species, migratory timing may be driven primarily by conditions or events during the non-breeding season (Both and Visser 2001; Visser et al. 2004; Crick 2004). Perceived predation risk at migratory stopover sites may drive the migratory timing and behaviors of some shorebirds (Lank et al. 2003; Ydenberg et al. 2004). In particular, the recent recovery of many raptor populations following decades of decline (Hoffman and Smith 2003) has the potential to affect the migratory patterns of their prey species, as migrating birds encounter greater numbers of predators on their journey. For example, Ydenberg et al. (2004) documented decreases in body mass and shortened stopover durations in shorebirds migrating through sites that have become more risky over the past decade. Furthermore, climate may influence the timing and abundance of raptors that prey upon migrating birds (Dawson and Bortolotti 2000), whose migrations may in fact be timed to coincide with that of their prey species (Lank et al. 2003). The mechanisms driving avian breeding and migration are abundant and complex, but it is evident that we must not only consider interactions between birds and their environment, but also the interactions between birds and their predators.
Here, we examine how regional weather at breeding sites and the presence of predators during migration interact to affect the timing of breeding and southward migration for small arctic-breeding shorebirds. Western sandpipers (Calidris mauri) are an ideal model system for examining the mechanisms driving these behaviors, as they migrate at an average of 10,000 km to breed in the arctic and sub-arctic regions (Wilson 1994). Peregrine falcons (Falco peregrinus) are a principal predator of C. mauri during the non-breeding season (Page and Whitacre 1975) as well as during migration (Lank et al. 2003; Ydenberg et al. 2004). The influence of falcon population dynamics on that of sandpipers is evident: the recent recovery of falcon numbers in western North America (Hoffman and Smith 2003) has already led to changes in some migratory behaviors of migrating sandpipers (Ydenberg et al. 2004). In this system, both predator and prey species breed at high latitudes before migrating to the coastal wintering sites, so northern climatic conditions may affect both species’ phenologies. We predict that the timing of the northward migration and breeding of C. mauri will be strongly correlated with snowmelt, and the timing of their southward migration will also be indirectly affected by snowmelt (when breeding has been delayed). We predict that the timing of the F. peregrinus migration back to the migratory stopover sites will correlate with snowmelt.
Materials and methods
Study species
Calidris mauri are long-distance migrants, travelling between non-breeding areas along the Caribbean and Pacific coastlines and a small breeding range centered in western Alaska and eastern Siberia (Wilson 1994). Most migrate southward along the Pacific Ocean to non-breeding sites ranging from the northwest coast of the United States to Peru (Wilson 1994; Clark and Butler 1999). Breeding takes place in late May to early July (Holmes 1972; Neville 2002; Ruthrauff 2002), and southbound adults move through British Columbian stopover sites beginning in July, followed a month later by juveniles (Butler et al. 1987).
The migration of F. peregrinus through these stopovers coincides with the sandpipers’ timing, both in spring and autumn (Butler et al. 2003). Like their shorebird prey, many F. peregrinus migrate between tundra and forest breeding sites in western Canada and Alaska and wintering sites in Central and South America. In fact, the southbound passage of adult and juvenile falcons along the west coast of North America has been likened to a ‘tsunami’ (Butler et al. 2003) that sandpipers must attempt to avoid, as falcons constitute a major predator of sandpipers during their migration (Ydenberg et al. 2002).
Timing of snowmelt in breeding regions
We derived an annual regional snowmelt index date from weather records (c.f. Foster 1989) available at three sites spanning the breeding range of C. mauri in Alaska (see Holmes 1972). Daily records of snow depth were available from Bethel (60°47.0′N, 161°50.0′W), Emmonak (62°47.0′N, 164°29.0′W), and Nome (64°30.0′N, 165° 25.8′W) for the years 1978 to 2000 (National Data Centers, National Oceanic and Atmospheric Administration 2000). We defined the date of snowmelt at each site as the last day on which >1.25 cm (0.5 in.) snow depth was registered, and took the average date of snowmelt across all sites as our regional snowmelt index date. This depth was selected according to the limitations of the meteorological data and was deemed to have minimal effect on sandpiper nesting capabilities. Snow depths were not recorded at Nome in 1998 or Emmonak in 1986, 1992, or 1994–2000, and thus the number of sites available on which to base the annual index was one (1998), two (1986, 1992, 1994–2000) or three (all other years). Timing of snowmelt was on average 3 weeks earlier at the Bethel site than the other two locales, which were very similar to each other, but the actual difference among the sites varied widely among years.
Overall, we believe that our composite index satisfactorily represents the timing of snowmelt western-coastal Alaska. However, we recognize that conditions could occasionally vary greatly between specific locales across the breeding range of C. mauri. For example, in one year our regional index date differed substantially from snowmelt dates reported by Babcock et al. (2002) at a particular site on the Yukon-Kuskokwim Delta (1998: snowmelt on the local day-of-the-year 94 vs. regional day 140). This strong discrepancy occurred in only one of the 17 years in this comparison, and with 1998 excluded there is a strong relationship between Babcock’s local estimates and our regional index (r = 0.70, P = 0.02, N = 16). Note that 1998 was the only year in which our regional snowmelt date had to be based on data from a single site. For comparative purposes, we also ran these analyses incorporating the snowmelt timing data reported by Babcock et al. (2002) and excluding the regional data from 1998.
Timing of hatch for small shorebirds
In 1982 and 1985–2000, intensive nest surveys were conducted in coastal areas of the Yukon-Kuskokwim Delta in western Alaska (Bowman et al. 2001), during which the hatch date was estimated for every nest of several avian species. In these surveys, ‘small shorebirds’ were grouped together, and included several Calidrine species (C. mauri, C. pusilla, C. alpina, C. ptilocnemis), as well as Phalaropus spp. and Arenaria spp., whose nests could not always be differentiated. The hatch dates for small shorebirds were similar to reported hatch timing of C. mauri in western Alaska (Holmes 1972; Sandercock 1997; Neville 2002; Ruthrauff 2002), and small shorebirds breeding in the same geographical location may exhibit similar temporal patterns of breeding (Sandercock 1997). In fact, the timing of hatch was similar for all species in the survey of Bowman et al. (2001), suggesting large-scale environmental effects (Ydenberg et al. 2005). On an average, 9.4 (± 3.7) small shorebird nests were found each year, and we took the mean estimated hatch date as an index of the hatch timing for C. mauri.
Timing of sandpiper stopover during southward migration
More than 11,000 southbound adult and juvenile C. mauri were mist-netted over 18 years between 1978 and 2000 at the Strait of Georgia, British Columbia (49° N, 123° W). This is the largest and the first major stopover on the southward migration after departure from Alaska (Butler and Kaiser 1995). Measurement methods and criteria for age and gender assignment are described in Butler et al. (1987) and Ydenberg et al. (2004). Mist-netting began early in July and continued until late August.
We used the mean capture dates of adults and juveniles as indices of passage timing. To minimize bias due to uneven capture, we restricted estimates for an age class to years in which at least 20 C. mauri of that age class were captured over at least a 10 day-time period. Based on these criteria, we used data from 14 years for adults: 1978–1983, 1985, 1990, and 1995–2000; and 18 years for juveniles: 1978–1983, 1985, 1989–1990, and 1992–2000. The average numbers of captures in a year was 316 (range: 23–1,221) adults and 384 (range: 38–1,734) juveniles.
Timing of falcon arrival at sandpiper stopover during southward migration
The presence of F. peregrinus in the Strait of Georgia has a strong seasonal rhythm. Standardized 1 h surveys were conducted nearly-daily beginning in 1986 at the Reifel Island Migratory Bird Sanctuary (Lank et al. 2003; Ydenberg et al. 2004), located adjacent to the large tidal mudflats used by sandpipers. These surveys show that F. peregrinus numbers are low during June and July, but numbers rise sharply in abundance beginning in the late summer and plateau in autumn (see Fig. 1 in Lank et al. 2003). The onset of this sharp increase appears to vary greatly between years. To estimate the timing of this onset, we derived a peregrine arrival timing index based on the surveys made between July 1 and October 31 (123 days) in the years 1986–2000 (∼1,100 surveys), during which about 1,250 F. peregrinus sightings were made. We estimated falcon abundance by combining sightings made over 3-day intervals, to smooth out day-to-day variation in presence. We then defined falcon arrival timing as the interval in which the number of falcons present at Reifel Island reached 50% of the maximum for that year.
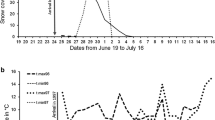
Annual variation in the phenology of Calidris mauri and Falco peregrinus is shown between northern nesting sites and a migratory stopover in British Columbia from 1978 to 2000. Regional snowmelt (black circles) is plotted against itself to form a 1:1 reference line, with points jittered to reveal any overlapping data. Above the reference line, the timing of hatch for small shorebirds in western Alaska is plotted (triangles) in relation to snowmelt timing. Finally, the arrival timing of adult sandpipers (filled squares), falcons (open dots) and juvenile sandpipers (hashed diamonds) at a migratory stopover site are plotted in relation to the snowmelt timing. Although snowmelt timing does not appear to influence the migratory phenology of sandpipers, it relates very strongly with the arrival timing of falcons at southbound stopover sites
Statistical analysis
We report results using Pearson product-moment correlations, and means ± 95% confidence intervals. Sample sizes vary among our analyses because not all years are represented in all four datasets.
Results
From 1978 to 2000, mean snowmelt occurred in western-coastal Alaska on day-of-the-year 120 (± 4.4 days), but varied among years by 43 days. Over the same time period, the mean hatch date for small shorebirds was about 1.5 months later, on day 174 (± 4.7 days). Overall, the hatch of small shorebirds took place earlier in years with earlier snowmelt (r = 0.60, P < 0.01, N = 17), and results were similar when the snowmelt data of Babcock et al. (2002) were included and 1998 was excluded (see Materials and methods) (r = 0.7, P = 0.01, N = 16). More specifically, the timing of hatch was not related to snowmelt (r = 0.0, N = 11) in years when snowmelt occurred prior to sandpiper arrival (day-of-the-year 125; see Discussion). However, hatch was significantly delayed in years when snowmelt took place after day 125 (r = 0.77, P < 0.05, N = 7), changing by 0.64 days (r = 0.77, P < 0.05, N = 7) for each day that snowmelt was delayed.
The mean timing of southward passage of adult C. mauri in the Strait of Georgia was day 198.3 (± 3.1 days) and occurred on average 25.7 ± 6.5 days after the index hatch date. Migratory timing showed no correlation with either snowmelt or hatch (both P > 0.5), likely because the timing of migration was the least variable of the factors that we considered. In fact, sandpiper passage timing varied by only 19 days, less than half the range recorded among years for snowmelt (Fig. 1). The consistency in migratory timing means that the observed interval between hatch and migratory passage dates shortened by 1.05 days for each day that hatch was delayed (r = −0.77, P < 0.01, N = 9). Juvenile C. mauri showed similar patterns to adults, with a mean southward migratory passage on day-of-the-year 229.6 (± 2.3 days) and varying among years by only 20 days (Fig. 1). Passage occurred on average 54.3 ± 3.6 days after the index hatch date (day 228) and was not related to either snowmelt or hatch timing (both P > 0.5). As observed with adults, the interval between the hatch index date and the passage index date shortened strongly (by 0.89 days) for each day that hatch was delayed (r = 0.85, P < 0.01, N = 9).
The arrival of F. peregrinus in the Strait of Georgia varied among years by 54 days, taking place on average 100.2 ± 5.3 days after the regional snowmelt index date (day-of-the-year 220). The timing of the falcon index varied significantly with snowmelt (r = 0.81, P < 0.001, N = 13), advancing by 1.09 days for each day that snowmelt was earlier, and the result did not differ when the snowmelt data of Babcock et al. (2002) were incorporated into the analyses (r = 0.67, P = 0.02, N = 12). The timing of the falcon migration did not vary directly with the timing of the sandpiper migration (P > 0.5).
Discussion
We examined how ecological factors may interact and influence the breeding and migratory behaviors of birds breeding at high latitude. The timing of breeding for the sandpipers was related to snowmelt, specifically for years in which snowmelt occurred after migratory arrival. However, even years of late snowmelt did not appear to produce any long-term delays in the southward migration of sandpipers. In fact, we found that the timing of southward migration by both adult and juvenile C. mauri was far less variable among years than the ecological events bounding the breeding season, namely snowmelt in the spring and the reappearance of F. peregrinus at southward stopover sites in the autumn. The relatively invariable southward migration timing of the sandpipers contrasted with that of their principal predator, which showed dramatic variability among years and in relation to snowmelt timing. Although there was no direct relationship between falcon arrival at stopover sites and the migratory passage of the sandpipers, sandpipers appear more likely to encounter falcons in years with early snowmelt (Fig. 1).
Snowmelt was highly variable among years in the breeding region of C. mauri, but the relationship between snowmelt and breeding depended strongly on the estimated timing of sandpipers’ arrival at the breeding sites. Northward passage of C. mauri through southwestern British Columbia, Canada, takes place between days 109 and 130, and peaks on day-of-the-year 120 (based on surveys in 7 years between 1992 and 2000; Butler personal communication; see also Butler et al. 1987). Radio-tracking work shows that individuals travel rapidly from British Columbia to the Copper River Delta in Alaska (Iverson et al. 1996), and movement to the breeding areas follows shortly afterward. Therefore, arrival at breeding sites probably peaks on day 125 or 130. We found a mean snowmelt date at day-of-the-year 120, indicating that the arrival of C. mauri across the breeding region appears to occur just after the expected timing of snowmelt. Presumably, C. mauri cannot predict the actual date of snowmelt when they set out on migration from their non-breeding areas. Northward migration is rapid, and with limited opportunity to advance the arrival date in the course of the migration, a schedule designed to bring them to the destination just after the expected (i.e. mean) snowmelt date appears advantageous.
The mean southward passage of both adult and juvenile sandpipers varied much less dramatically than snowmelt or the timing of breeding, and was not directly related to either of these events. Flexibility in the length of the breeding period may mask any season-long effects caused by delays in snowmelt. For example, in years of early snowmelt, the interval between the breeding and migratory passage indices was 35 days for adult C. mauri and 62 days for juveniles. In late snowmelt years, these intervals shortened to 15 and 42 days, respectively.
The specific mechanisms by which these changes occur are not clear, but likely result from behavioral adjustments to breeding or migratory strategies. Conditions at northern post-breeding sites or migratory stopovers do not appear to force the migratory timing of C. mauri. In Alaska, 90% of the bird species found in some pre-migratory staging areas in October are ‘small shorebirds’ (Gill and Handel 1990). In addition, evidence for declining food abundances at stopovers is equivocal. Food availability at southward migratory stopovers in eastern North America declines over the period of the southward migration for some shorebirds (Schneider and Harrington 1981; Boates and Smith 1989). In contrast, a study in British Columbia found a seasonal increase in food abundance for southward-migrating C. mauri (Wolf 2001). Possibly, the opportunities for breeding and re-nesting are reduced in years with late snowmelt for C. mauri, resulting in fewer adults with parental duties that delay southward departure, and fewer young hatched. Individual sandpipers could possibly speed up the rate of southward migration by spending less time fattening at early migratory stopovers, although this is unlikely to be the case. Assessment of inter-annual variability in body condition during the southward migration could elucidate some of these questions.
In contrast with the sandpipers, the timing of falcon arrival on stopover sites during southward migration shows a strong and consistent relationship with snowmelt. For F. peregrinus breeding in northern and western Canada and Alaska, snow cover is likely to play a strong role in the timing of food availability, and perhaps the longer incubation and parental care provided by breeding falcons limits the flexibility timing of their breeding season among years with varying snowmelt timing. Although we are uncertain what proportion of the falcons sighted in southwestern British Columbia are from Canadian or Alaskan populations, we hypothesize that large-scale climate factors such as position of the Aleutian Low affect the timing of snowmelt similarly for both predators (i.e., falcons) and prey (i.e., sandpipers) breeding at the northern latitudes.
Several recent studies have evaluated trends in the timing of breeding and migration with respect to changing climate (e.g., Crick et al. 1997; Stevenson and Bryant 2000; Both and Visser 2001; Jenni and Kéry 2003) or predator landscapes (e.g., Ydenberg et al. 2004). The data reported here suggest that advances in the date of snowmelt caused by climate change may produce species-specific effects on the migratory timing of some species of birds. In this case, the predatory species (F. peregrinus) appears to respond more strongly to annual variation in snowmelt than does the prey species (C. mauri). This means that early snowmelt years may correspond with relatively more dangerous southward migrations for C. mauri, because in these years the sandpipers may be more likely to encounter falcons. Our work highlights the need to consider the climate change effects in an ecological framework including inter-specific interactions. We believe that the future work on climate change will be of greater value if organisms are not merely considered in isolation, but as part of a greater predatory–prey ecosystem.
References
Alerstam T, Hedenström A, Åkesson S (2003) Long-distance migration: evolution and determinants. Oikos 103:247–260
Babcock CA, Fowler AC, Ely CR (2002) Nesting ecology of tundra swans on the coastal Yukon-Kuskokwim Delta, Alaska. Waterbirds 25(SP1):236–240
Boates JS, Smith PC (1989) Crawling behaviour of the amphipod Corophium volutator and foraging by Semipalmated Sandpipers, Calidris pusilla. Can J Zool 67:457–462
Both C, Visser ME 2001. Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature 411:296–298
Bowman T, Stehn R, Walters G (2001) Population size and production of geese and eiders nesting on the Yukon-Kuskokwim Delta, Alaska. In: Report U.S. Fish and Wildlife Service, Anchorage, AK
Butler R, Kaiser G (1995) Migration chronology, sex ratio, and body mass of least sandpipers in British Columbia. Wilson Bull 107:413–422
Butler RW, Kaiser GW, Smith GEJ (1987) Migration chronology, length of stay, sex ratio, and weight of western sandpipers, (Calidris mauri) on the south coast of British Columbia. J Field Ornithol 58:103–111
Butler R, RC Ydenberg DB Lank (2003) Wader migration on the changing predator landscape. Wader Study Group Bull 100:130–133
Clark CW, Butler RW (1999) Fitness components of avian migration: a dynamic model of western sandpiper migration. Evol Ecol Res 1(4):443–457
Coppack T, Pulido F (2004) Photoperiodic response and the adaptability of avian life cycles to environmental change. Birds Clim Chan Adv Ecol Res 35:131–150
Crick HQP (2004) The impact of climate change on birds. Ibis 146(supp 1):48–56
Crick HQP, Dudley C, Glue DE, Thompson DL (1997) UK birds are laying eggs earlier. Nature 411:296–298
Dawson RD, Bortolotti GR (2000) Reproductive success of American kestrels: the role of prey abundance and weather. Condor 102:814–822
Foster JL (1989) The significance of snow disappearance on the arctic tundra as a possible indicator of climate change. Arct Alp Res 21:60–70
Gill RE Jr, Handel CM (1990) The importance of subarctic intertidal habitats to shorebirds: a study of the central Yukon-Kuskokwim Delta, Alaska. Condor 92:709–725
Hoffman SW, Smith JP (2003) Population trends of migratory raptors in western North America, 1977–2001. Condor 105:397–419
Holmes RT (1972) Ecological factors influencing the breeding season schedule of western sandpipers (Calidris mauri) in subarctic Alaska. Am Midl Nat 87:472–491
Iverson GC, Warnock SE, Butler RW, Bishop MA, Warnock N (1996) Spring migration of western sandpipers (Calidris mauri) along the Pacific coast of North America: a telemetry study. Condor 98:10–22
Jenni L, Kéry M (2003) Timing of autumn bird migration under climate change: advances in long-distance migrants, delays in short-distance migrants. Proc R Acad Sci B 270:1467–1471
Lack D (1968) Bird migration and natural selection. Oikos 19:1–9
Lank DB, Butler RW, Ireland J, Ydenberg RC (2003) Effects of predation danger on migration strategies of sandpipers. Oikos 103:303–319
Martin K, Wiebe KL (2004) Coping mechanisms of alpine and arctic breeding birds: extreme weather and limitations to reproductive resilience. Integr Comp Biol 44(2):177–185
Neville JA (2002) Division of parental roles in the monogamous western sandpiper, Calidris mauri. MSc Thesis, University of Alaska
Page G, Whitacre DF (1975) Raptor predation on wintering shorebirds. Condor 77:73–83
Pearce-Higgins JW, Yalden DW, Whittingham MJ (2005) Warmer springs advance the breeding phenology of golden plovers Pluvialis apricaria and their prey (Tipulidae). Oecologia 143:470–476
Quinn TP, Adams DJ (1996) Environmental changes affecting the migratory timing of American shad and sockeye salmon. Ecology 77(4):1151–1162
Ruthrauff DR (2002) Seasonal and age-related trends in the reproductive output of western sandpipers (Calidris mauri) at Kanaryaraq, Alaska. MSc Thesis, Humboldt State University
Sandercock BK (1997) Factors affecting the breeding demography of western sandpipers (Calidris mauri) and semipalmated sandpipers (Calidris pusilla) at Nome, Alaska. PhD Thesis, Simon Fraser University
Schneider DC, Harrington BA (1981) Timing of shorebird migration in relation to prey depletion. Auk 98:801–811
Stevenson IR, Bryant DM (2000) Climate change and constraints on breeding. Nature 406:366–367
Visser ME, Both C, Lambrechts MM (2004) Global climate change leads to mistimed avian reproduction. Adv Ecol Res 35:89–100
Walter H (1979) Eleonora’s falcon: adaptations to prey and habitat in a social raptor. Chicago University Press, Chicago
Wolf N (2001) Foraging ecology and site selection in Western Sandpipers during their fall migration through southwestern British Columbia. MSc Thesis, Simon Fraser University
Wilson WH (1994) The Western Sandpiper (Calidris mauri). In: Poole A, Gill F (eds) The Birds of North America, No. 90. The Academy of Natural Sciences of Philadelphia, PA, and The American Ornithologists’ Union, Washington, DC pp 1–19
Ydenberg RC, Butler RW, Lank DB, Guglielmo C, Lemon MJF, Wolf N (2002) Trade-offs, condition dependence and stopover site selection by migrant sandpipers. J Avian Biol 33:47–55
Ydenberg RC, Butler RW, Lank DB, Smith BD, Ireland J (2004) Western sandpipers have altered migration tactics as peregrine populations have recovered. Proc R Soc Lond B 271:1263–1269
Ydenberg RC, Niehaus AC, Lank DB (2005) Interannual differences in the relative timing of southward migration of male and female western sandpipers (Calidris mauri). Naturwissenschaften 92:332–335
Young DD Jr, McIntyre CL, Bente PJ, McCabe TR, Ambrose RE (1995) Nesting by golden eagles on the north slope of the Brooks Range in northeastern Alaska. J Field Ornithol 66(3):373–379
Acknowledgments
We thank the many members of the Canadian Wildlife Service and the Centre for Wildlife Ecology at Simon Fraser University who helped in the field. We thank especially Tim Bowman providing us with his report on the breeding of birds in western Alaska, and John Ireland, Director of the Reifel Migratory Bird Refuge, for making his falcon sighting data available. The Alaska crew, David Lank, Nils Warnock, Tony Williams, Dan Ellis, Rob Nielson, Rolf Gradinger and anonymous reviewers provided valuable comments. Research was funded by a graduate fellowship from the National Science Foundation (to ACN), grants from the National Science and Engineering Research Council of Canada (to RCY) and the Centre for Wildlife Ecology at Simon Fraser University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niehaus, A.C., Ydenberg, R.C. Ecological factors associated with the breeding and migratory phenology of high-latitude breeding western sandpipers. Polar Biol 30, 11–17 (2006). https://doi.org/10.1007/s00300-006-0154-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-006-0154-1