Abstract
In the shorebird subfamily Calidridinae, one of the parents shortens parental care and initiates southward migration before the other. We estimated the difference in passage date between male and female western sandpipers (Calidris mauri) at their first major stopover on the southward migration from breeding areas in Alaska, in 18 years between 1978 and 2000. Overall, adult females preceded adult males by 1.22 days. A novel finding was that among juveniles, which migrate approximately a month later than adults, females preceded males by similar magnitude (1.14 days). There was wide variation among years, however, and males actually preceded females in years with late hatch. We relate these findings to hypotheses for female-first southward migration in sandpipers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the context of avian migration, the order with which males and females migrate has long been of interest. Males generally precede females on northward migration among shorebirds (Myers 1981; Ketterson and Nolan 1983; Butler et al. 1987) and passerines (Chandler and Mulvihill 1990; Swanson et al. 1999), presumably due to the importance of early arrival in acquiring good breeding territories (Kokko 1999). Supporting this hypothesis, females precede males on northward migration in role-reversed shorebird species, such as spotted sandpipers (Oring and Lank 1982) and phalaropes (Reynolds et al. 1986).
Among shorebirds, the order of migration when southbound is associated with the pattern of parental care (Myers 1981). Generally the non-caring parent departs the breeding grounds and initiates migration first. The evolutionary reasons for this pattern are not clear, and Reynolds and Székely (1997) and Myers (1981) both state that information on intra-specific variation in these factors would be especially revealing. Here we report on the difference in southward migratory timing between male and female western sandpipers (Calidris mauri) in 18 years between 1978 and 2000.
Methods
Migratory timing
Western sandpipers were mist-netted from 1978–2000 in the Strait of Georgia, British Columbia (49°05′ N, 123°00′W). This is the first stopover on southward migration after breeding (Butler and Kaiser 1995), and follows a ∼2900 km migratory jump over the Gulf of Alaska. Mist-netting began with the onset of adult migration early in July, and continued through the end of the juvenile migration period in late August. Methods are described by Butler et al. (1987) and Ydenberg et al. (2004). We restricted analyses to years in which at least 20 individuals of a particular age class were captured over at least 10d, admitting 14 years with on average 317 adults, and 18 years with on average 383 juveniles.
Passage timing
Migration timing (DeLong and Hoffman 1999; Swanson et al. 1999) is usually estimated based on median passage dates. However, the median date can easily be biased when capture effort is episodic, which, because good conditions are critical, is often the case when mist-netting shorebirds. We used a method developed by Morbey (2000) that estimates the difference in passage timing as the area between the cumulative percent distributions of female and male captures (termed Dm). We express female-first migration as negative values, and male-first migration as positive values. Dm can be interpreted as the expected number of days by which the capture of a randomly-selected male precedes that of a randomly-selected female. The likelihood that the value of Dm occurred by chance can be estimated by comparing the estimated value of Dm with a null distribution (i.e. that expected if the order of arrival were random with respect to gender). The null distribution is generated by randomly assigning a gender to each successive capture in proportion to each gender’s representation in the data set, computing Dm, and repeating 1000 times (Morbey 2000). We expected female-first migration, and therefore report the proportion of the null distribution lying below (i.e. females further ahead) the measured value of Dm—effectively a one-tailed test that more extreme values could arise by chance.
This procedure is inadequate to test the hypothesis that males are significantly advanced when, as we unexpectedly observed, they preceded females. In these cases the proportion of the null distribution lying below the measured value of Dm is large and may approach 1.00, but this does not mean that the difference is random. To better and more conservatively test whether males ever significantly preceded females, we took the inverse (i.e. 1 minus the probability), and doubled it to make a two-tailed test. The estimates of Dm and the associated one-tailed probability levels are summarized in Table 1.
Hatch timing
In 1982 and 1985–2000, intensive nest surveys were conducted in coastal areas of the Yukon-Kuskokwim Delta in western Alaska (Bowman et al. 2001), at the heart of the breeding range of western sandpipers. We used the data reported for ‘small shorebirds’ to estimate the hatch date of western sandpipers. On average, 9.4 (±3.7 95% CI) nests were found. We used general linear models with nested age classes to evaluate the relationship between hatch date and Dm.
Bowman et al.’s (2001) methodology was not designed specifically for shorebirds, and their ‘small shorebird’ group includes the nests of several species (C. mauri, C. pusilla, C. alpina, C. ptilocnemis, Phalaropus spp., Arenaria spp.), which could not always be distinguished. Although Arctic shorebirds breeding in the same locale exhibit similar temporal patterns of breeding (Sandercock 1997), and the reported hatch dates are similar to those based on breeding studies of western sandpipers (Holmes 1972; Sandercock 1997; Neville 2002; Ruthrauff 2002), the estimated hatch dates may not be very accurate. Bowman et al.’s (2001) data show strong correlations in hatch date among almost all species, indicating that large-scale (likely weather-related) factors drive the timing of breeding for most avian species. To be less dependent on the accuracy of Bowman et al.’s estimated hatch dates, we also classified years merely as ‘early’ or ‘late’, relative to the mean hatch date of Julian day 173.9, and compared hatch in early and late years with t-tests.
The data used in our analyses are summarized in Table 1. Note that estimates for all parameters are not available for every year, accounting for the sample size differences between the comparisons that follow.
Results
As expected, the southward migration of western sandpiper adults was female-first in 11 (79%) of the 14 years in which Dm could be estimated, and was significant in 4 years. A surprising result was that male-first migration was observed in 3 of 14 years (significant in 2 years). The latter outcome is not a sampling error, as both significant years have good samples, and one (1980) has the largest sample (n=1221) of all. The overall annual average value of Dm is −1.22d (n=14), not significantly different from zero (95% CI ± 1.44). The annual estimates range from −7.83 (female-first) to +2.82 (male-first).
The southward migration of juveniles shows similar patterns, with female-first migration observed in 12 (68%) of 18 years, attaining significance in 6 years (and near-significance in 3 other years). Male-first migration was observed in 6 of 18 years, but was significant in none. The overall annual average value of Dm is −1.14d (n=18), which is significantly less than zero (95% CI ± 0.83). Annual estimates vary from −5.08 to 1.21. Using a two-tailed procedure instead of the one-tailed tests reported above when males were significantly advanced does not change any of the results (see Table 1).
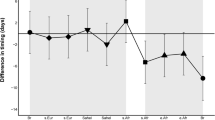
We have estimates for both hatch date and Dm in 9 years for adults and 13 years for juveniles. Using a general linear model with age class nested within hatch dates shows that the effect of hatch date on the value of Dm is positive and significant (F2,21=4.93, p=0.019; i.e. females further ahead in years with earlier hatch). The value of Dm is positive (i.e. males-first) in years with late hatch. If we restrict the analysis to the 9 years for which we have estimates of Dm for both adults and juveniles, the result remains significant (F2,17=3.92, p=0.035). Figure 1 plots the relation between hatch date and Dm, showing that the effect is similar within each age class.
This conclusion does not change if hatch is more simply classified as ‘early’ or ‘late’. The mean adult male-female difference in early years is −3.4d (SD=1.44, n=3), while in late years the difference is −0.7d (SD = 0.34, n=5; t=4.34, p<0.01). In early years the male-female difference among juveniles is −2.2d (SD = 2.27, n=6), while in late years the difference is −0.32d (SD = 1.41, n=8; t=2.84, p<0.02). If we pool age groups the differences are −2.6d (SD = 2.02, n=9) in early years, and -0.48d (SD = 1.12, n=13; t=3.15, p<0.01) in late years.
Discussion
The southward migrations of both adult and juvenile western sandpipers are on average female-first, and by similar magnitude. Female-first southward migration of adults has been previously described for western sandpipers and other calidridines (Butler et al. 1987; Jehl et al. 1979; Gratto-Trevor 1991; Butler and Kaiser 1995), but we could locate no reports on this phenomenon for juveniles of any calidridine species (though see Butler et al. 1987). A second discovery was that the timing difference varied widely among years in both adults and juveniles, and in some years male-first migration was observed. Finally, the data indicate that females are further ahead in years with early hatch.
Niehaus (2003) reviewed five main hypotheses to explain why females migrate first. Briefly, these are the breeding recovery hypothesis (females must recover from breeding), the territoriality hypothesis (males stay to defend territories), the migration distance hypothesis (females migrate further), the molt deadline hypothesis (molt must completed by a deadline), and the escape performance hypothesis (females have poorer predator-escape abilities). She concluded that the case was strongest for the escape performance hypothesis, which states that female (both adult and juvenile) western sandpipers have relatively poor escape performance due to their larger size. They therefore terminate arctic residence sooner than do males in order to migrate southward earlier in the face of seasonally-rising predation danger (see Lank et al. 2003, their Fig. 3).
Based on his comparative analysis, Myers (1981) concluded that “... these [parental care] patterns are consistent with a hypothesis that through early departure an individual can decrease the risks of long-distance migration ...”. Myers evidently associated ‘risk’ with the challenges of accumulating large energy reserves, but the idea that the risks stem from the danger posed by predators during or after migration (Lank et al. 2003) fits well into this basic hypothesis. However, neither Myers (1981) nor Reynolds and Székely (1997) explicitly considered predation danger as a possible cost of extended Arctic residence for long-distance migrants.
Our observation of female-first migration among juveniles weakens the case for hypotheses invoking molt, breeding or territorial defense, as juvenile western sandpipers do none of these. The wide variability in passage timing among years indicates that the conditions underlying the decision to initiate migration vary widely between years, weakening the case for the migration distance hypothesis. Finally, our finding that the difference in male-female timing is associated with hatch date suggests that whatever the changing conditions are, they differentially affect males and females. This observation could be explained if migration was more dangerous in years with early hatch, so that females terminated Arctic residence sooner (relative to males) than in late hatch years.
References
Bowman T, Stehn R, Walters G (2001) Population size and production of geese and eiders nesting on the Yukon-Kuskokwim Delta, Alaska, in 2001. US Fish and Wildlife Service, Anchorage, AK. Field Report
Butler R, Kaiser G (1995) Migration chronology, sex ratio, and body mass of least sandpipers in British Columbia. Wils Bull 107:413–422
Butler RW, Kaiser GW, Smith GEJ (1987) Migration chronology, length of stay, sex ratio, and weight of western sandpipers, (Calidris mauri) on the south coast of British Columbia. J Field Ornithol 58:103–111
Chandler CR, Mulvihill RS (1990) Interpreting differential timing of capture of sex classes during spring migration. J Field Ornithol 61:85–89
DeLong J, Hoffman SW (1999) Differential autumn migration of sharp-shinned and Cooper’s hawks in western North America. Condor 101:674–678
Gratto-Trevor CL (1991) Parental care in semipalmated sandpipers Calidris pusilla: brood desertion by females. Ibis 133:394–399
Holmes RT (1972) Ecological factors influencing the breeding season schedule of western sandpipers (Calidris mauri) in subarctic Alaska. Amer Midland Nat 87:472–491
Jehl JR Jr (1979) The autumnal migration of Baird’s sandpiper. In: Pitelka FA (ed) Studies in avian biology No. 2, Cooper Ornithological Society. Allen Press, Lawrence, Kansas, pp 55–68
Ketterson ED, Nolan V Jr (1983) The evolution of differential migration. Current Ornithol 1:357–402
Kokko H (1999) Competition for early arrival in birds. J Anim Ecol 68:940–950
Lank DB, Butler RW, Ireland J, Ydenberg RC (2003) Effects of predation danger on migration strategies of sandpipers. Oikos 103:303–319
Morbey Y (2000) Protandry in Pacific salmon. Can J Fish Aquatic Sci 57:1252–1257
Myers JP (1981) Cross-seasonal interactions in the evolution of sandpiper social systems. Beh Ecol Sociobiol 8:195–202
Neville JA (2002) Division of parental roles in the monogamous western sandpiper, Calidris mauri. MSc thesis, Univ Alaska, Fairbanks, AK
Niehaus AC (2003) Ecology of migratory timing for southbound male and female western sandpipers (Calidris mauri). MSc thesis, Simon Fraser University, Burnaby, BC
Oring LW, Lank DB (1982) Sexual selection, arrival times, philopatry, and site-fidelity in the polyandrous spotted sandpiper. Behav Ecol Sociobiol 10:185–191
Reynolds JD, Colwell MA, Cooke F (1986) Sexual selection and spring arrival times of red-necked and Wilson’s phalaropes. Behav Ecol Sociobiol 18:303–310
Reynolds JD, Székely T (1997) The evolution of parental care in shorebirds: life histories, ecology, and sexual selection. Behav Ecol 8:126–134
Ruthrauff DR (2002) Seasonal and age-related trends in the reproductive output of western sandpipers (Calidris mauri) at Kanaryaraq, Alaska. MSc thesis, Humboldt State University, Arcata, CA
Sandercock BW (1997) Incubation capacity and clutch size determination in two calidrine sandpipers: a test of the four-egg hypothesis. Oecologia 110:50–59
Swanson DL, Liknes ET, Dean KL (1999) Differences in migratory timing and energetic condition among sex/age classes in migrant ruby-crowned kinglets. Wils Bull 111:61–69
Ydenberg RC, Butler RW, Lank DB, Smith BD, Ireland J (2004) Western sandpipers have altered migration tactics as peregrine populations have recovered. Proc Roy Soc Lond B 271:1263–1269
Acknowledgments
We thank the Canadian Wildlife Service, Environment Canada, and the Centre for Wildlife Ecology at Simon Fraser University for support. We especially thank Tim Bowman for sharing his reports with us. Our research was supported by a Pre-doctoral Fellowship from the National Science Foundation to AN, and grants from the Environment Canada and NSERC Canada to RY. Permits were granted by SFU, the State of Alaska, and the US Fish and Wildlife Service
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ydenberg, R.C., Niehaus, A.C. & Lank, D.B. Interannual differences in the relative timing of southward migration of male and female western sandpipers (Calidris mauri). Naturwissenschaften 92, 332–335 (2005). https://doi.org/10.1007/s00114-005-0637-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-005-0637-x