Abstract
Key message
Microwounding pre-treatment facilitates agroinfiltration and transient gene expression in hard-to-agroinfiltrate citrus varieties.
Abstract
Agrobacterium infiltration is a widely used method for transient expression studies in plants, but this method is not used extensively in citrus because of its low efficiency. In this study, we developed an easy, cheap, and reliable agroinfiltration method for transient gene expression in citrus. A microneedle roller was used to create microscopic wounds in the leaf epidermis to facilitate agroinfiltration. Several optimization parameters were explored in this study, including the density of wounds per cm2 of abaxial leaf area, the leaf maturity grade, the effect of the Agrobacterium strain, and the length of the incubation period. Increasing the density of wounds on the leaf surface had a positive effect on transient expression. Higher transient expression levels were observed in well-expanded young leaves in comparison with older leaves. The Agrobacterium strain GV2260 was the most suitable to express a large amount of recombinant protein, and an eight- to ten-day incubation period resulted in the highest expression. Endoplasmic reticulum and cytoskeleton-targeted GFP were both successfully localized, confirming that this protocol can be used for protein subcellular localization in citrus. Finally, up to 100 ng of GFP per milligram of agroinfiltrated leaf tissue was estimated to be expressed using this method. This protocol was tested for GFP expression in five different citrus varieties with no significant statistical differences among them. This simple and easy method can speed up functional genomic studies in citrus and may be applied to other recalcitrant species with extensive epidermal cuticular wax.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Citrus is among the most cultivated fruit crops in tropical and subtropical regions. Due to a devastating disease (Huanglongbing, HLB) caused by a phloem-restricted pathogen (Candidatus Liberibacter asiaticus, CLas), citrus production has been affected in many regions around the world. In the USA alone, the annual production of sweet orange has decreased from almost 13 million tons in 2000 to about 4 million tons in 2018 (NASS/USDA 2019).
Transient gene expression in plants has allowed the study of protein functions involved in important agronomic traits like pathogen defense and abiotic stress tolerance. Several protocols for stable genetic transformation from mature and juvenile citrus explants have been developed using Agrobacterium tumefaciens (Cervera et al. 2005; Orbovic and Grosser 2006; Wu et al. 2015). Most recently, a biolistic-mediated transformation method was developed (Wu et al. 2016, 2019). Nevertheless, transgenic citrus production is a time- and labor-intensive procedure that can be particularly inefficient for some important citrus varieties which are recalcitrant to tissue culture and agrobacterium-mediated transformation.
Transient gene expression through agroinfiltration represents a cost-effective procedure for studying gene function when transgenic plants prove difficult to produce. Although agroinfiltration induces the expression of defense-related genes, this procedure is widely used in plants to study gene function. In citrus, transient gene expression is achieved through several approaches including polyethylene glycol (PEG)-mediated transformation of protoplasts and particle bombardment of leaves.
Transient gene expression can eliminate many limitations inherent to the transgenic citrus production method such as the low transgenic regeneration efficiency and laborious tissue culture procedures. Citrus has been considered very recalcitrant to transient gene expression through agroinfiltration, therefore this procedure has only been used in a limited number of citrus varieties. In grapefruit (Citrus paradisi), agroinfiltration allowed the characterization of genes involved in hypersensitive response and their subcellular localization in chloroplasts (Figueiredo et al. 2011). A Xanthomonas citri subsp. citri pre-treatment before agroinfiltration facilitated transient protein expression in different citrus varieties (Jia and Wang 2014). The main limitation of this protocol is that it requires the use of a microorganism which is pathogenic in citrus, which could also induce undesirable alterations in the agroinfiltrated tissue.
Recently, an efficient biolistic transformation method was developed for transient gene expression in citrus leaves (Levy et al. 2018; Acanda et al. 2019) using a hand-held gene gun device. This procedure works well for a wide variety of citrus, but it is very expensive and is not easily available in many laboratories.
Microneedle rollers are routinely employed to deliver therapeutic solutions in humans by creating micron-sized pores in the skin to facilitate infusion (Hussein et al. 2020). Microneedle arrays have also been fabricated to penetrate plant tissues for precise delivery of agrochemicals into the vascular tissue to treat plant systemic pathogens (Figueiredo et al. 2011). Recently, micromilled needles were also used to deliver agrichemicals to vascular bundles in citrus (Kundu et al. 2019). The microneedles can increase the plant uptake of a therapeutic amount of Zinkicide™ up to 7.5-fold. We applied a similar tool (DERMAROLL, commercialized by Prosper Beauty; Hussein et al. 2020) to create microwounds in the epidermis of the citrus leaf to facilitate the infiltration of Agrobacterium cells and improve transient gene expression.
In this work, we describe a simple, cheap, efficient, and reliable method for transient gene expression in citrus leaves through microneedle-facilitated agroinfiltration. This protocol can be successfully applied for functional genomic, protein–protein interaction and subcellular localization of foreign proteins expressed in different citrus varieties without the need to produce transgenic citrus plants.
Materials and methods
Plant material, Agrobacterium strains and plasmid constructs
The plants used in this study were 6-month-old seedlings of ‘Carrizo’ citrange (Citrus sinensis Osb. x Poncirus trifoliata L. Raf.), Citrus macrophylla, ‘Duncan’ grapefruit (C. paradisi), ‘Pineapple’ sweet orange and ‘Sugar Belle’ (Sweet Clementine x Minneola Tangelo), maintained under greenhouse conditions at the Citrus Research and Education Center (Lake Alfred, FL). Production of transgenic ‘Duncan’ grapefruit plants overexpressing Histone-CFP was previously described (Levy et al. 2018).
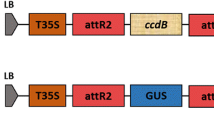
The GFPhdel coding region, containing the second intron (IV2) of the potato ST-LS1 gene, was PCR-amplified from the pTLAB21 plasmid (Orbović et al. 2007) and assembled under the control of the CaMV 35S promoter and the NOS terminator into the pCR-blunt cloning vector. The 35S-GFPhdel-NOS expression cassette was then XbaI/HindIII-excised and inserted into pCAMBIA2301, which contains kanamycin selection and GUS reporter genes, to generate the vector pCAMBIA2301:GFPhdel encoding for endoplasmic reticulum-targeted GFP. Another construction, pSITE-UtrCH-GFP (Levy et al. 2015), was used to target the actin cytoskeleton. The UtrCH-GFP binds to actin through the calponin-binding domain of utrophin (UtrCH), a probe reported to mark F-actin without altering the balance of actin assembly/disassembly. Plasmids were transferred to three different electro-competent Agrobacterium strains (EHA105, AGL1 and GV2260) for agroinfiltration in citrus leaves.
Microneedle wounding and agroinfiltration in citrus leaves
Prior to agroinfiltration experiments, a single colony of each Agrobacterium strain was inoculated in 20 ml of LB medium supplemented with 20 µM acetosyringone (AS) (5-dimethoxy-4-hydroxyaceto-phenone) and the respective antibiotics. The cultures were incubated overnight on an orbital shaker at 250 rpm at 28 °C and then centrifugated at 5000 rpm for 5 min. The bacterial pellets were finally resuspended in agroinfiltration medium (AIM) (4 g/l Murashige and Tucker Medium (salts and vitamins), 4 g/l sucrose, 20 mM MES, 5 mM MgCl2, 150 µM AS, 0.1% v/v Plant Preservative Mixture (PPM), pH 5.3) to a final cell density (OD600) of 0.6 and activated with 1 h of shaking at room temperature.
Well-expanded young citrus leaves (light green color) were selected for agroinfiltration. After carefully cleaning the leaves with dish detergent and water, a 0.5-mm microneedle roller was rolled over the abaxial surface of the leaves to cause wounds in a density of 70 wounds/cm2 as shown in Fig. 1a, b. This tool is originally intended to puncture the human epidermis during dermatological procedures, and it is commercially available in different materials and sizes.
Citrus leaf agroinfiltration procedure using a microneedle roller. a, b Pre-application of the microneedle roller to produce wounding on the surface of the abaxial side of the citrus leaf. c A rubber band adapter placed on a 10 ml needless syringe is used to apply high pressure to the citrus leaf without causing physical damage during agroinfiltration. d Agroinfiltrated leaves incubated for 6 to 10 days at RT on agar-solidified medium for transient gene expression. e GFP transient expression in citrus leaf cells 10 days after agroinfiltration. f Agroinfiltration negative control (empty plasmid, pCambia2301). Scale bar: 500 µm in a–d, 60 µm in e–f
The Agrobacterium suspension was then infiltrated into the leaf using a 10-ml needleless syringe, with a rubber band adapter as shown in Fig. 1c. Detached leaves were rinsed with sterile DI water after agroinfiltration and placed in Petri dishes containing AIM supplemented with 30 g/l sucrose and solidified with 4 g/l Gelzan™ (CP Kelco, Atlanta, GA) (Fig. 1d). The leaves were incubated under dark conditions at room temperature for 6 to 8 days. Plants used for agroinfiltration of attached leaves were covered with a plastic bag after agroinfiltration to maintain high humidity and prevent dehydration. The plants were kept in the greenhouse for 6–8 days.
Confocal microscopy
A small piece of leaf (approx. 0.2 cm2) was mounted on a glass slide and a drop of water was used as the mounting medium. GFP and CFP expression was visualized using a Leica SP8 laser-scanning confocal microscope (Leica Microsystems Inc., Buffalo Grove, IL) with a 488 nm argon excitation laser for GFP and a near-UV diode 405 nm excitation laser for CFP. Emission signals of GFP were detected at 500–530 nm and for CFP at 475–501 nm. Chloroplast autofluorescence was visualized by excitation at 488 nm and emission detection at 700 nm. Leica Application Suite-LAS X (Leica Microsystems Inc.) was used to collect z-stacks composed of optical sections with 1024 × 1024 resolution. Images were exported as TIFF files to be analyzed using the ImageJ software with a customized macro.
ImageJ macro to analyze GFP expression area from confocal images
Data were collected from the confocal images using a method which was modified from previously published work (Zavaliev and Epel 2015). A simple for-loop was written so that ImageJ would open images from their storage directory, apply the functions detailed below, and collect the data in an automated manner. The global scale was set using a scale bar created with the LAS X software. Each image was converted to an 8-bit format. Uneven background material was corrected by applying the Subtract Background function with a rolling ball radius of 50. Noise was reduced by applying the Mean Filter function with a radius of 2. The GFP-expressing tissue was separated from the background using the Auto Local Threshold function with the Bernsen algorithm and a radius of 15. Using the Set Measurements function, ImageJ was instructed to measure the area of the region of interest, defined by the threshold, in pixels. Using the Analyze Particles function, ImageJ was instructed to measure the area of the threshold-defined fluorescent region in each image. The area values were exported to Excel, where the area of fluorescent pixels was divided by the total area of the image in pixels to get percentage values.
Histochemical GUS assay
The GUS assay was performed by incubating leaf disks in GUS staining buffer (1 mM X-Gluc, 50 mM PBS pH 6.8, 20% v/v methanol, 1% v/v Triton X-100) for 24 h at room temperature in the dark. Leaf disks were then bleached with a solution of ethanol and acetone (4:1) for 48 h at 65 °C to remove the chlorophyll.
Western blotting
A crude protein extract was prepared from 110 mg of agroinfiltrated leaf tissue ground with liquid nitrogen and resuspended in 600 µl of 1X Laemmli Sample Buffer (Bio-Rad, Hercules, CA) containing 0.1% w/v polyvinylpyrrolidone and 2% v/v β-mercaptoethanol. The mixture was incubated at 95 °C for 5 min and then centrifugated at maximum speed in a countertop Eppendorf centrifuge for 10 min. The supernatant was recovered and centrifuged again for 5 min to eliminate cell debris and then stored at − 80 °C.
Twenty microliters of the crude protein extract were separated by 12% SDS-PAGE and then transferred to a nitrocellulose membrane. The membrane was blocked for 1 h at RT in blocking buffer (1X TBST with 5% w/v nonfat dry milk) and incubated for 2 h at RT with a primary rabbit monoclonal anti-GFP antibody (1:1000 dilution in blocking buffer) (Sigma, St. Louis, MO). After washing the membrane three times for 10 min each in 1X TBST, it was incubated for 1.5 h at RT with a secondary goat anti-rabbit IgG conjugated to HRP (1:100 dilution in blocking buffer) (Invitrogen, Carlsbad, CA). The membrane was then washed three times for 10 min each in 1X TBST. Immediately after, 500 μl of SuperSignal™ West Pico PLUS Chemiluminescent Substrate (Thermo Scientific, Waltham, MA) was spread over the membrane to visualize the peroxidase activity in a CL-XPosure™ Film (Thermo Scientific). Films were exposed to the membranes for 10 min.
To estimate the amount of GFP expressed in the agroinfiltrated leaves, known amounts of recombinant GFP (4, 40, 400 and 4000 ng) (Novus Biologicals, CO) in 20 μl samples, were used as standards in western blotting. Crude protein extracts from 8-day incubation agroinfiltrated leaves were prepared as described above and separated by 12% SDS-PAGE. The gel was transferred to a nitrocellulose membrane and the amount of expressed GFP was estimated in a western blot.
Statistics
Statistical analysis of GFP-expressing areas from confocal images was performed using the SPSS Statistics version 26 (IBM, Armonk, NY). Differences in the tissue area (%) expressing GFP were analyzed using an independent-samples Kruskal–Wallis test at a significance level of 0.05.
Results and discussion
The transference of T-DNA from Agrobacterium to plant cells is a complex process that involves several plant- and bacteria-encoded genes. Different factors, including plant species, Agrobacterium strain, agroinfiltration medium, and incubation conditions can affect transient gene expression in plant cells. Here, we focused on enhancing the penetration of the bacteria into the leaf tissues, by creating microwounds on the leaf surface with a microneedle roller before agroinfiltration (Fig. 1).
First, we determined if wounding with the microneedles facilitates agroinfiltration and enhances gene expression levels. C. macrophylla leaves were infiltrated with GV2260 Agrobacterium strain harboring the expression vector pCAMBIA2301:GFPhdel, which encode for ER-localized GFP, either with or without the microneedle wounding (Fig. 1). Western blot analysis to detect GFP resulted in a clear protein band when the microneedle roller was applied, but no protein band appeared when a similar protocol, without microneedle roller wounding, was applied (Fig. 2a). Next, we determined if the number of wounds affects gene expression levels. We compared rolling the microneedle roller over the abaxial surface of the leaf in a single and a double rolling session. A single pass with the roller produces wounds in a density of 70 wounds/cm2, while two passes with the roller produce twice the number of wounds (140 wounds/cm2). A larger amount of GFP was detected on western blotting when the leaf surface was rolled twice, indicating that number of wounds affected gene expression (Fig. 2a). Indeed, confocal images showed that the highest number of GFP-expressing cells was located surrounding the wounds (Fig. 2b–g). Our results show that generating microwounds on the surface of citrus leaves dramatically increases agroinfiltration efficiency and that doubling the number of wounds had a positive effect on the transient gene expression. A third, higher density of wounds was also tested but it caused too much damage on the leaf tissues, leading to necrosis and bacterial overgrowth, affecting gene expression (data not shown).
Effect of the number of wounds and the leaf age on transient GFP expression in C. macrophylla leaves. a Western blot to examine GFP expression 10 days after agroinfiltration with GV2260 carrying pCambia2301:GFPhdel vector. iBright™ protein ladder was used for scale (Lane 1). A band of ~ 30 kDa corresponding to GFP is detected when the microneedle roller is applied twice to produce 140 wounds/cm2 (Lane 2, lower panel) or once to produce 70 wounds/cm2 (Lane 3, lower panel). GFP is not detected when the microneedle roller is not applied (Lane 4, lower panel). A sample from a leaf agroinfiltrated with the empty vector pCambia2301 was used as a negative control (Lane 5, lower panel). Agroinfiltration in well-expanded young leaves allows better GFP expression than in old leaves (Lanes 5 and 6, lower panel). The image above the western blot is the Coomassie-stained sister gel. b–d GFP-expressing cells visualized under the GFP channel (b, green) are mostly located around the wounds (white arrows in c and f). The merged channels for GFP and chlorophyll are shown in d. No GFP-expressing cells are detected in negative control agroinfiltrated leaf (pCambia2301, e–f) Scale bar: 500 µm
The leaf maturity grade was also found to affect GFP transient expression. Western blot analysis determined that agroinfiltration of well-expanded young leaves located near the end of the branches produced higher transient expression levels in comparison with agroinfiltration of older leaves (Fig. 2a, lanes 5–6). Leaf age also affected transient expression in Theobroma cacao and Fragaria vesca, probably due to physiological and physical differences between leaves of different ages (Fister et al. 2016; Cui et al. 2017).
Next, three different Agrobacterium strains (EHA105, AGL1 and GV2260) harboring the expression vector pCAMBIA2301:GFPhdel, were used to agroinfiltrate detached, well-expanded young leaves of C. macrophylla. Evaluation of the agroinfiltration efficiency of each strain was conducted by western blot detection of GFP in the crude protein extracts from the agroinfiltrated tissues ten days after infiltration. The largest band of GFP was detected in the western blot when GV2260 was used, and GV2260 was selected as the most suitable strain for agroinfiltration in citrus (Fig. 3a). EHA105, a disarmed succinamopine strain which is most frequently used to produce transgenic citrus, was also successful for agroinfiltration but was less efficient than GV2260. Mesophyll and epidermal cells expressing GFP were also observed under the confocal microscope 8 days after agroinfiltration with each Agrobacterium strain (Fig. 3b–e). The visualization of 12 sections of agroinfiltrated leaves under the confocal microscope confirmed that GV2260 is the most suitable strain to achieve transient expression in citrus. The competence of GV2260 for transient expression was also confirmed in several citrus varieties (see below). Another Agrobacterium strain commonly used to produce transgenic citrus, AGL1, did not perform well for agroinfiltration.
GFP expression in C. macrophylla leaves which were infiltrated with different Agrobacterium strains carrying pCambia 2301:GFPhdel plasmid. a Western blot of GFP expression (lower panel) on leaf tissues agroinfiltrated with Agrobacterium strains AGL1, EHA105 and GV2260 (Lanes 2 – 4). iBright™ protein ladder was used for scale (Lane 1). The same Agrobacterium strains carrying the empty vector (EV) pCambia2301 were used as negative controls for GFP western blotting (Lanes 5–7). The image above the western blot is the Coomassie-stained sister gel. b–e: Confocal images of GFP expression in leaves using AGL1 (b), EHA105 (c), and GV2260 (d). e A magnified image to show cell structure. Scale bars in b–d = 100 µm, scale bar in e = 10 µm
Although EHA105 and AGL1 are the most frequently used Agrobacterium strains for sweet orange scion and rootstock transformation, the effect of the Agrobacterium strain on stable and transient transformation efficiency has not been extensively studied in citrus. ‘Carrizo’ citrange and grapefruit are very competent for transformation with the highly virulent EHA101 strain (Orbović et al. 2007), but sweet oranges are more competent with the EHA105 or AGL-1 strains for transformation (Orbovic and Grosser 2006).
To identify the optimal incubation period, detached young leaves of ‘Duncan’ grapefruit, ‘Pineapple’ sweet orange and C. macrophylla were agroinfiltrated with GV2260 Agrobacterium strain carrying the expression vector pCAMBIA2301:GFPhdel. Leaf disks collected after 6-, 8- and 10-day incubation periods were analyzed under confocal microscope for GFP expression. A longer incubation period significantly increased GFP transient expression in all three citrus genotypes. Eight to ten days were necessary to visualize GFP expression in about 20% of the agroinfiltrated area, while up to 70% was observed at 10 days in C. macrophylla (Fig. 4a). GUS assay and GFP western blotting also confirmed that an 8- to 10-day incubation period is necessary for gene expression in C. macrophylla (Fig. 4b, c).
Dynamics of gene transient expression in citrus. a GFP-expressing area (%) in ‘Duncan’ grapefruit (Dun), ‘Pineapple’ sweet orange (PA) and C. macrophylla (Cmac) leaves after 6, 8 and 10 days after agroinfiltration. b GUS-stained C. macrophylla leaf sections incubated for 6, 8 and 10 days after infiltration with GV2260 Agrobacterium strain carrying pCambia2301:GFPhdel vector. This construction also contains the gusA gene from the original pCambia2301. c GFP western blot of C. macrophylla leaves incubated for 6, 8 and 10 days after agroinfiltration (Lanes 2–4). Lane 5: GV2260 without plasmid as a negative control (C- 10d). iBright™ protein ladder was used for scale (Lane 1). The image above the western blot is the Coomassie-stained sister gel. Transient gene expression is highest after an 8- to 10-day incubation period. Scale bar = 2 mm
To transfer T-DNA to a plant cell, Agrobacterium cells need activation. Molecules involved in that activation come from the wounded plant tissues and play a central role in determining the competence of plant tissues for transformation. Usually, epicotyl explants from etiolated seedlings are used to produce transgenic citrus plants. It is possible to detect both transient and stable transformation events on epicotyl segments 2 or 3 days after Agrobacterium inoculation. In agroinfiltrated leaves, we were unable to detect any transformation event (cells expressing GFP) during a 2- or 3-day incubation period after agroinfiltration. It is well known that plant tissues have different competencies for agrotransformation. Although both epicotyl and leaf tissue are suitable for transient gene expression in citrus, a longer incubation time is necessary for expression in leaf cells.
Wounding in plant tissues induces the release of molecules such as acetosyringone that can activate the transfer of the T-DNA from Agrobacterium to the plant cell. Physical wounding such as a short vortex pre-treatment of the explants with sand or aluminum borate whisker powder significantly increased Agrobacterium-mediated transformation in Jatropha curcas and Cucurbita moschata (Khemkladngoen et al. 2011; Nanasato et al. 2011). In citrus, the transformation efficiency was also improved by a microwounding pre-treatment with sonication and vacuum-assisted Agrobacterium infiltration (Oliveira et al. 2009).
Next, we used this agroinfiltration protocol to visualize cellular organelles in citrus. GFPhdel and UtrCH-GFP were localized in the endoplasmic reticulum and the actin filaments, respectively, in agroinfiltrated C. macrophylla leaves (Fig. 5). The simplicity and affordability of this protocol make it an effective alternative to the gene gun to perform this kind of study in citrus.
Subcellular localization of endoplasmic reticulum-targeted GFP (GFP-HDEL) and actin-targeted GFP (UtrCH-GFP) visualized under the confocal microscope 8 days after infiltration with GV2260 Agrobacterium strain harboring respective plasmid constructions. Cell nuclei and chloroplasts are visualized in the CFP and chlorophyll channels, respectively. Control images are from an agroinfiltrated citrus leaf with empty plasmid pCambia2301. Scale bar = 5 µm
Stable genetic transformation protocols are available for several citrus varieties. Multiple factors, including the citrus genotype, have been demonstrated to affect transformation efficiency. Some highly vigorous genotypes with a very good morphogenesis potential, like Carrizo citrange, are easier to transform than others that have shown recalcitrance to tissue culture or have poor shoot regeneration potential. To test whether transient gene expression is also affected by the genotype, we used this agroinfiltration protocol for transient expression of GFP in five different citrus genotypes. The analysis of 10 confocal images from different agroinfiltrated leaves from each genotype showed no significant statistical differences in the percentages of GFP-expressing areas (Fig. 6a), suggesting that the effectiveness of this protocol is not affected by the genotype. Finally, to estimate the amount of protein expressed with this method, we compared the band intensity of agroinfiltrated samples with recombinant GFP standards using a western blot (Fig. 6b). We obtained approximately 100 ng of GFP per milligram of fresh tissue in agroinfiltrated C. macrophylla leaves.
GFP transient expression in different citrus genotypes. a Area of GFP expression (%) in confocal images taken 8 days after infiltration with GV2260 Agrobacterium strain carrying pCambia2301:GFPhdel. No statistical difference was found among the genotypes. Carrizo: ‘Carrizo’ citrange (C. sinensis x Poncirus trifoliata), Cmac: C. macrophylla, Duncan: ‘Duncan’ grapefruit (C. paradisi), PA: ‘Pineapple’ sweet orange, SB: ‘Sugar Belle’ (Sweet Clementine x Minneola Tangelo). b Estimation of GFP content in leaf crude extract (20 μL) from three different agroinfiltrated leaves compared to known amounts of recombinant GFP. iBright™: protein ladder
In conclusion, we present a simple and efficient agroinfiltration method for transient gene expression in citrus that is applicable and reproducible in many varieties. With the aid of a microneedle roller, we created a wounded surface to facilitate agroinfiltration into the leaf tissues. This protocol can be used to express a substantial amount of foreign protein and study its subcellular localization in citrus as an alternative to other more laborious and expensive methods like particle bombardment and protoplast transformation. The simplicity and efficacy of this method also make it very convenient to be used for the validation of plasmid constructions for genetic transformation, and sgRNA targeting efficiency for CRISPR/Cas9 gene editing in economically important citrus varieties. Moreover, this simple method may be applied to help transformation in other recalcitrant plants, especially those with extensive epidermal cuticular wax, such as woody trees and monocot species.
Data availability
Not applicable.
Code availability
Not applicable.
References
Acanda Y, Chunxia W, Levy A (2019) Gene expression in citrus plant cells using Helios® gene gun system for particle bombardment. In: Catara AF (eds) Citrus Tristeza Virus: methods and protocols. Methods Mol Biol 2015:219–228. Clifton, N.J. https://doi.org/https://doi.org/10.1007/978-1-4939-9558-5_16.
Cervera M, Juárez J, Navarro L, Peña L (2005) Genetic transformation of mature citrus plants. In: Peña L. (eds) Transgenic plants: methods and protocols. Methods Mol Biol 286:177–187. Clifton, N.J. https://doi.org/https://doi.org/10.1385/1-59259-827-7:177
Cui MY, Wei W, Kuan G, Yin GX, Ye G (2017) A rapid and efficient Agrobacterium-mediated transient gene expression system for Strawberry leaves and the study of disease resistance proteins. PCTOC 131(2):233–246. https://doi.org/10.1007/s11240-017-1279-3
Figueiredo JFL, Romer P, Lahaye T, Graham JG, White FF, Jones JB (2011) Agrobacterium-mediated transient expression in Citrus leaves: a rapid tool for gene expression and functional gene assay. Plant Cell Rep 30:1339–1345. https://doi.org/10.1007/s00299-011-1045-7
Fister AS, Shi Z, Zhang Y, Helliwell EE, Maximova SN, Guiltinan MJ (2016) Protocol: transient expression system for functional genomics in the tropical tree Theobroma cacao L. Plant Methods 2016:12–19. https://doi.org/10.1186/s13007-016-0119-5
Jia H, Wang N (2014) Xcc-facilitated agroinfiltration of Citrus leaves: a tool for rapid functional analysis of transgenes in Citrus leaves. Plant Cell Rep 33:1993–2001. https://doi.org/10.1007/s00299-014-1673-9
Hussein MA, Zaki AA, Abdelkhaliq AA, Wilson AM (2020) Evaluation of micro-needling as a flap preconditioning modality: a split-flap study. Egypt J Plast Reconstr Surg 44(3):415–419
Khemkladngoen N, Cartagena JA, Fukui K (2011) Physical wounding-assisted Agrobacterium-mediated transformation of juvenile cotyledons of a biodiesel-producing plant, Jatropha curcas L. Plant Biotechnol Rep 5:235–243. https://doi.org/10.1007/s11816-011-0177-0
Kundu A, Nogueira MGC, Santra S, Rajaraman S (2019) Precision vascular delivery of agrochemicals with micromilled microneedles (µ MMNs ). Sci Rep 9:14008. https://doi.org/10.1038/s41598-019-50386-8
Levy A, Zheng JY, Lazarowitz SG (2015) Synaptotagmin SYTA forms ER-plasma membrane junctions that are recruited to plasmodesmata for plant virus movement. Curr Biol 25(15):2018–2025. https://doi.org/10.1016/j.cub.2015.06.015
Levy A, El-Mochtar C, Wang C, Goodin M, Orbovic V (2018) A new toolset for protein expression and subcellular localization studies in Citrus and its application to Citrus Tristeza virus proteins. Plant Methods 14(1):2. https://doi.org/10.1186/s13007-017-0270-7
Nanasato Y, Konagaya KI, Okuzaki A, Tsuda M, Tabei Y (2011) Agrobacterium-mediated transformation of Kabocha squash (Cucurbita moschata Duch) induced by wounding with aluminum borate whiskers”. Plant Cell Rep 30:1455–1464. https://doi.org/10.1007/s00299-011-1054-6
Oliveira MLP, Febres JV, Costa MGC, Moore GA, Otoni WC (2009) High-efficiency Agrobacterium-mediated transformation of Citrus via sonication and vacuum infiltration. Plant Cell Rep 28:387–395. https://doi.org/10.1007/s00299-008-0646-2
Orbovic V, Grosser JW (2006) Citrus: sweet orange (Citrus sinensis L. Osbeck ‘Valencia’) and Carrizo citrange [Citrus sinensis (L.) Osbeck x Poncirus trifoliata (L.) Raf.]. In: Wang K (ed) Agrobacterium protocol, Methods in molecular biology. Humana Press Inc, Totowa, NJ, 177–189
Orbović V, Pasquali G, Grosser JW (2007) A GFP-containing binary vector for Agrobacterium tumefaciens-mediated plant transformation. Acta Hortic (738):245–253
USDA—Agricultural statistics 2019. Orange production in the United States from 2000 to 2018. https://www.statista.com/statistics/193297/us-total-orange-production-since-2000/
Wu H, Acanda Y, Shankar A, Peeples M, Hubbard C, Orbovic V, Zale J (2015) Genetic transformation of commercially important mature Citrus scions. Crop Sci 55(6):2786–2797. https://doi.org/10.2135/cropsci2015.01.0013
Wu H, Acanda Y, Hongge J, Wang N, Zale J (2016) Biolistic transformation of Carrizo citrange (Citrus sinensis Osb. 3 Poncirus trifoliata L. Raf.)”. Plant Cell Rep 35(9):1955–1962. https://doi.org/10.1007/s00299-016-2010-2
Wu H, Acanda Y, Canton M, Zale J (2019) Efficient biolistic transformation of immature citrus rootstocks using phosphomannose-isomerase selection. Plants 8 (10):390
Zavaliev R, Epel BL (2015) Imaging callose at plasmodesmata using aniline blue : Quantitative confocal microscopy. In: Heinlein M (ed) Plasmodesmata: Methods and Protocols. Methods Mol Biol 1217:105–119. Clifton, N.J. https://doi.org/https://doi.org/10.1007/978-1-4939-1523-1_7.
Acknowledgements
The authors are grateful to Dr. Fernanda Nogales for her contributions in the conception of the original idea of this paper. This research was funded by the National Institute of Food and Agriculture, Grant numbers 2018-70016-27392 and 2019-70016-29096 to AL.
Funding
National Institute of Food and Agriculture, Grant numbers 2018-70016-27392 and 2019-70016-29096.
Author information
Authors and Affiliations
Contributions
YA and AL conceived and designed research. YA conducted experiments. SW and VO contributed new reagents or analytical tools. YA and SW analyzed data. YA and AL wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Leandro Peña.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Acanda, Y., Welker, S., Orbović, V. et al. A simple and efficient agroinfiltration method for transient gene expression in Citrus. Plant Cell Rep 40, 1171–1179 (2021). https://doi.org/10.1007/s00299-021-02700-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-021-02700-w