Abstract
Key message
Our study is the first to demonstrate that PSK1 , a SKP1 -like gene homologue, is involved in salinity tolerance. Our functional characterization of PSK1 provides new insights into tree peony development.
Abstract
A homologous gene of S-phase kinase-associated protein1 (SKP1) was cloned from tree peony (Paeonia suffruticosa) and denoted as PSK1. The 462-bp open reading frame of PSK1 was predicted to encode a protein comprising 153 amino acids, with a molecular mass of 17 kDa. The full-length gene was 1,634 bp long and included a large 904-bp intron. PSK1 transcription was detected in all tissues, with the highest level observed in sepals, followed by leaves. Under salinity stress, overexpression of PSK1 in Arabidopsis resulted in increased germination percentages, cotyledon greening, and fresh weights relative to wild-type plants. Furthermore, transgenic Arabidopsis lines containing 35S::PSK1 displayed increased expression of genes that would be essential for reproduction and growth under salinity stress: ASK1, LEAFY, FT, and CO involved in flower development and flowering time as well as P5CS, RAB18, DREB, and SOD1-3 contributing to salinity tolerance. Our functional characterization of PSK1 adds to global knowledge of the multiple functions of previously explored SKP1-like genes in plants and sheds light on the molecular mechanism underlying its role in salinity tolerance. Our findings also provide information on the function and molecular mechanism of PSK1 in tree peony flower development, thereby revealing a theoretical basis for regulation of flowering and conferral of salinity tolerance in tree peony.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
S-phase kinase-associated protein1 (SKP1) was initially identified in yeast (Kong et al. 2004). Although only one functional SKP1 protein present in Homo sapiens and yeast (Kong et al. 2004), many animal and plant species possess multiple homologues, including 7 in Drosophila melanogaster, 21 in Caenorhabditis elegans, 21 in Arabidopsis (denoted as ASKs), and 32 in rice (denoted as OSKs) (Vierstra 2009; Nayak et al. 2002; Kong et al. 2007). SKP1 proteins found in various organisms have diverse functions associated with a myriad of vital biological processes (Takahashi et al. 2004). Much attention has been focused on SKP1 proteins because they are one of the most important components of the SKP1/Cullin/F-box (SCF) complex, an E3 ubiquitin ligase. Through its interaction with different F-box proteins to ubiquinate different substrates, SKP1 performs various functions including control of cell cycle progression, transcriptional regulation, signal transduction, and other cellular processes (Hellmann and Estelle 2002).
Among studied SKP1-like homologues in plants, the greatest progress has centered around Arabidopsis SKP1-like proteins (ASK proteins), this is especially true for ASK1, the most studied ASK at present. ASK1 is a component of SCFTIR1, SCFCOI1, and SCFSLEEPY1 complexes that, respectively, regulate auxin (Gray et al. 1999), jasmonic acid (Xie et al. 1998) and gibberellic acid (McGinnis et al. 2003) signal transduction pathways. Recent studies demonstrated that ASK1, together with other SCF complex members, plays an important role in control of circadian rhythm (Somers et al. 2000), flowering timing (Imaizumi et al. 2005), light signaling (Dieterle et al. 2001), defense response (Wang et al. 2013), and cross-pollen compatibility in S-RNase-based self-incompatibility systems (Zhao et al. 2010). Genetic studies on ASK1/ASK11 have revealed its important functions in vegetative growth and male meiosis (Yang et al. 1999; Gusti et al. 2009). ASK1 also regulates B-function gene (B subfamily MADS-box gene) expression in cooperation with UFO and LEAFY to regulate flower development and floral organ identity (Zhao et al. 1999, 2003).
Apart from Arabidopsis, SKP1 functional studies of other plant species, including rice OSKs (Kahloul et al. 2013) and wild tomato SSKs (Zhang et al. 2015), have made some progress. For example, an examination of tissue-specific expression patterns revealed that some SSKs were expressed in a large number of tissues (e.g., fruits, petals, and sepals). In addition, expressions of SSKs (SSK4, SSK7, and SSK12) were found to be responsive to different abiotic stresses, including heat and salinity, with expression levels of nearly 50% of analyzed SSKs up-regulated by abscisic acid (ABA) and salicylic acid (SA) treatments (Zhang et al. 2015). The transcriptional profiles of these SSKs under abiotic stress treatment provided new insights to the function of SKP1 homologous genes. In another functional study, that of wheat TSK1, the specific expression pattern and interaction between TaSKPs and selected F-box proteins demonstrated the possible function of these proteins in various growth and flower development processes (Hong et al. 2013). Preliminary research carried out in woody plants such as pummelo (Citrus grandis, CgSKP1), citrus (C. reticulate, CrWSKP1), and sweet cherry (Prunus avium, PacSSK1) suggests that their SKP1-like genes are involved in flower development and self-incompatibility reactions (Chai et al. 2010; Li et al. 2015; Matsumoto et al. 2012).
Except for the above-mentioned species, little is known about SKP1-like genes in plants, especially those without genomic information. Tree peony (Paeonia suffruticosa), a member of section Moutan in the genus Paeonia of Paeoniaceae (Li 1999), is a perennial deciduous shrub with excellent medicinal, ornamental, and seed oil properties (Li et al. 2010; Li et al. 2014). The root bark of tree peony is a valuable ingredient in Chinese traditional medicine. In addition, this ornamental species is world-renowned for its large showy flowers in various colors and shapes. Because of the high content of unsaturated fatty acids (92%) especially α-linolenic acid (42% of total fatty acids), as well as phenolic and monoterpene glycosides, tree peony seeds are used for food oil and pharmaceuticals (Li et al. 2014). Given the multiple uses of this species, aspects of tree peony, such as flower color, root compounds, oil content and abiotic stress response, are of great research interest. Although SKP1 family genes play vital roles in flower development, reproduction, seed set, hormone signaling, and other processes (Zhang et al. 2015), little is known about the function of these genes in tree peony. To explore the function of SKP1-like genes in tree peony, we isolated a SKP1-like gene homologue (denoted as PSK1) in this study and analyzed its expression profile in different tissues and its subcellular localization. We also carried out an ectopic expression analysis of its function in Arabidopsis. The results of this study provide a basis for future investigations of SKP1-like genes in tree peony and related species. In regard to functional conservation and diversification, our findings are also beneficial to help clarify the functions of SKP1-like genes in non-model plants.
Materials and methods
Isolation of PSK1 gene and bioinformatic analysis
A SKP1-like gene homologue was previously isolated from a flower-bud cDNA library of P. suffruticosa and denoted as PSK1 (GenBank accession no. FE529999) (Shu et al. 2009). Genomic DNA was prepared from young leaves according to (Han et al. 2008). The genomic DNA sequence was amplified using the primers listed in ESM_S1. PSK1 homologues were identified by BLASTX analysis against the NCBI database. Predicted amino acid sequences of tree peony and related plant species were aligned in Clustal X (Thompson et al. 1997) followed by manual refinements, and a phylogenetic tree was constructed in MEGA4.1 with 1,000 bootstrap replicates (Tamura et al. 2007). Conserved domains of PSK were predicted by Pfam and SMART programs (Letunic et al. 2002; Punta et al. 2012), while, subcellular localization of PSK1 was predicted using the Plant-mPLoc program (Chou and Shen 2010).
Quantitative real-time PCR (qPCR) analysis of PSK1
The expression pattern of PSK1 was studied in various tissues and petals at different developmental stages of Paeonia suffruticosa ‘Gunpohden’ under natural conditions, collected from the Beijing Botanical Garden, Institute of Botany, Chinese Academy of Sciences. After isolation of total RNA from various tissues with an RNAprep pure kit (Tiangen, Beijing, China), cDNA was synthesized using a Fast Quant Kit (Tiangen) according to the manufacturer’s protocol. Flower petals at different developmental stages (Du et al. 2015), namely, flower-bud (S1), swollen flower-bud (S2), bloom initiation (S3), half-blooming (S4), and blooming (S5) stages, as well as leaves, stems, and sepals at the blooming stage (S5) were collected as samples in triplicate. Following treatment of 3-week-old plants of PSK1 transgenic lines and wild-type (WT) Arabidopsis with 150 mM NaCl for 0 h, 4 h, 8 h, and 12 h, leaves were collected in triplicate. Total RNA and cDNA were obtained as described above. Quantitative assays were performed using SuperReal qPCR PreMix (SYBR Green, Tiangen, China) and a StepOnePlus™ Real-Time PCR system (Applied Biosystems, Life Technologies, USA) following the manufacture’s protocol. Each 20-µl qPCR mixture was subjected to the following program: 95 °C for 15 min, followed by t 40 cycles of 94 °C for 5 s, 56–60 °C for 20 s, and 72 °C for 32 s. The qPCR assay was carried out on three biological replicates with three technical replicates performed per sample. Relative quantification of mRNA transcripts was performed in triplicate with normalization to β-tubulin of P. suffruticosa (accession no. EF608942) or ACTIN of Arabidopsis thaliana (AY120779). All primers used in the qPCR analysis are listed in ESM_S1. Relative expression levels were calculated by comparing 2−ΔΔCt values based on with threshold cycle (Ct) (Livak and Schmittgen 2001; Yoshida et al. 2003).
Transformation vector construction and transgenic plant generation
To generate PSK1-overexpressing transgenic plants, the full-length coding sequence of PSK1, was amplified using forward primer 5′-ACTCTAGAATGGTTAGGGTCGTGACTTTG-3′ (XbaI site underlined) and reverse primer 5′-AAGGATCCCTCAAATGCCCACTGGTTC-3′ (BamHI site underlined), was inserted into the XbaI/BamHI site of a pPZP vector (Chen et al. 2007) under the control of the cauliflower mosaic virus (CaMV) 35S promoter. Transformation of Arabidopsis was performed by the floral dip method using Agrobacterium tumefaciens (EHA105) cells (Clough and Bent 1998). Seeds from transgenic and WT Arabidopsis sown in half-strength Murashige-Skoog (MS) medium or pots were maintained at 4 °C for 3 days in the dark to break residual dormancy; the germinated seedlings were then grown in a culture room at 22 °C under a 16/8 h light/dark photoperiod and 60% relative humidity. PSK1 expression levels in transgenic Arabidopsis lines were checked by qPCR; lines with the highest expression levels were used for subsequent analyses. For phenotypic analyses, we used T3 homozygous lines selected from T2 plants on half-strength MS medium with 50 µg ml−1 kanamycin. Three T3 generations of Arabidopsis transgenic lines were selected for stress tolerance studies.
Analysis of transgenic plants under salinity stress
To test salinity tolerance of transgenic Arabidopsis plants, germination assays were carried out in triplicate by sowing at least 40 seeds from each transgenic lines or WT line on plates containing half-strength MS medium supplemented with various concentrations (0, 100 or 125 mM) of NaCl. After incubation at 4 °C for 3 days, the seeds were maintained in a culture room at 21–22 °C until germination. Upon germination, each seedling was transferred to 22 °C conditions and the day of germination was recorded as day 1. Fully emerged radicle tips or fully opened cotyledons were used as a reference for counting germinated seeds. After growing for 2 weeks, seeds developing green cotyledons were counted. For seedling growth measurements, 20 seedlings from each line were weighed and fresh weight was calculated as the average value. To determine the effect of NaCl on root growth, 7-day-old plants were transplanted to vertically standing half-strength MS plates containing NaCl with root length measured after 1 week. All of the above experiments were repeated at least three times. The relative expression level of stress-associated genes after NaCl treatment was analyzed using 3-week-old seedlings treated with 150 mM NaCl. The seedlings were sampled after treatment for 0–12 h and subjected to qPCR assay for AtDREB, AtRAB18, AtSOS1-3, and AtP5CS, with physiological parameters also measured. To determine the extent of lipid peroxidation, malondialdehyde (MDA) levels were assayed according to Kramer et al. (1991) with a few minor modifications, namely, 0.5 g of leaf tissue was homogenized, and 1 ml instead of supernatant rather than 2 ml was combined with 2 ml of glucosinolates barbituric acid (TBA) reagent [0.5% (w/v) TBA in 20% (w/v) trichloroacetic acid (TCA)] and heated at 100 °C for 10 min instead of 20. Proline and soluble sugar contents were determined following protocols described by Shan et al. (2007) and Bailey (1958). To further explore PSK1 function in flowering development, we used qPCR to investigate the expression of genes involved in the flower development pathway including AtCO, AtFT, AtLEAFY, AtUFO, and ASK1 in transgenic and control Arabidopsis plants. Primers used for the qPCR analyses are indicated in ESM_S1. Flower-bud formation and flowering time were also observed and calculated from 20 plants of three transgenic lines.
Statistical analysis
Data in this study including data from analysis of gene expression, germination, measurements of cotyledon greening root length and fresh weight, and plant physiological parameters (MDA, proline and soluble sugar contents) were subjected to analysis of variance (ANOVA) using SPSS 21.0. Differences were considered to be significant at p < 0.05. Figures were drawn in SigmaPlot 10.0. Each data point represented three replicates with error bars used to indicate SD.
Results
Sequence analysis of PSK1
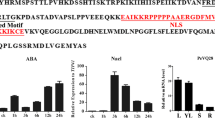
A full-length gene (accession no. FE529999) isolated from a flower-bud cDNA library of tree peony (Shu et al. 2009) was revealed by BLASTX analysis against the NCBI database to be a close homologue of plant, SKP1-like genes. We accordingly designated this as PSK1 (for P aeonia suffruticosa SKP1-like1). The cDNA of PSK1 was 730 bp long, with a 462-bp open reading frame (ORF) encoding a predicted protein of 153 amino acids and a molecular mass of 17 kDa. The full-length gene was 1,634 bp long and included a large 904-bp intron (Fig. 1a). The predicted protein contained a SKP1-POZ domain. Alignment of PSK1 with homologues from Theobroma cacao, Gossypium hirsutum, and Vitis vinifera demonstrated the presence of conserved amino acids and motifs shared by plant SKP1-like family members (Fig. 1b). A few conserved motifs were identified among the aligned amino acids. At the N-terminus (positions 40–50), for example, a PEST motif rich in proline, glutamic acid, serine, and threonine residues for protein turn over (Rogers et al. 1986) was uncovered. At the C-terminus (positions 135–140), a P-loop for binding GTP/ATP was identified: as indicated in Fig. 1b, the conserved motif was AXXXXGRT/S except in yeast, where arginine (R) was replaced by lysine (K) to give the conserved motif AXXXXGKT/S (Winkler et al. 2000). Phylogenetic analysis indicated that PSK1 is homologous to SKP1 proteins from Amborella trichopoda, T. cacao, and G. hirsutum (Fig. 2).
Analyses of gene structure and sequence alignment of PSK1. a Genomic structure of PSK1, containing two exons (black boxes), one intron (solid line), and 462-bp long ORF. Dotted lines correspond to untranslated regions. b Amino acid sequence alignment of PSK1 and SKP1 sequences from Theobroma cacao (EOX90784.1), Gossypium hirsutum (AFM75900.1), Vitis vinifera (XP_002270061.1), and Arabidopsis thaliana (AEE35780.1). Identified sequence regions are indicated by blocks. Blue-asterisks below the aligned sequences indicate amino acid residues closely associated with the interaction between SKP1 and F-box proteins (color figure online)
Phylogenetic tree of PSK1 and SKP1 proteins from Theobroma cacao (EOX90784.1), Gossypium hirsutum (AFM75900.1), Vitis vinifera (XP_002270061.1), Arabidopsis thaliana (AEE35780.1), Arabidopsis lyrata subsp. lyrata (XP_002863814.1), Amborella trichopoda (ERN09550.1), Brachypodium distachyon (XP_003577587.1), Cucumis sativus (XP_004143010.1), Fragaria vesca subsp. vesca (XP_004287783.1), Glycine max (XP_003517160.1), Medicago truncatula (XP_003612227.1), Oryza sativa (AY598357), Petunia integrifolia subsp. inflata (ABB77425.1), and Triticum urartu (EMS35771.1). Numbers above and below branches are bootstrap percentages based on 1000 replicates using MEGA 4.1
Wide expression of PSK1 in various tissues of P. suffruticosa ‘Gunpohden’
PSK1 expression levels were monitored in stems, sepals, leaves, and petals at the flower-blooming stage (S5). At S5, PSK1 was expressed in all tissues. The highest level was in sepals, followed by leaves, with no significant difference (p < 0.05) observed between petals and stems (Fig. 3). When expression levels in petals from S1 to S5 were traced, however, the highest expression level was observed in petals at S3, followed by S1. Because expression level at S1 was nearly two times higher than that of S5, PSK1 expression appears to be down-regulated, except during transition from S2 to S3, as floral development processes (Fig. 3).
Expression pattern of PSK1 at the different developmental stages (S1–S5) and in different tissues measured by qRT-PCR with the β-tublin gene used as a reference. Developmental stages are as follows: S1 flower-bud stage, S2 swollen flower-bud stage, S3 bloom initiation stage, S4 half-blooming, and S5 blooming stage; Stems, leaves, and sepals at S5
Increased salinity tolerance of PSK1-overexpressing transgenic Arabidopsis plants
In total, nine positive transgenic lines were obtained. Lines 7-9, which showed the highest PSK1 expression levels were used for further study of salinity tolerance (ESM_S2). All transgenic seeds displayed tolerance to salinity stress, and 90% of seeds germinated on the first day at 22 °C (Fig. 4a–c). In contrast, WT seeds germinated two days later than those of transgenic lines, with the transgenic lines displaying significant difference (p < 0.05) in germination compared with the WT at 22 °C on the first 3 days (ESM_S3-A). The transgenic lines exhibited significantly higher cotyledon greening rates (ESM_S3-B) than those of WT plants after NaCl treatment. For example, 90.3, 91.3, and 90.3% of L7, L8, and L9 seedlings displayed green cotyledons on medium with 125 mM NaCl, compared with 58.7% of the WT. In addition, a seedling growth assay was carried out to further test the phenotypes of transgenic lines under salinity stress. Significant increases in fresh weights of transgenic lines occurred in the presence of salinity. Even in the absence of salinity, transgenic lines showed a greater increase in fresh weights compared with those of WT plants, thus indicating that PSK1 may promote the growth of transgenic Arabidopsis plants (Fig. 4d).
Germination response of PSK1-overexpressing transgenic lines (lines 7–9) and WT plants to salinity stress. The growth was assayed on medium containing 0 mM (a) or 100 mM NaCl (b). The germination process was analyzed within 16 days after sowing in medium containing 100 mM NaCl, with the day when seedlings were transferred from 4 to 22 °C (c). Fresh weights of 20 plants growing in medium containing 100 mM NaCl was measured (d). For c and d, each column represents the average of three replicates, and the bar indicates SD. Data from each treatment were subjected to analysis of variance using SPSS 21.0. The different characters above each column represent various significant differences at p < 0.05
To further investigate the role of PSK1 in the conferral of salinity stress tolerance, root length was measured after NaCl treatment. Although no significant differences were observed between transgenic lines and WT plants at lower concentration of salinity (0–100 mM NaCl), a marked reduction in root elongation was noted in WT plants compared with transgenic lines on medium containing 125 mM NaCl (ESM_S3-C). Therefore, owing to early germination, high germination percentage, increased cotyledon greening rates, and accelerated seedling growth, including shoot and root growth, PSK1 overexpression was able to enhance plant tolerance to salinity stress.
Increased proline and soluble sugar content of PSK1-overexpressing transgenic Arabidopsis plants under salinity stress
To explore the effect of PSK1 overexpression on salinity tolerance of Arabidopsis plants, we investigated the following physiological parameters: proline, soluble sugar, and MDA contents. In the absence of salinity stress (0 h), all transgenic lines accumulated higher levels of proline than the WT. When subjected to salinity stress for 12 h, an obvious increase in proline content was observed in transgenic lines (L7 and L9) relative to the WT (Fig. 5a). MDA, a product of lipid peroxidation in biomembranes, was measured as an indicator of salinity stress damage due to reactive oxygen species (ROS) (Niu et al. 2012), but little difference was observed between transgenic lines and the WT after salinity treatment (Fig. 5b). PSK1-overexpressing plants were assayed for soluble sugar content, which was found to be higher in transgenic lines than WT plants with or without salinity stress; this was especially true after salinity treatment for 8 h, when a significant difference (p < 0.05) was observed (Fig. 5c).
Measurements of proline, soluble sugar and MDA contents and expression analysis of related genes in WT and PSK1-overexpressing Arabidopsis plants treated with 150 mM NaCl for 0–12 h. a Proline content. b MDA content. c Soluble sugar content. Expression levels of DREB (d), RAB18 (e) and P5CS (f) between WT and PSK1-overexpressing Arabidopsis plants. Each column represents the average of three replicates, and the bar indicates SD. Data from each treatment were subjected to analysis of variance using SPSS 21.0. The different characters above each column represent various significant differences at p < 0.05
Variable expression of salinity-related genes up-regulated in PSK1-overexpressing transgenic Arabidopsis lines and their possible roles in salinity tolerance
To investigate the mechanism through which PSK1 participates in enhancement of salinity stress tolerance in transgenic plants, we assayed the relative expression levels of several salinity stress-related genes after NaCl treatment (150 mM). Among several known salinity defense pathway, the salinity overly sensitive pathway (SOS) appears to be a first line of defense with SOS1, SOS2, and SOS3 genes playing a major role in this system. In the absence of salinity stress, expression levels of these three genes were higher in transgenic lines than those in the WT; this was especially the case for L7 and L8, which displayed, respectively, 2.8- and 3.2-fold higher expression of SOS1, 1.3- and 2.8-fold higher expression of SOS3, and 2.0- and 1.9-fold higher expression of SOS2 relative to the WT (ESM_S3). After salinity stress for 12 h, the expression levels of these three genes were higher in PSK1-overexpressing lines than those in WT plants (ESM_S4). Transcription factors, including DREB and the functional gene RAB18, displayed opposite patterns of expression. The AtDREB gene showed up-regulated in L8 and the WT under salinity treatment, with the highest level at 8 h; in contrast, expression in L7 initially decreased and then rose to its highest level after 8 h treatment, whereas the highest level of AtDREB in L9 was detected after 4 h of salinity treatment (Fig. 5d). Expression levels of AtRAB18 were higher in transgenic lines than those in the WT, with significant differences (p < 0.05) after salinity treatment for 12 h (Fig. 5e), while AtP5CS expression levels exhibited a significant increase (p < 0.05) in all transgenic lines compared with those of WT after salinity treatment for 8 h (Fig. 5f).
Possible promotion of flowering by higher expression levels of flowering developmental genes in PSK1-overexpressing transgenic Arabidopsis lines
To further explore the function of PSK1 in flower development, the expression levels of genes involved in the flower development pathway, including CONSTANS (CO), Flowering Locus T (FT), LEAFY, and ASK1, were investigated in transgenic Arabidopsis and WT plants. Relative expression levels of all detected genes were higher in transgenic plants than in the WT. Average expression level were 3.5 times higher for FT, 6.2 times higher for LEAFY, 4.1 times higher for CO, and 5.7 times higher for ASK1 (Fig. 6a) in line 7–9 than in WT plants. In this study, PSK1-overexpressing Arabidopsis plants (L7–9) exhibited early formation of flower-bud formation and flowered 1 week earlier (ESM_S5) than WT plants (Fig. 6b), indicating that increased expression of the above-mentioned genes may promote early flowering in transgenic plants.
Analysis of expression levels of flower-related genes and demonstration of early flowering. a Expression levels of genes including ASK1, LEAFY, CO, and FT in WT and PSK1-overexpressing Arabidopsis plants (lines 7–9). Each column represents the average of three replicates, and the bar indicates SD. Data from each treatment were subjected to analysis of variance using SPSS 21.0. The different characters above each column represent various significant differences at p < 0.05. b Flowering of WT and PSK1-overexpressing Arabidopsis plants (lines 7–9)
Discussion
In this study, we obtained a SKP1-like gene homologue from a tree peony cDNA library. Denoted as PSK1, this gene was predicted to encode 153 amino acids. According to phylogenetic analysis, PSK1 is most closely related to homologues from Amborella trichopoda, T. cacao, and G. hirsutumf (Fig. 1c). PSK1 also possesses conserved amino acids and motifs (indicated by a blue star in Fig. 1b) that are important for the interaction of SKP1 with F-box proteins (Schulman et al. 2000). PSK1 showed wide expression in tree peony tissues, consistent with previous studies in Arabidopsis (Zhao et al. 2003; Takahashi et al. 2004; Dezfulian et al. 2012), in addition, it displayed increased expression as flower-bud development and flowering progressed, which indicates PSK1 may be functionally important to tree peony flower development and blooming. To confirm this possible function, additional studies are needed.
A previous phylogenetic analysis (Kong et al. 2007) has suggested that all Arabidopsis and rice SKP1-like genes are derived from a single ancestral gene and can be classified into three types, namely, type Ia with one intron and two exons, type Ib lacking introns, and type II containing several introns. As revealed by analysis of its genomic structure, PSK1 contains only a single long intron at a conserved position. In the phylogenetic tree of Kong et al. (2007), ASKs and OSKs with one conserved intron occupied the same basal position, whereas intronless genes generally formed terminal clades. OSK1 and OSK20 both have one intron at a conserved position, thereby demonstrating their orthologous relationship. A detailed analysis of plant, animal, and insect genomes has suggested that genuine orthologous genes have more conserved intron positions than those of non-orthologous genes (Henricson et al. 2010). In contrast, nearly 50% (144/288) of plant SKP1-like genes are intronless, which indicates an active retroposition phenomenon (Kahloul et al. 2013). Despite these findings, investigation of the evolution and genomic structure of all SKP1-like genes from tree peony is still needed to understand their functional conservation and diversification.
Plant displayed various responses to salinity stress, including early germination, vigorous growth, and early development and rapid completion of the life cycle. At the cellular level, concentrations of many metabolites are increased when plant confronts salinity stress. In this study, we found that PSK1-overexpressing transgenic Arabidopsis plants increased salinity tolerance as a consequence of elevated accumulation of proline and soluble sugars (Fig. 5a, c). In contrast, MDA content, an indicator of salinity stress damage (Cheng et al. 2013), did not change significantly. We deduce that the conferral of salinity stress on Arabidopsis plants by overexpression of PSK1 due to increased proline and soluble sugar accumulation, as soluble sugar would prevent cell dehydration (Niu et al. 2012), while, free proline has multiple functions in cells, such as ROS scavenging and osmoprotection (Zsigmond et al. 2012; Niu et al. 2012). Furthermore, transgenic lines demonstrated vigorous growth as reflected by increased fresh weight (Fig. 4d). As ASK1 has been confirmed to play a vital role in early development (Liu et al. 2004), vigorous growth may help plants overcome the disadvantage of salinity stress, thereby aiding their reproduction and benefiting future generations (Kim et al. 2013).
Expression profiles of genes responsible for salinity stress were also analyzed. This analysis revealed that expression levels of detected genes, including AtSOS1-3, AtRAB18, AtDREB, and AtP5CS, were up-regulated in PSK1-overexpressing transgenic lines compared with those in WT after NaCl treatment (Fig. 5d–f; ESM_S4), but with slight discrepancy displayed by the three transgenic lines. Previous studies have demonstrated that the above-mentioned genes can be induced under saline conditions and may play important roles in salinity tolerance (Yamaguchi-Shinozaki 2006; Yang et al. 2009). The expression levels of three genes were higher in PSK1-overexpressing lines than those in WT plants after salinity stress for 12 h (ESM_S4). A SKP1-like gene from Trticum aestivum (TSK1) has been confirmed to be involved in ABA signaling and may positively regulate this process (Li et al. 2012), while AtRAB18 and AtP5CS have been verified to be active in an ABA-dependent regulatory pathway and also induced by abiotic stress (Szabados and Savouré 2010; Cheng et al. 2013). P5CS expression, which has been shown to be induced by salinity stress, is mainly responsible for proline accumulation during salinity or drought stress (Ziaf et al. 2011; Szabados and Savouré 2010). Lines 7–9, especially L7, displayed extremely high AtRAB18 expression levels, even in the absence of salinity stress (Fig. 5e). Although this observation implies that this gene may be involved in ABA signaling or another salt tolerance pathway, further studies are needed to uncover its contribution to salinity tolerance. These results suggest the existence of transcriptional regulatory mechanism for stress tolerance in PSK1-overexpressing Arabidopsis operated by elevating the expression of transcriptional factors and enzymatic genes, including DREB, RAB18, SOS1-3, and P5CS. In Arabidopsis ask1 mutant flowers, increased expression of two proteins has been detected. One of these proteins is a class I heat shock protein, which is stress induced and confers cytoprotection against oxidative injury; the other is an oxygen-evolving complex-related protein, which indicates that mutation of ASK1 may directly or indirectly cause oxidative stress in flower organs (Wang et al. 2006). This finding suggests that overexpression of PSK1 in Arabidopsis increases salinity tolerance through up-regulation of salt-stress-related genes as well as accumulation of some functional proteins. Previous research on plant SKP1-like genes has mainly focused on their function in male fertility and flower development addressed their role in the hormone signaling pathway via SCF-mediated protein degradation in model plants (rice and Arabidopsis). Little information has been made available concerning the function of these genes in abiotic stress. Our study represents the first attempt to uncover additional functions of SKP1-like gene, but much work is still required to fully understand all possible functions in plants.
Plant SKP1 genes in eudicots and monocots have evolved by multiple duplication events from a single ancestral gene. Such gene duplication events are important for gene family evolution with duplicate genes providing a good material for the evolution of new genes as well as physiological and morphological novelties (Kong et al. 2007). In regard to ancestral genes, birth rate much higher than the death rate has aided the expansion of the SKP1 family in plants (Kong et al. 2007). A total of 21, 30 and 19 SKP1-like gene exhibiting a variety of expression patterns are found in Arabidopsis (ASK1-21), rice (OSK1-30), and wild tomato (SSK1-19), respectively, but not all of them can interact with the SCF complex to perform a function. In particular, ASK1 and ASK2 and possibly ASK11 have been found to participate in most positive interactions with F-box proteins in Arabidopsis thaliana, which suggests that ASKs play diverse roles extending beyond serving as components of the SCF complex (Zhao et al. 2003; Takahashi et al. 2004; Kuroda et al. 2012; Kahloul et al. 2013; Zhang et al. 2015). Tissue-specific expression patterns of wild tomato SSKs have revealed some to additionally be responsive to heat stress and salicylic acid treatment (Zhang et al. 2015). Despite these findings, not all SKP1-like genes have been thoroughly studied in a given plant species, even in Arabidopsis; future functional characterization of each of these genes would be beneficial to understand the functional diversification of duplicated genes in plants. The number of SKP1 family members in tree peony is unknown; to gain a comprehensive knowledge on their diverse function and evolution of SKP1-like genes in this woody species, aspects such as their structures, expression patterns, localization, and interacting proteins remain to be further characterized.
In Arabidopsis, ASKs are essential for flower development (Liu et al. 2004; Zhao et al. 2010) and male gametophyte sterility (Marrocco et al. 2003), while SSKs may be involved in tomato fruit development (Zhang et al. 2015) and TSKs regulate male meiosis in wheat (Li et al. 2006). These functions indicate that SKP1-like genes play an important role in reproductive development. In this study, we found that overexpression of PSK1 increased the expression of flowering-related genes including CO, LEAFY, and FT (Fig. 6a), and promoted flower formation and early flowering (Fig. 6b; ESM_S5). This effect was similar to that of ASK1 which interacts with B function proteins (B subfamily MADS-box gene products) and cooperates with UFO and LEAFY involved in flower development and floral organ identity (Zhao et al. 1999, 2010). A similar involvement of SKP1-like genes in flower development has been reported in woody plants such as pummelo (CgSKP1), citrus (CrWSKP1), and sweet cherry (PacSSK1) (Chai et al. 2010; Matsumoto et al. 2012; Li et al. 2015). Taking into account the current progress on SKP1-like genes, PSK1 may possess a conserved function in regulation of flower development and flowering. To fully elucidate the evolution and functional diversification of SKP1-like genes in tree peony, a future study focused on their cloning and functional characterization is planned. Tree peony is a woody shrub, with 3 -5 years required for the transition from vegetative to reproductive growth. PSK1 overexpression increased the transcription of genes involved in the flower development pathway. To shorten its juvenile phase, overexpression of PSK1 in tree peony may promote early flowering. Functional characterization of PSK1 may not only illuminate the molecular mechanism underlying salinity tolerance and its importance, but may also provide global knowledge concerning the multiple functions of SKP1-like family genes under different physiological conditions and developmental stage.
Conclusions
In this study, a SKP1-like gene homologue was cloned from tree peony and functionally analyzed in transgenic Arabidopsis. Overexpression of PSK1 improved their salinity tolerance of the transgenic Arabidopsis plants, which indicated a novel function of PSK1. In PSK1-overexpressing transgenic plants, genes positively related to flower development and flowering time were up-regulated thereby promoting flower formation and early flowering. This study has yielded new information on the function of PSK1 and its possible molecular mechanism during tree peony flower development and conferral of salinity tolerance, thus providing a theoretical basis for the regulation of flowering and abiotic stress tolerance. Because tree peony is a globally popular ornamental flowering plant, our gained insights into flowering will also be beneficial for future breeding of cultivars and new germplasm resources.
Author contribution statement
HQ and RHX performed salinity stress analyses; ZJ and HSC conducted the qRT-PCR analysis; WLS and LZA carried out plants germination and growth analysis; GZM and SQY performed PSK1 cloning, vector construction, and transformation of Arabidopsis, and wrote the article.
Abbreviations
- CaMV:
-
Cauliflower mosaic virus
- DREB:
-
Dehydration-responsive element-binding protein
- MDA:
-
Malondialdehyde
- MS:
-
Murashige and Skoog
- ORF:
-
Open reading frame
- P5CS:
-
Δ1-Pyrroline-5-carboxylate synthase
- qPCR:
-
Quantitative real-time PCR
- RAB18:
-
Ras-related protein
- RACE:
-
Rapid amplification of cDNA ends
- ROS:
-
Reactive oxygen species
- RT-PCR:
-
Reverse transcription-PCR
- SOD:
-
Superoxide dismutase
- WT:
-
Wild type
- CO :
-
CONSTANS
- FT :
-
Flowering locus T
References
Bailey RW (1958) The reaction of pentoses with anthrone. Biochem J 68:669–672
Chai LJ, Biswas MK, Ge XX, Deng XX (2010) Isolation, characterization, and expression analysis of an SKP1-like gene from ‘Shatian’ Pummelo (Citrus grandis Osbeck). Plant Mol Biol Rep 28:569–577
Chen ZL, Yu Y, Liu LN, Xia GX (2007) Isolation, characterization and functional analysis of a cdc48 homologue from tobacco BY-2 cells. Progress Nat Sci 17:156–162
Cheng MC, Liao PM, Kuo WW, Lin TP (2013) The Arabidopsis ETHYLENE RESPONSE FACTOR1 regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiol 162(3):1566–1582
Chou KC, Shen HB (2010) Plant-mPLoc: a top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS One 5:e11335
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16(6):735–743
Dezfulian MH, Soulliere DM, Dhaliwal RK, Sareen M, Crosby WL (2012) The SKP1-like gene family of Arabidopsis exhibits a high degree of differential gene expression and gene product interaction during development. PLoS One 7:e50984
Dieterle M, Zhou YC, Schafer E, Funk M, Kretsch T (2001) EID1, an F-box protein involved in phytochrome A-specific light signaling. Genes Dev 15:939–944
Du H, Wu J, Jia KX, Zeng QY, Bhuiyad MW, Su S, Shu QY, Ren HX, Liu ZA, Wang LS (2015) Methylation mediated by an anthocyanin O-methyltransferase, is involved in purple flower coloration in Paeonia. J Exp Bot 66:6563–6577
Gray WM, del Pozo JC, Walker L, Hobbie L, Risseeuw E, Banks T, Crosby WL, Yang M, Ma H, Estelle M (1999) Identification of an SCF ubiquitin-ligase complex required for auxin response in Arabidopsis thaliana. Genes Dev 13:1678–1691
Gusti A, Baumberger N, Nowack M, Pusch S, Eisler H, Potuschak T, De Veylder L, Schnittger A, Genschik P (2009) The Arabidopsis thaliana F-box protein FBL17 is essential for progression through the second mitosis during pollen development. PLoS One 4:e4780
Han XY, Wang LS, Liu ZA, De Riek J, Shu QY (2008) Characterization of sequence-related amplified polymorphism marker analysis of tree peony bud sports. Sci Horti (Amsterdam) 115:261–267
Hellmann H, Estelle M (2002) Plant development: regulation by protein degradation. Science 297:793–797
Henricson A, Forslund K, Sonnhamme E (2010) Orthology confers intron position conservation. BMC Genom 11:412
Hong MJ, Kim DY, Seo YW (2013) SKP1-like-related genes interact with various F-box proteins and may form SCF complexes with Cullin-F-box proteins in wheat. Mol Biol Rep 40:969–981
Imaizumi T, Schultz TF, Harmon FG, Ho LA, Kay SA (2005) FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science 309:293–297
Kahloul S, HajSalahElBeji I, Boulaflous A, Ferchichi A, Kong H, Mouzeyar S, Bouzidi MF (2013) Structural, expression and interaction analysis of rice SKP1-like genes. DNA Res 20:67–78
Kim WY, Ali Z, Park HJ, Park SJ, Cha JY, Perez-Hormaeche J, Quintero FJ, Shin G, Kim MR, Qiang Z, Ning L, Park HC, Lee SY, Bressan RA, Pardo JM, Bohnert HJ, Yun DJ (2013) Release of SOS2 kinase from sequestration with GIGANTEA determines salt tolerance in Arabidopsis. Nat Commun 4:1352
Kong H, Leebens-Mack J, Ni W, dePamphilis CW, Ma H (2004) Highly heterogeneous rates of evolution in the SKP1 gene family in plants and animals: functional and evolutionary implications. Mol Biol Evol 21:117–128
Kong H, Landherr LL, Frohlich MW, Leebens-Mack J, Ma H, de Pamphilis CW (2007) Patterns of gene duplication in the plant SKP1 gene family in angiosperms: evidence for multiple mechanisms of rapid gene birth. Plant J 50:873–885
Kramer GF, Norman HA, Krizek DT, Mirecki RM (1991) Influence of UV-B radiation on polyamines, lipid peroxidation and membrane lipids in cucumber. Phytochem 30:2101–2108
Kuroda H, Yanagawa Y, Takahashi N, Horii Y, Matsui M (2012) A comprehensive analysis of interaction and localization of Arabidopsis SKP1-like (ASK) and F-box (FBX) proteins. PLoS One 7e:50009
Letunic I, Goodstadt L, Dickens NJ, Doerks T, Schultz J, Mott R, Ciccarelli F, Copley RR, Ponting CP, Bork P (2002) Recent improvements to the SMART domain-based sequence annotation resource. Nucleic Acids Res 30:D142–D144
Li JJ (1999) Chinese Tree Peony and Herbaceous Peony. Chinese Forestry Press, Beijing, p 17
Li C, Liang Y, Chen C, Li J, Xu Y, Xu Z, Ma H, Chong K (2006) Cloning and expression analysis of TSK1, a wheat SKP1 homologue, and functional comparison with Arabidopsis ASK1 in male meiosis and auxin signaling. Funct Plant Biol 33:381–390
Li ZX, Qin GW, He JH, Cao XY (2010) Comparative analysis of fatty acid composition in seed kernel and coat of Paeonia rockii seeds. Seed 29:34–36
Li C, Liu Z, Zhang Q, Wang R, Xiao L, Ma H, Chong K, Xu Y (2012) SKP1 is involved in abscisic acid signaling to regulate seed germination, stomatal opening and root growth in Arabidopsis thaliana. Plant Cell Environ 35:952–965
Li SS, Yuan RY, Chen LG, Wang LS, Hao XH, Wang LJ, Zheng XC, Du H (2014) Systematic qualitative and quantitative assessment of fatty acids in the seeds of 60 tree peony (Paeonia section Moutan DC.) cultivars by GC-MS. Food Chem 173:133–140
Li P, Miao HX, Ma YW, Wang L, Hu GB, Ye ZX, Zhao JT, Qin YH (2015) CrWSKP1, a SKP1-like gene, is Involved in the self-Incompatibility reaction of “Wuzishatangju” (Citrus reticulata Blanco). Int J Mol Sci 16:21695–21710
Liu F, Ni W, Griffith ME, Huang Z, Chang C, Peng W, Ma H, Xie D (2004) The ASK1 and ASK2 genes are essential for Arabidopsis early development. Plant Cell 16:5–20
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods 25:402–408
Marrocco K, Lecureuil A, Nicolas P, Guerche P (2003) The Arabidopsis SKP1-like genes present a spectrum of expression profiles. Plant Mol Biol 527:15–727
Matsumoto D, Yamane H, Abe K, Tao R (2012) Identification of a Skp1-like protein interaction with SFB, the pollen S determinant of the gametophytic self-incompatibility in Prunus. Plant Physiol 159:1252–1262
McGinnis KM, Thomas SG, Soule JD, Strader LC, Zale JM, Sun TP, Steber CM (2003) The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. Plant Cell 15:1120–1130
Nayak S, Santiago FE, Jin H, Lin D, Schedl T, Kipreos ET (2002) The Caenorhabditis elegans Skp1-related gene family: diverse functions in cell proliferation, morphogenesis, and meiosis. Curr Biol 12:277–287
Niu CF, Wei W, Zhou QY, Tian AG, Hao YJ, Zhang WK, Ma B, Lin Q, Zhang ZB, Zhang JS, Chen SY (2012) Wheat WRKY genes TaWRKY2 and TaWRKY19 regulate abiotic stress tolerance in transgenic Arabidopsis plants. Plant Cell Environ 35:1156–1170
Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, Boursnell C, Pang N, Forslund K, Ceric G, Clements J, Heger A, Holm L, Sonnhammer EL, Eddy SR, Bateman A, Finn RD (2012) The Pfam protein families database. Nucleic Acids Res 40:D290–D301
Rogers SW, Well R, Rechsteiner M (1986) Amino acid sequences act as quantitative enchancers of gene expression in transgenic tobacco and potato plants. Plant Mol Biol 37:885–897
Schulman BA, Carrano AC, Jeffrey PD, Bowen Z, Kinnucan ER, Finnin MS, Elledge SJ, Harper JW, Pagano M, Pavletich NP (2000) Insights into SCF ubiquitin ligases from the structure of the Skp1-Skp2 complex. Nature 408:381–386
Shan DP, Huang JG, Yang YT, Guo YH, Wu CA, Yang GD, Gao Z, Zheng CC (2007) Cotton GhDREB1 increases plant tolerance to low temperature and is negatively regulated by gibberellic acid. New Phytol 176:70–81
Shu QY, Wischnitzki E, Liu ZA, Ren HX, Han XY, Hao Q, Gao FF, Xu SX, Wang LS (2009) Analysis and functional annotation of expressed sequence tags for Tree Peony for the understanding of molecular mechanism controlling flower bud development. Physiol Plant 135:436–449
Somers DE, Schultz TF, Milnamow M, Kay SA (2000) ZEITLUPE encodes a novel clock-associated PAS protein from Arabidopsis. Cell 101:319–329
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Takahashi N, Kuroda H, Kuromori T, Hirayama T, Seki M, Shinozaki K, Shimada H, Matsui M (2004) Expression and interaction analysis of Arabidopsis Skp1-related genes. Plant Cell Physiol 45:83–91
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4.1: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Vierstra RD (2009) The ubiquitin-26S proteasome system at the nexus of plant biology. Nat Rev Mol Cell Biol 10:385–397
Wang X, Ni W, Ge X, Zhang J, Ma H, Cao KM (2006) Proteomic identification of potential target proteins regulated by an ASK1-mediated proteolysis pathway. Cell Res 16:489–498
Wang Q, Tao T, Han Y, Chen X, Fan Z, Li D, Yu J, Han C (2013) Nonstructural protein P7–2 encoded by Rice black-streaked dwarf virus interacts with SKP1, a core subunit of SCF ubiquitin ligase. Virol J 10(1):325
Winkler AA, Goedegebure RH, Zonneveld BJ, Steensma HY, Hooykaas PJ (2000) Isolation and partial characterization of the Kluyveromyces lactis homologue of SKP1. Curr Genet 38:8–16
Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG (1998) COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280:1091–1094
Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57:781–803
Yang M, Hu Y, Lodhi M, McCombie WR, Ma H (1999) The Arabidopsis SKP1-LIKE1 gene is essential for male meiosis and may control homologue separation. Proc Natl Acad Sci USA 96:11416–11421
Yang Q, Chen ZZ, Zhou XF, Yin HB, Li X, Xin XF, Hong XH, Zhu JK, Gong Z (2009) Overexpression of SOS (Salt Overly Sensitive) genes increases the salt tolerance in transgenic Arabidopsis. Mol Plant 2:22–31
Yoshida A, Suzuki N, Nakano Y, Kawada M, Oho T, Koga T (2003) Development of a 5′ nuclease-based real-time PCR assay for quantitative detection of cariogenic dental pathogens Streptococcus mutans and Streptococcus sobrinus. J Clin Microbiol 41:4438–4441
Zhang YQ, Wang CP, Lin QF, Gao FH, Ma Y, Zhang M, Lin YH, Ma QH, Hua XJ (2015) Genome-wide analysis of phylogeny, expression profile and sub-cellular localization of SKP1-Like genes in wild tomato. Plant Sci 238:105–114
Zhao D, Yang M, Solava J, Ma H (1999) The ASK1 gene regulates development and interacts with the UFO gene to control floral organ identity in Arabidopsis. Dev Genet 25:209–223
Zhao D, Ni W, Feng B, Han T, Petrasek MG, Ma H (2003) Members of the Arabidopsis-SKP1-like gene family exhibit a variety of expression patterns and may play diverse roles in Arabidopsis. Plant Physiol 133:203–217
Zhao L, Huang J, Zhao ZH, Li Q, Sims TL, Xue YB (2010) The SKP1-like protein SSK1 is required for cross-pollen compatibility in S-RNase-based self-incompatibility. Plant J 62:52–63
Ziaf K, Loukehaich R, Gong P, Liu H, Han Q, Wang T, Li H, Ye Z (2011) A multiple stress responsive gene ERD15 from Solanum pennelli confers stress tolerance in tobacco. Plant Cell Physiol 52:1055–1067
Zsigmond L, Szepesi A, Tari I, Rigo´ G, Kira´ly A, Szabados L (2012) Overexpression of the mitochondrial PPR40 gene improves salt tolerance in Arabidopsis. Plant Sci 182:87–93
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Grant Nos. 31272201 and 31471909).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by B. Li.
Qing Hao and Hongxu Ren contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
299_2016_2066_MOESM3_ESM.jpg
ESM_S3. Comparison of stress response of PSK1-overexpressing Arabidopsis transgenic plants (lines 7 - 9) and the WT. (A) Germination percentages of transgenic lines and WT plants calculated 1 days after keeping at 22 ºC in medium containing 0-125 mM NaCl. (B) Cotyledon greening rates of transgenic lines and WT plants growing in medium containing 0–125 mM NaCl for 1 week. (C) Root elongation measurement of 7-day-old plants maintained in medium for 1 week. Each column represents the average of three replicates, and the bar indicates SDs. The different characters above each column represent various significant differences at p < 0.05 (JPEG 3118 kb)
299_2016_2066_MOESM4_ESM.jpg
ESM_S4. Expression analysis of SOS1-3 in Arabidopsis plants (lines 7-9 and the WT) treated with 150 mM NaCl for 0–12 h. (A) SOS1 (B) SOS2 (C) SOS3. The different characters above each column represent various significant differences at p < 0.05 (JPEG 2450 kb)
Rights and permissions
About this article
Cite this article
Hao, Q., Ren, H., Zhu, J. et al. Overexpression of PSK1, a SKP1-like gene homologue, from Paeonia suffruticosa, confers salinity tolerance in Arabidopsis. Plant Cell Rep 36, 151–162 (2017). https://doi.org/10.1007/s00299-016-2066-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-016-2066-z