Abstract
Key message
The C-terminal extension region of SlGAD3 is likely involved in autoinhibition, and removing this domain increases GABA levels in tomato fruits.
Abstract
γ-Aminobutyric acid (GABA) is a ubiquitous non-protein amino acid with several health-promoting benefits. In many plants including tomato, GABA is synthesized via decarboxylation of glutamate in a reaction catalyzed by glutamate decarboxylase (GAD), which generally contains a C-terminal autoinhibitory domain. We previously generated transgenic tomato plants in which tomato GAD3 (SlGAD3) was expressed using the 35S promoter/NOS terminator expression cassette (35S-SlGAD3-NOS), yielding a four- to fivefold increase in GABA levels in red-ripe fruits compared to the control. In this study, to further increase GABA accumulation in tomato fruits, we expressed SlGAD3 with (SlGAD3 OX) or without (SlGAD3ΔC OX) a putative autoinhibitory domain in tomato using the fruit ripening-specific E8 promoter and the Arabidopsis heat shock protein 18.2 (HSP) terminator. Although the GABA levels in SlGAD3 OX fruits were equivalent to those in 35S-SlGAD3-NOS fruits, GABA levels in SlGAD3ΔC OX fruits increased by 11- to 18-fold compared to control plants, indicating that removing the autoinhibitory domain increases GABA biosynthesis activity. Furthermore, the increased GABA levels were accompanied by a drastic reduction in glutamate and aspartate levels, indicating that enhanced GABA biosynthesis affects amino acid metabolism in ripe-fruits. Moreover, SlGAD3ΔC OX fruits exhibited an orange-ripe phenotype, which was associated with reduced levels of both carotenoid and mRNA transcripts of ethylene-responsive carotenogenic genes, suggesting that over activation of GAD influences ethylene sensitivity. Our strategy utilizing the E8 promoter and HSP terminator expression cassette, together with SlGAD3 C-terminal deletion, would facilitate the production of tomato fruits with increased GABA levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
γ-Aminobutyric acid (GABA) is a four-carbon, non-protein amino acid that widely occurs in animals, plants, and bacteria. In humans, GABA functions as an inhibitory neurotransmitter (Owens and Kriegstein 2002) as well as a functional compound that confers several health benefits, such as lowering blood pressure, inducing relaxation, and reducing sleeplessness, depression, and autonomic disorders (Okada et al. 2000; Inoue et al. 2003; Abdou et al. 2006); therefore, a variety of GABA-enriched foods have been developed (Tsushida et al. 1987; Okada et al. 2000; Inoue et al. 2003; Park and Oh 2007).
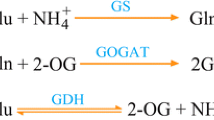
Among natural food resources, tomatoes accumulate relatively high levels of GABA in the fruits (Matsumoto et al. 1997), perhaps due to their high capacity to biosynthesize and/or store GABA. We previously investigated the mechanism underlying GABA accumulation in tomato fruits and attempted to further increase GABA accumulation in tomatoes (Akihiro et al. 2008; Yin et al. 2010; Koike et al. 2013; Takayama et al. 2015). In various higher plants, GABA is primarily biosynthesized from glutamate by the enzyme glutamate decarboxylase (GAD) and is catabolized to succinic semialdehyde (SSA) by GABA transaminase (GABA-T) (Bouché and Fromm 2004). At least three homologs of GAD and GABA-T genes are present in the tomato genome, i.e., SlGAD1-3 and SlGABA-T1-3, respectively (Akihiro et al. 2008). Genetic suppression analyses of these genes revealed that SlGAD2/3 and SlGABA-T1 are likely the key regulatory genes for GABA biosynthesis and catabolism, respectively, in the fruit (Koike et al. 2013; Takayama et al. 2015). Although suppressing SlGABA-T1 under the control of the cauliflower mosaic virus (CaMV) 35S promoter was effective for increasing fruit GABA levels (up to 9.2-fold wild-type levels at the red stage), this process also induced severe dwarfism and infertility (Koike et al. 2013). To avoid the negative effects of systemic suppression of SlGABA-T1, we also generated transgenic tomato plants in which SlGABA-T1 was suppressed under the control of the E8 promoter, a strong, inducible promoter specific to ripening tomato fruits (Deikman et al. 1998). The resulting transgenic plants exhibited normal development, but the GABA levels in red-ripe fruits were lower than those in transgenic fruits in which SlGABA-T1 was suppressed driven by the 35S promoter, even though the levels were still higher than those in the wild-type (WT) (Koike et al. 2013).
Increasing GABA biosynthesis appears to represent an effective alternative approach for improving GABA levels in tomato fruits. Indeed, we previously reported that overexpressing SlGAD3 in tomato fruits using the CaMV 35S promoter/nopaline synthase (NOS) terminator expression cassette led to an increase in GABA levels (up to 5.2-fold wild-type levels) at the red-ripe stage, demonstrating that SlGAD3 is a good candidate for genetic engineering to increase GABA accumulation in tomatoes (Takayama et al. 2015).
Plant GAD genes generally possess an extension region (consisting of 30–50 amino acid residues) at the C-terminus known as the Ca2+/calmodulin (CaM) binding domain, which autoinhibits the activity of this enzyme at physiological pH, although its autoinhibitory function is de-repressed under low cellular pH or by binding of Ca2+/CaM to this domain (Snedden et al. 1995; Snedden et al. 1996; Yap et al. 2003). Therefore, when plants are exposed to stresses that induce the accumulation of high concentrations of H+ (low pH) and/or Ca2+ in plant cells, GABA biosynthesis becomes more active due to the suppression of GAD autoinhibition (Shelp et al. 1999). Transgenic studies revealed that removing this autoinhibitory domain results in extremely high levels of GABA production in plant cells, most likely resulting from increased GAD enzyme activity (Baum et al. 1996; Akama and Takaiwa 2007). However, the role of the autoinhibitory domain in tomato GAD genes has not been investigated to date. In the present study, we generated transgenic tomato plants in which the full-length coding sequence of SlGAD3 with or without the C-terminal extension region (SlGAD3ΔC) was overexpressed under the control of the fruit-specific E8 promoter and the heat shock protein 18.2 (HSP) terminator from Arabidopsis thaliana, which probably improves mRNA stability via a post-transcriptional process and was shown to increase gene expression in tomato (Nagaya et al. 2010; Hirai et al. 2011b; Kurokawa et al. 2013). We found that deleting the C-terminal autoinhibitory domain from SlGAD3 greatly increased GABA levels in fruits, accompanied by both delayed ethylene production and reduced ethylene sensitivity. We discuss the methodology used to increase GABA levels by genetic engineering and propose that there is a molecular connection between GABA and ethylene.
Materials and methods
Plant material and growth condition
The tomato (Solanum lycopersicum L.) cv. ‘Micro-Tom’ seeds used in this study were provided by the National Bioresource Project (Saito et al. 2011). Seeds of WT and T1 transgenic plants were germinated on moistened filter paper and transferred to Rockwool cubes (5 × 5 × 5 cm). The plants were grown under fluorescent light with a 16 h light (60 μmol/m2/s)/8 h dark photoperiod at 25 °C and were supplied with standard nutrient solution (Otsuka A; Otsuka Chemical Co., Ltd., Osaka, Japan). Fruits at the mature green (MG; 24 days after flowering), breaker (Br), Br + 3 days (Br + 3), Br + 7 days (Br + 7), Br + 10 days (Br + 10), Br + 15 days (Br + 15), and Br + 30 days (Br + 30) stage were used in this study.
Vector construction and tomato transformation
Two binary vectors derived from the previously reported binary vector E8-MIR-HSP containing the E8 promoter and HSP terminator (Kurokawa et al. 2013) were constructed in this study. The full-length coding sequence of SlGAD3 (Solyc01g005000) and this sequence lacking the C-terminal 87 nucleotides (designated SlGAD3ΔC) was amplified by PCR using gene-specific primers containing some additional sequences at their 5′ ends (details are shown in Fig. S1 and Table S1). The E8-MIR-HSP plasmid was digested with BamHI and SacI, and the amplified fragments of SlGAD3 or SlGAD3ΔC were fused by the In-Fusion cloning reaction (Clontech, CA, USA) according to the manufacturer’s instructions. The resulting constructs were designated SlGAD3 OX and SlGAD3ΔC OX, respectively (Fig. 2a). These constructs were transformed into Agrobacterium tumefaciens strain GV2260 (Deblaere et al. 1985) and used for tomato transformation (Sun et al. 2006). Regenerated plants that survived on Murashige and Skoog (MS) medium containing kanamycin (100 mg l−1) were transferred to Rockwool cubes (5 × 5 × 5 cm) and grown under the conditions described above.
qRT-PCR analysis
Fruits at the MG, Br + 3, and Br + 10 stages were harvested and frozen in liquid nitrogen after removing seeds and jelly tissues. Frozen fruits were ground to a fine powder in liquid nitrogen using a mortar and pestle. Total RNA was extracted using an RNeasy Plant Mini kit (Qiagen, Tokyo, Japan) with RNase-free DNase (Qiagen, Tokyo, Japan) according to the manufacturer’s instructions. First-strand cDNA was synthesized from 1 μg of total RNA using a SuperScript VILO cDNA Synthesis Kit (Invitrogen, USA). The resulting first-strand cDNA was diluted tenfold in RNase-free water and used as a template for qRT-PCR. The qRT-PCR was performed using a Takara Thermal Cycler Dice Real Time System TP800 with SYBR Premix Ex Taq II (Takara-Bio Inc., Otsu, Japan). The reaction conditions were as follows: 95 °C for 30 s for initial denaturation, followed by 40 cycles of 5 s of denaturation at 95 °C and 30 s of annealing/extension at 60 °C (54 °C for SlGAD3). The tomato ubiquitin gene (SlUBI3; GenBank accession no. X58253) was used as an internal control. The gene-specific primers used in this experiment are shown in Table S1.
Determination of free amino acid contents
Free amino acid contents in MG and Br + 10 fruits were determined using an amino acid analyzer (JLC-500/V2, Japan Electron Optics Laboratory) as described previously (Koike et al. 2013). As a quick method for screening GABA contents in fruits, the GABase enzymatic assay was also carried out as described by Jakoby (1962) and Saito et al. (2008).
Determination of GAD activity
Br + 10 fruits were harvested from T0 plants and immediately frozen in liquid nitrogen after removing seeds and jelly tissues. Frozen fruits were then ground to a fine powder in liquid nitrogen using a mortar and pestle. To extract crude protein, powdered fruits were homogenized in fivefold volumes of ice-cold extraction buffer containing 50 mM Tris–HCl buffer (pH 8.2), 3 mM dithiothreitol (DTT), 1.25 mM EDTA, 2.5 mM MgCl2, 10 % (v/v) glycerol, 6 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulphonate, 2 % (w/v) polyvinylpyrrolidone, 2 μg ml−1 pridoxal-5-phosphate, 1 mM phenylmethylsulphonyl fluoride and 2.5 μg ml−1 leupeptin and pepstatin as described in Clark et al. (2009). The homogenates were then centrifuged at 10,000×g for 15 min at 4 °C. The supernatant was concentrated using Amicon ultra-4 (10 kDa, Millipore) and desalted with PD-10 columns (GE Healthcare). GAD enzymatic activity was assayed based on GABA production during the glutamate-dependent GAD reaction as described by Akama and Takaiwa (2007) and Akihiro et al. (2008) with slight modifications. The reaction was initiated by adding 60 μl of the crude protein (adjusted to 0.4–0.6 mg ml−1) in 240 μl of GAD reaction buffer containing 100 mM of an adequate buffer (Bis–Tris for pH 7.0; MES for pH 5.8), 1 mM DTT, 5 mM glutamate, 0.5 mM pyridoxal-5-phosphate, 0.5 mM CaCl2, 0.1 M bovine calmodulin (Sigma-Aldrich, Missouri) and 10 % (v/v) glycerol. The mixture was incubated at 30 °C for 180 min and boiled for 10 min to stop the reaction. GABA production was measured by the ‘GABase’ method as described by Jakoby (1962) with slight modifications.
Determination of lycopene and β-carotene contents
Lycopene and β-carotene contents in Br + 10 fruits were measured as described previously (Kitagawa et al. 2005; Mubarok et al. 2015). Pigments were extracted from powdered fruits with acetone-hexane (4:6) solution, and the optical density of the supernatant was simultaneously measured in a spectrophotometer at 663 nm (A 663), 645 nm (A 645), 505 nm (A 505), and 453 nm (A 453). Lycopene (C LYC) and β-carotene (C CAR) were calculated according to the following formula:
Determination of ethylene production
Fruits were harvested at different developmental stages (MG, Br, Br + 3, Br + 10, and Br + 15). Each fruit was placed in a 50 ml glass vial and pre-incubated for more than 3 h to reduce ethylene evolution triggered by harvest shock. Subsequently, each vial was sealed with a cap containing a septum and incubated for 1 h. Finally, 1 ml of headspace gas was sampled from the vial, and the ethylene production rate was measured by injecting it into a gas chromatograph (GC-14B, Shimadzu).
Results
Comparison of the C-terminal region of GAD proteins from various plant species
Tomato genome contains at least three GAD genes (SlGAD1-3; Akihiro et al. 2008). Like other higher plants, the encoded proteins possess an extension region at the C-terminus, which generally functions as a CaM-binding domain as well as an autoinhibitory domain (Baum et al. 1993; Arazi et al. 1995; Gut et al. 2009). When the amino acid sequences of the C-terminal region of SlGADs are compared with those from previously characterized plants, SlGADs represent several typical features involving CaM-binding (Fig. 1). For example, SlGAD1 and SlGAD2 conserve a tryptophan (W) residue and lysine (K) clusters that are likely involved in hydrophobic and electrostatic interactions, respectively, in CaM/GAD complex formation (Arazi et al. 1995; Gut et al. 2009). These residues are highly conserved in PhGAD, AtGAD1, OsGAD1 and MdGAD1 that are the typical isoforms with CaM-binding ability, but not (or less) in MdGAD3 and OsGAD2, the isoforms without CaM-binding ability (Akama and Takaiwa 2007; Trobacher et al. 2013). Although SlGAD3 contains only one lysine residue, it conserves a tryptophan residue (Fig. 1). In addition, all SlGADs possess one of the two putative pseudo-substrate glutamate (E) residues characterized in PhGAD (Fig. 1; Yap et al. 2003), suggesting that they might have function as an autoinhibitory domain. In this study, we first focused on SlGAD3, which is the main isoform involving GABA biosynthesis in tomato fruits, and examined the effects of its C-terminal truncation on GABA accumulation in tomato fruits.
Multiple alignment of the C-terminal extension regions of plant GADs. The C-terminal regions of tomato GADs (SlGAD1-3) were compared with those from previously characterized plant, such as petunia (PhGAD), Arabidopsis (AtGAD1), rice (OsGAD1 and 2) and apple (MdGAD1-3). The conserved tryptophan (W) residue and lysine (K) clusters involved in CaM-binding (Arazi et al. 1995; Gut et al. 2009) are indicated by an asterisk and a black line, respectively. The two pseudo-substrate glutamate (E) residues that are likely involved in autoinhibition in PhGAD (Yap et al. 2003) are indicated as filled circles
Generation of GABA hyper-accumulating transgenic tomato lines
To investigate the role of the C-terminal extension region in SlGAD3, we constructed two types of plant expression vectors, in which the full-length coding sequence of SlGAD3 with or without the C-terminal 87 nucleotides (corresponding to the putative CaM-binding/autoinhibitory domain) was inserted into the E8 promoter/HSP terminator cassette (designated SlGAD3 OX and SlGAD3ΔC OX, respectively; Fig. 2a). Using Agrobacterium-mediated method, ten and fifteen transgenic plants were obtained for SlGAD3 OX and SlGAD3ΔC OX, respectively (Fig. S2). Among them, three independent lines with significantly increased levels of SlGAD3 (ΔC) expression in Br + 10 fruits were selected (Fig. 2b). To examine the effects of increased SlGAD3 or SlGAD3ΔC expression on fruit GABA accumulation, we determined the GABA levels using the GABase enzymatic assay (Jakoby 1962; Saito et al. 2008). The GABA levels in Br + 10 fruits of SlGAD3 OX (lines 8b, 9, and A) were significantly higher than those of the WT, ranging from 12–15 μmol gFW−1, which corresponded to six to sevenfold higher levels than those of the WT (Fig. 2c). Notably, SlGAD3ΔC OX lines (lines 68, 140a, and 153) accumulated much higher levels of GABA, i.e., 23–26 μmol gFW−1, corresponding to 11- to 12-fold higher levels than those of the WT and 1.9-fold higher levels than those of the SlGAD3 OX lines (Fig. 2c). To confirm whether the increased GABA levels in transgenic tomato fruits are correlated with the GAD enzymatic activity, we extracted crude proteins from Br + 10 fruits and used them for GAD enzymatic assay (Fig. 3). As expected, higher GAD activity was found in the fruit of SlGAD3 OX (lines 8b, 9, and A) and SlGAD3ΔC OX (lines 68, 140a, and 153). Notably, SlGAD3ΔC OX fruits exhibited the highest activity in all conditions we tested (pH 7.0 or pH 5.8, with or without Ca2+/CaM); the average activity was 80.5- and 48.9-fold higher at pH 7.0 without Ca2+/CaM, 45.2- and 8.8-fold higher at pH 7.0 with Ca2+/CaM, 15.5- and 6.1-fold higher at pH 5.8 without Ca2+/CaM, and 7.3- and 4.3-fold higher at pH 5.8 with Ca2+/CaM, when compared with that of the WT and SlGAD3 OX lines, respectively (Fig. 3).
Generation of SlGAD3 and SlGAD3ΔC overexpressing lines. a Maps of T-DNA used for tomato transformation. RB and LB, right and left borders of T-DNA; NPTII, neomycin phosphotransferase II gene; E8 pro., tomato E8 gene promoter; HSP ter., Arabidopsis heat shock protein gene terminator. b qRT-PCR analysis of SlGAD3 in T0 transgenic fruits at the Br + 10 stage. The expression levels of WT fruits were set to a baseline value of 1. Slubiquitin3 (UBI) was used for normalization. c GABA contents determined by the GABase enzymatic assay using the fruit samples used in b. The mean ± SE of three biological replicates are shown. Asterisks indicate a significant difference between WT and transgenic lines according to Student’s t test (*P < 0.05 and **P < 0.01)
GAD activity in T0 SlGAD3 OX and T0 SlGAD3ΔC OX fruits. Crude proteins extracted from Br + 10 fruits were used for GAD enzymatic assay. The mean ± SE of three biological replicates are shown. Asterisks indicate a significant difference between WT and transgenic lines according to Student’s t test (*P < 0.05 and **P < 0.01)
Although the fruit appearance of SlGAD3 OX (lines 8b, 9, and A) was indistinguishable from that of the WT (Fig. 4a), all three SlGAD3ΔC OX (lines 68, 140a, and 153) did not form red-ripe fruits, but formed fruits exhibiting orange-ripe phenotype at the Br + 10 stage (Fig. 4b). The lycopene levels in Br + 10 fruits of SlGAD3ΔC OX were 37–63 % those of the WT, whereas no significant decrease was observed in those of SlGAD3 OX (Fig. 4c).
Fruit appearance of T0 SlGAD3 OX and T0 SlGAD3ΔC OX lines. Br + 10 fruits of SlGAD3 OX (a) and SlGAD3ΔC OX (b) lines are shown. Bar 1 cm. c Lycopene contents in the fruits of WT and T0 transgenic lines at the Br + 10 stage. The mean ± SE of three biological replicates are shown. Asterisks indicate a significant difference between WT and transgenic lines according to Student’s t test (*P < 0.05 and **P < 0.01)
SlGAD3 (ΔC) expression and free amino acid contents in T1 SlGAD3ΔC OX fruits
To analyze the stability of GABA accumulation, we compared the T1 progenies of three SlGAD3ΔC OX lines (68, 140a, and 153) with those of the WT control. Using fruits at the MG and Br + 10 stages, we analyzed SlGAD3 (ΔC) mRNA transcript levels to confirm that mRNA induction occurred in T1 SlGAD3ΔC OX fruits. The SlGAD3 (ΔC) mRNA levels in transgenic lines 68 and 140a were significantly higher than those of the WT at the MG stage, and all three transgenic lines had much higher levels at the Br + 10 stage, ranging from 800- to 1500-fold WT levels (Fig. 5a). Next, we determined the GABA levels in MG and Br + 10 fruits using an amino acid analyzer. In general, GABA levels in WT ‘Micro-Tom’ fruits are high at the MG stage and decrease rapidly as the fruit reaches maturation during the ripening stage (Akihiro et al. 2008). Consistent with this finding, the GABA level in WT fruits decreased by 82 % from MG to the Br + 10 stage (Fig. 5b). By contrast, all transgenic lines accumulated much higher levels of GABA at the Br + 10 stage, ranging from 19 to 29 μmol gFW−1, which corresponded to 12- to 18-fold higher levels than WT (1.6 μmol gFW−1) at the Br + 10 stage (Fig. 5b).
SlGAD3 mRNA levels and GABA contents in T1 SlGAD3ΔC OX fruits. Fruits at the MG and Br + 10 stage were analyzed. a qRT-PCR analysis of SlGAD3. The expression levels in WT MG fruits were set to a baseline value of 1. Slubiquitin3 (UBI) was used for normalization. b GABA contents determined using an amino acid analyzer. The same fruit samples used in a were used. The mean ± SE of three biological replicates are shown. Asterisks indicate a significant difference between WT and transgenic lines according to Student’s t test (*P < 0.05 and **P < 0.01). MG mature green, Br + 10 10 days after breaker
We also analyzed the levels of other free amino acids in Br + 10 fruits to determine whether enhanced GABA biosynthesis affected amino acid metabolism (Table 1). Notably, the levels of aspartate and glutamate were significantly lower in all transgenic lines compared to WT, i.e., 5–12 and 5–9 % of WT levels, respectively (Table 1). By contrast, the levels of alanine, cysteine, and phenylalanine were significantly higher than those of the WT, even though these levels accounted for less than 6 % of total free amino acids in the transgenic lines. In the transgenic lines, GABA comprised up to 81 % of total free amino acids at the Br + 10 stage. This percentage was much higher than that of the WT, not only at the Br + 10 stage (6.2 %; Table 1), but also at the MG stage (33 %; data not shown), when GABA reached maximum levels in WT fruits.
We also determined pH and total soluble solid (TSS) contents in Br + 10 fruits of SlGAD3ΔC OX lines. pH and TSS are commonly used as the indicators to estimate organic acid and sugar levels, respectively. Compared with WT, all three transgenic lines showed significantly higher pH, suggesting that over production of GABA also affected organic acid levels (Fig. S3a). By contrast, no significant difference was observed in TSS contents (Fig. S3b).
Fruit pigmentation of T1 SlGAD3ΔC OX lines
Although the overall plant growth (plant height and days to flowering) and fruit development (days from flowering to the Br stage, and fruit weight) of the T1 SlGAD3ΔC OX lines were indistinguishable from those of the WT (Fig. S4), transgenic mature fruits failed to progress to the red-ripe stage, instead exhibiting an orange-ripe phenotype similar to that of the T0 generation (Fig. 6a). At the Br + 10 stage, three SlGAD3ΔC OX lines showed significantly low levels of lycopene, i.e., 28–57 % of WT levels, while β-carotene levels were equivalent to the WT level except for line 153 (Fig. 6c). Even when the fruits remained on the vine until 30 days after the Br stage, the exocarp and mesocarp tissue of the three SlGAD3ΔC OX lines did not turn red completely (Fig. 6b). However, some transgenic seeds had germinated in the Br + 30 fruit like in the WT, most likely due to over-ripening, suggesting that fruit coloration was defective, whereas seed maturation and development were functional in SlGAD3ΔC OX lines (Fig. 6b). Also, when the off vine harvested fruits were stored at 25 °C, both SlGAD3ΔC OX and WT fruits began to shrink in two weeks (Fig. S5), indicating that the fruit shelf life of SlGAD3ΔC OX lines is unchanged.
Appearance of WT and T1 SlGAD3ΔC OX fruits. Fruits were ripened on the vine and harvested at the Br + 10 (a) and Br + 30 (b) stages. Bar 1 cm. c Lycopene and β-carotene contents in the fruits of WT and T1 SlGAD3ΔC OX transgenic lines at the Br + 10 stage. The mean ± SE of three biological replicates are shown. Asterisks indicate a significant difference between WT and transgenic lines according to Student’s t test (*P < 0.05 and **P < 0.01)
Delayed ethylene production in SlGAD3ΔC OX fruits
The plant hormone ethylene plays an essential role in tomato fruit ripening, and inhibiting its biosynthesis represses many aspects of ripening-related physiological processes, such as softening, aroma development, and red-ripe coloration (Oeller et al. 1991; Klee et al. 1991). To determine whether the reduced lycopene levels in SlGAD3ΔC OX fruits could be attributed to suppressed ethylene production, we analyzed the ethylene production rates at different stages of fruit development. In WT fruits, a typical climacteric rise in ethylene production was observed, peaking at the Br + 3 stage and then declining (Fig. 7a). By contrast, in SlGAD3ΔC OX fruits, a climacteric rise in ethylene production was detected from the Br stage (like WT), but ethylene levels increased slowly and peaked at the Br + 10 stage (Fig. 7a). However, ethylene production in transgenic fruits at the Br + 10 stage was almost equivalent to that of the WT at the Br + 3 stage (Fig. 7a).
Ethylene production in the fruits of WT and T1 SlGAD3ΔC OX transgenic lines. a Ethylene production rates measured at different developmental stages. The mean ± SE of five biological replicates are shown. b Relative expression levels of ethylene biosynthesis genes (ACS2, ACS4, and ACO1) determined by qRT-PCR. The expression levels in the WT MG fruits were set to a baseline value of 1. Slubiquitin3 (UBI) was used for normalization. The mean ± SE of three biological replicates are shown
To examine whether delayed ethylene production resulted from reduced production of mRNA transcripts of ethylene biosynthetic genes, we analyzed the mRNA levels of two 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS) genes, ACS2 and ACS4, and one ACC oxidase (ACO) gene, ACO1, in fruits at three developmental stages, MG, Br + 3, and Br + 10 (Van de Poel et al. 2012). In accordance with ethylene production, in WT fruits, the mRNA levels of ACS2, ACS4, and ACO1 peaked at the Br + 3 stage and then declined (Fig. 7a, b). By contrast, in SlGAD3ΔC OX fruits, the mRNA levels of these genes were highest at the Br + 10 stage, except for ACO1 in line 153, which peaked at the Br + 3 stage (Fig. 7a, b).
Expression of carotenoid biosynthesis genes in T1 SlGAD3ΔC OX fruits
Although the timing of the climacteric rise in ethylene production was delayed in SlGAD3ΔC OX fruits, ethylene levels in these fruits were equivalent to those of the WT until the Br + 10 stage (Fig. 7a). However, SlGAD3ΔC OX fruits never reached the red-ripe stage, instead remaining orange, even at the Br + 30 stage (Fig. 6b). To examine whether this phenotype is associated with reduced carotenoid accumulation, we analyzed the mRNA levels of the phytoene synthase 1 (PSY1), phytoene desaturase (PDS), ζ-carotene desaturase (ZDS), carotene isomerase (CRTISO), chloroplast-specific lycopene β-cyclase (LCY-B), and chromoplast-specific lycopene β-cyclase (CYC-B) genes, all of which are involved in carotenoid biosynthesis (Pecker et al. 1996; Ronen et al. 2000; Liu et al. 2015). In WT fruits, the mRNA levels of all six genes peaked at the Br + 3 stage and then declined (Fig. 8a). By contrast, in transgenic fruits, the expression of all genes except LCY-B remained at low levels until the Br + 3 stage and then increased slightly at the Br + 10 stage (Fig. 8a). These results suggest that SlGAD3ΔC overexpression widely affects the expressions of carotenoid biosynthesis genes in ripening fruits.
Relative expression levels of carotenoid biosynthesis genes in the fruit of WT and T1 SlGAD3ΔC OX transgenic lines. The mRNA levels of carotenoid biosynthesis genes (PSY1, PDS, ZDS, CRTISO, LCY-B and CYC-B) (a) and ripening-related transcription factors (RIN, TAGL1, FUL1, FUL2, and SlERF6) (b) were determined by qRT-PCR. The expression levels in WT MG fruits were set to a baseline value of 1. Slubiquitin3 (UBI) was used for normalization. The mean ± SE of three biological replicates are shown
We also examined the mRNA levels of RIN, TAGL1, FUL1 (TDR4), FUL2 (MBP7), and SlERF6 that encode transcription factors having a broad effect on fruit ripening including carotenogenesis. In WT fruits, these genes were all upregulated from MG to Br + 3 stage. By contrast, in transgenic fruits, the mRNA levels of these genes were consistently low throughout ripening, except that the expressions of RIN and ERF6 were upregulated from Br + 3 to Br + 10 stage (Fig. 8b).
Discussion
The E8 promoter/HSP terminator expression cassette strongly induces the expression of SlGAD3 in ripening tomato fruit
GAD mRNA expression and GAD enzyme activity are generally high in green tomato fruits, and they decline after Br stage. In addition, GABA accumulation is well correlated with these mRNA and enzyme profiles; the final GABA content in mature red-ripe fruits of cv. ‘Micro-Tom’ is 1–3 µmol/gFW (Akihiro et al. 2008; Sorrequieta et al. 2013; Koike et al. 2013). Therefore, producing tomato plants with increased GAD mRNA levels or GAD enzyme activity at the ripening stage via genetic engineering represents a feasible strategy for improving GABA levels in red-ripe tomato fruits. Previously, we generated transgenic tomato plants in which SlGAD3 was expressed using the CaMV 35S promoter/NOS terminator expression cassette (35S-SlGAD3-NOS; Takayama et al. 2015). The GABA levels in these transgenic fruits were 5.2-fold higher than those of WT at the red-ripe stage (Takayama et al. 2015), indicating that overexpressing SlGAD3 is an effective approach for increasing GABA accumulation in tomato fruits. In the current study, we further explored the possibility of increasing GABA levels in red-ripe tomato fruits using alternative approaches.
First, we modified the expression cassette. To induce the SlGAD3 expression more strongly and specifically in ripening fruits, we replaced CaMV35S promoter into fruit-specific E8 promoter, and NOS terminator into Arabidopsis HSP terminator. As a result, SlGAD3 OX plants exhibited significantly higher levels (300-fold of WT levels) of SlGAD3 expression in Br + 10 fruits (Fig. 2b). Although the E8 promoter generally induces lower levels of mRNA accumulation compared to the CaMV 35S promoter in red stage fruits (Nambeesan et al. 2010; Hirai et al. 2011a), the level of SlGAD3 upregulation in SlGAD3 OX fruits was substantially higher than that of the previously generated 35S-SlGAD3-NOS lines, which exhibited an approximately 200-fold increase in SlGAD3 mRNA levels compared to the WT (Takayama et al. 2015). As studies in tobacco, rice, and tomato have shown that the Arabidopsis HSP terminator is more effective than the NOS terminator in increasing mRNA accumulation of transgene (Nagaya et al. 2010; Hirai et al. 2011b; Kurokawa et al. 2013), it is suggested that the strong induction of SlGAD3 in SlGAD3 OX fruits was achieved by utilizing HSP terminator. However, although SlGAD3 OX fruits had higher levels of SlGAD3 mRNA, the GABA levels were comparable to those of previously generated 35S-SlGAD3-NOS fruits at the red stage (Fig. 2c; Takayama et al. 2015), indicating that GABA levels are not solely determined by SlGAD3 mRNA levels in red-ripe fruits.
C-terminal truncation of SlGAD3 increases GABA production in tomato fruits
We next examined the effects of C-terminal truncation of SlGAD3 on GABA accumulation. Although we found no prominent difference between SlGAD3 (ΔC) mRNA levels of SlGAD3 OX and SlGAD3ΔC OX lines (approximately 300-fold WT levels; Fig. 2b), the GABA levels in SlGAD3ΔC OX fruits were 1.9-fold higher than those of SlGAD3 OX lines at the Br + 10 stage (Fig. 2c). In addition, Br + 10 fruits of SlGAD3ΔC OX lines exhibited higher GAD activities compared with those of SlGAD3 OX lines in all conditions we tested (Fig. 3). These results suggest that the C-terminal extension region of SlGAD3 also functions as an autoinhibitory domain, like that of other plant GADs (Baum et al. 1996; Akama and Takaiwa 2007). The high productivity of GABA in SlGAD3ΔC OX fruits was maintained in T1 progenies that accumulated 19–29 μmol gFW−1 at the Br + 10 stage (Fig. 5b; Table 1). These levels of GABA were much higher than those of the WT (1.6 μmol gFW−1), as well as those of the previously generated 35S-SlGAD3-NOS plants and plants in which SlGABA-T1 was suppressed by RNAi under the control of the CaMV 35S promoter and NOS terminator, which exhibited levels up to 5.2- and 9.2-fold higher than that of the WT, respectively (Koike et al. 2013; Takayama et al. 2015). These results indicate that removal of the C-terminal extension region of SlGAD3 is a useful way to strongly increase GABA accumulation in tomato fruits.
Enhanced GABA biosynthesis in tomato fruits influences ethylene production and sensitivity
In the present study, we did not find any morphological abnormalities in both SlGAD3 OX and SlGAD3ΔC OX lines, probably due to the use of fruit-specific E8 promoter. However, SlGAD3ΔC OX lines showed an abnormality in fruit ripening. Unlike the fruit of the WT which became red-ripe until Br + 10 stage, that of SlGAD3ΔC OX lines remained orange even at the Br + 30 stage when the fruits were ripen on the vine (Fig. 4). This orange-ripe phenotype was accompanied by a reduction in lycopene levels, which was correlated with the reduced mRNA levels of carotenogenic genes (Figs. 4, 6, 8a). Taking consideration of that SlGAD3 OX fruits displayed a normal red-ripe phenotype, it is hypothesized that the orange-ripe phenotype in SlGAD3ΔC OX lines might be attributed to the metabolic disturbance caused by over activation of GAD via C-terminal truncation. In fruits of the WT, GABA comprised up to 6.2 % of total free amino acids at the Br + 10 stage, whereas GABA in T1 SlGAD3ΔC OX fruits comprised up to 81 % of total free amino acids at the same stage (Table 1). By contrast, the levels of aspartate and glutamate, the two major amino acids in WT fruits, were considerably decreased in all transgenic lines (Table 1). The decline in glutamate level could be caused by enhanced GAD activity, because glutamate is the direct precursor of GABA. Although aspartate is not the direct precursor of GABA, it is mainly biosynthesized from glutamate by aspartate aminotransferase (Forde and Lea 2007). Thus, rapid consumption of glutamate might have affected the aspartate biosynthesis in SlGAD3ΔC OX fruits. It is reported that transgenic tobacco plants expressing a petunia GAD lacking the C-terminal extension region possessed extremely high GABA and low glutamate levels (Baum et al. 1996). These transgenic plants exhibited severe developmental abnormalities correlated with attenuated cell elongation in stems. As the possible causes of this phenotype, the authors pointed to the lower levels of glutamate, because glutamate is directly or indirectly involved in a number of pathways, including biosynthesis of gibberellins as well as post-translational modifications of cell wall proteins (Baum et al. 1996). Similarly, transgenic rice plants expressing a C-terminal truncated OsGAD2 had aberrant phenotypes such as dwarfism, etiolated leaves and sterility (Akama and Takaiwa 2007). As high levels of GABA were detected in those transgenic plants, the authors suggested that an imbalance of amino acids in cells would be involved in aberrant phenotypes. Interestingly, transgenic tomato fruits in which transcription factors FUL1 and FUL2 were simultaneously suppressed exhibited the orange-ripe phenotype, with highly reduced lycopene levels (Bemer et al. 2012). These fruits also exhibited increased GABA levels (twofold higher than WT levels) and strongly reduced glutamate levels (eightfold lower than WT levels), possibly due to the upregulated expression of three SlGAD genes (SlGAD1, SlGAD2, and SlGAD3) (Bemer et al. 2012). As the GABA levels in FUL1/FUL2 suppressed fruits were less elevated compared with those of the red-ripen GAD3 OX fruits, it is possible that reduction in glutamate levels is also attributed to the orange-ripe phenotype. However, the relationships between glutamate and carotenoid accumulation are still unclear. In tomato fruits, ethylene plays a crucial role in accelerating fruit ripening and carotenoid accumulation (Oeller et al. 1991; Klee et al. 1991; Wilkinson et al. 1997). We found that although the climacteric ethylene production in SlGAD3ΔC OX fruits was delayed, this phytohormone eventually reached the maximum level at the Br + 10 stage, which was equivalent to the results from the WT at the Br + 3 stage (Fig. 7a). The ethylene production rates were, at least in part, correlated with the expression of two ethylene biosynthesis genes, ACS2 and ACS4 (Fig. 7b), suggesting that over activation of GAD via C-terminal truncation affects ethylene production through transcriptional control of these biosynthesis genes.
We also found that SlGAD3ΔC OX fruits did not turn red-ripe even at the Br + 30 stage when the fruits had already been exposed to high levels of ethylene (at Br + 10 stage for SlGAD3ΔC OX fruits) that were almost equivalent to the WT maximum levels (at Br + 3 stage for WT fruits) (Figs. 6b, 7a). Indeed, mature fruits of the ethylene insensitive mutants Sletr1, Never ripe (Nr), and ripening inhibitor (rin), as well as transgenic tomato in which an ethylene biosynthesis gene is suppressed, also fail to produce fruit with red-ripe coloration (Oeller et al. 1991; Alba et al. 2005; Okabe et al. 2011). Therefore, it is possible that the failure of SlGAD3ΔC OX to form red-ripe fruit is not only due to delayed ethylene production, but also to reduced ethylene sensitivity.
Enhanced GABA biosynthesis in tomato fruits affects the expression of ripening-associated genes
In addition to ethylene, MADS-box transcription factors are also key regulators of tomato fruit ripening. For example, RIN is one of the earliest acting factors that regulate fruit ripening in both ethylene-dependent and -independent pathways (Fujisawa et al. 2013). The recently identified transcription factors TAGL1, FUL1, and FUL2 also play crucial roles in regulating ripening processes such as ethylene biosynthesis and lycopene accumulation (Itkin et al. 2009; Bemer et al. 2012; Shima et al. 2013; Fujisawa et al. 2014). SlERF6 is known as a negative regulator of both carotenoid accumulations and ethylene synthesis (Lee et al. 2012). Thus, in the present study, we examined the mRNA levels of these transcription factors in SlGAD3ΔC OX fruits (Fig. 8b). Compared to the WT, SlGAD3ΔC OX fruits exhibited strong reductions in TAGL1, FUL1, and FUL2 expression at the Br + 3 and Br + 10 stages, while RIN was only suppressed at Br + 3, and ERF6 were upregulated from Br + 3 to Br + 10 stage (Fig. 8b). RIN, TAGL1, FUL1, and FUL2 can bind to the promoter region of ACS2 (Itkin et al. 2009; Fujisawa et al. 2011; Shima et al. 2013), suggesting that the reduced levels of ACS2 expression observed in SlGAD3ΔC OX fruits were caused by the reduced expression of these transcription factor genes (Figs. 7b, 8b). Similarly, carotenogenic genes PSY1 and CRTISO are targeted by RIN and FUL1 and by RIN, FUL1, and FUL2, respectively (Fujisawa et al. 2014). Thus, the reduced expression of these transcription factors might affect the expression of carotenogenic genes in SlGAD3ΔC OX fruits (Fig. 8). Although the mechanism by which RIN, TAGL1, FUL, and FUL2 are suppressed in SlGAD3ΔC OX fruits remains unknown, we found that over activation of GAD via C-terminal truncation alters the process of fruit ripening by modulating the expression of multiple genes involved in fruit ripening.
Conclusion
This study demonstrates that hyperaccumulation of GABA in tomato fruits could be achieved using the E8 promoter and HSP terminator expression cassette coupled with C-terminal truncation of SlGAD3. Using this strategy, we succeeded in increasing GABA accumulation in tomato fruits to a level of 29 μmol gFW−1 (295 mg 100 gFW−1). This level is much higher than those of previously generated transgenic tomato fruits with increased levels of GABA (Koike et al. 2013; Takayama et al. 2015) and is higher than that of S. pennellii, a wild species of tomato that accumulates high levels of GABA (approximately 200 mg 100 gFW−1 when determined by our protocol; data not shown) (Schauer et al. 2005). Daily intake of 10–20 mg of GABA is effective in reducing blood pressure in adults with mild hypertension (Fukuwatari et al. 2001; Kazami et al. 2002; Inoue et al. 2003). This amount of GABA is equivalent to the level present in 3.4–6.8 gFW of transgenic fruit (corresponding to 1–3 fruits), suggesting that the transgenic tomato fruit produced in the current study contains sufficient levels of GABA to contribute to human health.
Author contribution statement
HE, CM, and TA conceived and designed research. MT conducted experiments and analyzed data. MT, CM, TA, and HE wrote manuscript. All authors read and approved the manuscript.
References
Abdou AM, Higashiguchi S, Horie K, Kim M, Hatta H, Yokogoshi H (2006) Relaxation and immunity enhancement effects of gamma-aminobutyric acid (GABA) administration in humans. BioFactors 26:201–208
Akama K, Takaiwa F (2007) C-terminal extension of rice glutamate decarboxylase (OsGAD2) functions as an autoinhibitory domain and overexpression of a truncated mutant results in the accumulation of extremely high levels of GABA in plant cells. J Exp Bot 58:2699–2707. doi:10.1093/jxb/erm120
Akihiro T, Koike S, Tani R, Tominaga T, Watanabe S, Iijima Y, Aoki K, Shibata D, Ashihara H, Matsukura C, Akama K, Fujimura T, Ezura H (2008) Biochemical mechanism on GABA accumulation during fruit development in tomato. Plant Cell Physiol 49:1378–1389. doi:10.1093/pcp/pcn113
Alba R, Payton P, Fei Z, McQuinn R, Debbie P, Martin GB, Tanksley SD, Giovannoni JJ (2005) Transcriptome and selected metabolite analyses reveal multiple points of ethylene control during tomato fruit development. Plant Cell 17:2954–2965. doi:10.1105/tpc.105.036053
Arazi T, Baum G, Snedden WA, Shelp BJ, Fromm H (1995) Molecular and biochemical analysis of calmodulin interactions with the calmodulin-binding domain of plant glutamate decarboxylase. Plant Physiol 108:551–561. doi:10.1104/pp.108.2.551
Baum G, Chen Y, Arazi T, Takatsuji H, Fromm H (1993) A plant glutamate decarboxylase containing a calmodulin binding domain. Cloning, sequence, and functional analysis. J Biol Chem 268:19610–19617
Baum G, Lev-Yadun S, Fridmann Y, Arazi T, Katsnelson H, Zik M, Fromm H (1996) Calmodulin binding to glutamate decarboxylase is required for regulation of glutamate and GABA metabolism and normal development in plants. EMBO J 15:2988–2996
Bemer M, Karlova R, Ballester AR, Tikunov YM, Bovy AG, Wolters-Arts M, Rossetto Pde B, Angenent GC, de Maagd RA (2012) The tomato FRUITFULL homologs TDR4/FUL1 and MBP7/FUL2 regulate ethylene-independent aspects of fruit ripening. Plant Cell 24:4437–4451. doi:10.1105/tpc.112.103283
Bouché N, Fromm H (2004) GABA in plants: just a metabolite? Trends Plant Sci 9:110–115. doi:10.1016/j.tplants.2004.01.006
Clark SM, Di Leo R, Van Cauwenberghe OR, Mullen RT, Shelp BJ (2009) Subcellular localization and expression of multiple tomato γ-aminobutyrate transaminases that utilize both pyruvate and glyoxylate. J Exp Bot 60:3255–3267. doi:10.1093/jxb/erp161
Deblaere R, Bytebier B, De Greve H, Deboeck F, Schell J, Van Montagu M, Leemans J (1985) Efficient octopine Ti plasmid-derived vectors for Agrobacterium-mediated gene transfer to plants. Nucleic Acids Res 13:4777–4788
Deikman J, Xu R, Kneissl ML, Ciardi JA, Kim KN, Pelah D (1998) Separation of cis elements responsive to ethylene, fruit development, and ripening in the 5′-flanking region of the ripening-related E8 gene. Plant Mol Biol 37:1001–1011
Forde BG, Lea PJ (2007) Glutamate in plants: metabolism, regulation, and signaling. J Exp Bot 58:2339–2358. doi:10.1093/jxb/erm121
Fujisawa M, Nakano T, Ito Y (2011) Identification of potential target genes for the tomato fruit-ripening regulator RIN by chromatin immunoprecipitation. BMC Plant Biol 11:26
Fujisawa M, Nakano T, Shima Y, Ito Y (2013) A large-scale identification of direct targets of the tomato MADS Box transcription factor RIPENING INHIBITOR reveals the regulation of fruit ripening. Plant Cell 25:371–386. doi:10.1105/tpc.112.108118
Fujisawa M, Shima Y, Nakagawa H, Kitagawa M, Kimbara J, Nakano T, Kasumi T, Ito Y (2014) Transcriptional regulation of fruit ripening by tomato FRUITFULL homologs and associated MADS box proteins. Plant Cell 26:89–101. doi:10.1105/tpc.113.119453
Fukuwatari Y, Sato N, Kawamori R, Watanabe Y, Yoshida K, Ying R, Matsuda K, Fujii A, Uzawa M, Sato R (2001) A study on the antihypertensive action and safety of tablets containing γ-aminobutyric acid (GABA). Eastern Med (in Japanese) 17:1–7
Gut H, Dominici P, Pilati S, Astegno A, Petoukhov MV, Svergun DI, Grütter MG, Capitani G (2009) A common structural basis for pH- and calmodulin-mediated regulation in plant glutamate decarboxylase. J Mol Biol 392:334–351. doi:10.1016/j.jmb.2009.06.080
Hirai T, Kim YW, Kato K, Hiwasa-Tanase K, Ezura H (2011a) Uniform accumulation of recombinant miraculin protein in transgenic tomato fruit using a fruit-ripening-specific E8 promoter. Transgenic Res 20:1285–1292. doi:10.1007/s11248-011-9495-9
Hirai T, Kurokawa N, Duhita N, Hiwasa-Tanase K, Kato K, Kato K, Ezura H (2011b) The HSP terminator of Arabidopsis thaliana induces a high level of miraculin accumulation in transgenic tomatoes. J Agric Food Chem 59:9942–9949. doi:10.1021/jf202501e
Inoue K, Shirai T, Ochiai H, Kasao M, Hayakawa K, Kimura M, Sansawa H (2003) Blood-pressure-lowering effect of a novel fermented milk containing gamma-aminobutyric acid (GABA) in mild hypertensives. Eur J Clin Nutr 57:490–495
Itkin M, Seybold H, Breitel D, Rogachev I, Meir S, Aharoni A (2009) TOMATO AGAMOUS-LIKE 1 is a component of the fruit ripening regulatory network. Plant J 60:1081–1095. doi:10.1111/j.1365-313X.2009.04064
Jakoby WD (1962) Enzyme of γ-aminobutyrate metabolism (bacterial). Methods Enzymol 5:765–778
Kazami D, Ogura N, Fukuchi T, Tsuji K, Anazawa M, Maeda H (2002) Antihypertensive effect of Japanese taste seasoning containing γ-amino butyric acid on mildly hypertensive and high-normal blood pressure subjects and normal subjects. Nippon Shokuhin Kagaku Kogaku Kaishi (in Japanese) 49:409–415
Kitagawa M, Ito H, Shiina T, Nakamura N, Inakuma T, Kasumi T, Ishiguro Y, Yabe K, Ito Y (2005) Characterization of tomato fruit ripening and analysis of gene expression in F1 hybrids of the ripening inhibitor (rin) mutant. Physiol Plant 123:331–338. doi:10.1111/j.1399-3054.2005.00460.x
Klee HJ, Hayford MB, Kretzmer KA, Barry GF, Kishore GM (1991) Control of ethylene synthesis by expression of a bacterial enzyme in transgenic tomato plants. Plant Cell 3:1187–1193. doi:10.1105/tpc.3.11.1187
Koike S, Matsukura C, Takayama M, Asamizu E, Ezura H (2013) Suppression of γ-aminobutyric acid (GABA) transaminases induces prominent GABA accumulation, dwarfism and infertility in the tomato (Solanum lycopersicum L.). Plant Cell Physiol 54:793–807. doi:10.1093/pcp/pct035
Kurokawa N, Hirai T, Takayama M, Hiwasa-Tanase K, Ezura H (2013) An E8 promoter-HSP terminator cassette promotes the high-level accumulation of recombinant protein predominantly in transgenic tomato fruits: a case study of miraculin. Plant Cell Rep 32:529–536. doi:10.1007/s00299-013-1384-7
Lee JM, Joung JG, McQuinn R, Chung MY, Fei Z, Tieman D, Klee H, Giovannoni J (2012) Combined transcriptone, genetic diversity and metabolite profiling in tomato fruit reveals that the ethylene response factor SlERF6 plays an important role in ripening and carotenoid accumulation. Plant J 70:191–204. doi:10.1111/j.1365-313X.2011.04863.x
Liu L, Shao Z, Zhang M, Wang Q (2015) Regulation of carotenoid metabolism in tomato. Mol Plant 8:28–39. doi:10.1016/j.molp.2014.11.006
Matsumoto Y, Ohno K, Hiraoka Y (1997) Studies on the utilization of functional food materials containing high levels of gamma-aminobutyric acid (Part 1). Ehime Kougi Kenkyu Houkoku (in Japanese) 35:97–100
Mubarok S, Okabe Y, Fukuda N, Ariizumi T, Ezura H (2015) Potential use of a weak ethylene receptor mutant, Sletr1-2, as breeding material to extend fruit shelf life of tomato. J Agric Food Chem 63:7995–8007. doi:10.1021/acs.jafc.5b02742
Nagaya S, Kawamura K, Shinmyo A, Kato K (2010) The HSP terminator of Arabidopsis thaliana increases gene expression in plant cells. Plant Cell Physiol 51:328–332. doi:10.1093/pcp/pcp188
Nambeesan S, Datsenka T, Ferruzzi MG, Malladi A, Mattoo AK, Handa AK (2010) Overexpression of yeast spermidine synthase impacts ripening, senescence and decay symptoms in tomato. Plant J 63:836–847. doi:10.1111/j.1365-313X.2010.04286.x
Oeller PW, Min-Wong L, Taylor LP, Pike DA, Theologis A (1991) Reversible inhibition of tomato fruit senescence by antisense RNA. Science 254:437–439
Okabe Y, Asamizu E, Saito T, Matsukura C, Ariizumi T, Brès C, Rothan C, Mizoguchi T, Ezura H (2011) Tomato TILLING technology: development of a reverse genetics tool for the efficient isolation of mutants from Micro-Tom mutant libraries. Plant Cell Physiol 52:1994–2005. doi:10.1093/pcp/pcr134
Okada T, Sugishita T, Murakami T, Murai H, Saikusa T, Horino T, Onoda A, Kajimoto O, Takahashi R, Takahashi T (2000) Effect of the defatted rice germ enriched with GABA for sleeplessness, depression, autonomic disorder by oral administration. Nippon Shokuhin Kagaku Kogaku Kaishi (in Japanese) 47:596–603
Owens DF, Kriegstein AR (2002) Is there more to GABA than synaptic inhibition? Nat Rev Neurosci 3:715–727. doi:10.1038/nrn919
Park KB, Oh SH (2007) Production of yogurt with enhanced levels of gamma-aminobutyric acid and valuable nutrients using lactic acid bacteria and germinated soybean extract. Bioresour Technol 98:1675–1679. doi:10.1016/j.biortech.2006.06.006
Pecker I, Gabbay R, Cunningham FX, Hirschberg J (1996) Cloning and characterization of the cDNA for β-cyclase from tomato reveals decrease in its expression during fruit ripening. Plant Mol Biol 30:807–819. doi:10.1007/BF00019013
Ronen G, Carmel-Goren L, Zamir D, Hirschberg J (2000) An alternative pathway to β-carotene formation in plant chromoplasts discovered by map-based cloning of Beta and old-gold color mutations in tomato. Proc Natl Acad Sci USA 97:11102–11107. doi:10.1073/pnas.190177497
Saito T, Matsukura C, Sugiyama M, Watahiki A, Ohshima I, Iijima Y, Konishi C, Fujii T, Inai S, Fukuda N, Nishimura S, Ezura H (2008) Screening for γ-aminobutyric acid (GABA)-rich tomato varieties. J Japan Soc Hort Sci 77:242–250
Saito T, Ariizumi T, Okabe Y, Asamizu E, Hiwasa-Tanase K, Fukuda N, Mizoguchi T, Yamazaki Y, Aoki K, Ezura H (2011) TOMATOMA: a novel tomato mutant database distributing Micro-Tom mutant collections. Plant Cell Physiol 52:283–296. doi:10.1093/pcp/pcr004
Schauer N, Zamir D, Fernie A (2005) Metabolic profiling of leaves and fruit of wild species tomato: a survey of the Solanum lycopersicum complex. J Exp Bot 56:297–307. doi:10.1093/jxb/eri057
Shelp BJ, Bown AW, McLean MD (1999) Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci 4:446–452. doi:10.1016/s1360-1385(99)01486-7
Shima Y, Kitagawa M, Fujisawa M, Nakano T, Kato H, Kimbara J, Kasumi T, Ito Y (2013) Tomato FRUITFULL homologues act in fruit ripening via forming MADS-box transcription factor complexes with RIN. Plant Mol Biol 82:427–438. doi:10.1007/s11103-013-0071-y
Snedden WA, Arazi T, Fromm H, Shelp BJ (1995) Calcium/calmodulin activation of soybean glutamate decarboxylase. Plant Physiol 108:543–549. doi:10.1104/pp.108.2.543
Snedden WA, Koutsia N, Baum G, Fromm H (1996) Activation of a recombinant petunia glutamate decarboxylase by calcium/calmodulin or by a monoclonal antibody which recognizes the calmodulin binding domain. J Biol Chem 271:4148–4153
Sorrequieta A, Abriata LA, Boggio SB, Valle EM (2013) Off-the-vine ripening of tomato fruit causes alteration in the primary metabolite composition. Metabolites 3:967–978. doi:10.3390/metabo3040967
Sun HJ, Uchii S, Watanabe S, Ezura H (2006) A highly efficient transformation protocol for Micro-Tom, a model cultivar for tomato functional genomics. Plant Cell Physiol 47:426–431. doi:10.1093/pcp/pci251
Takayama M, Koike S, Kusano M, Matsukura C, Saito K, Ariizumi T, Ezura H (2015) Tomato glutamate decarboxylase genes SlGAD2 and SlGAD3 play key roles in regulating γ-aminobutyric acid levels in tomato (Solanum lycopersicum). Plant Cell Physiol 56:1533–1545. doi:10.1093/pcp/pcv075
Trobacher CP, Zarei A, Liu J, Clark SM, Bozzo GG, Shelp BJ (2013) Calmodulin-dependent and calmodulin-independent glutamate decarboxylases in apple fruit. BMC Plant Biol 13:144. doi:10.1186/1471-2229-13-144
Tsushida T, Murai T, Omori M, Okamoto J (1987) Production of a new type tea containing a high level of γ-aminobutyric acid. Nippon Nogeikagaku Kaishi (in Japanese) 7:817–822
Van de Poel B, Bulens I, Markoula A, Hertog ML, Dreesen R, Wirtz M, Vandoninck S, Oppermann Y, Keulemans J, Hell R, Waelkens E, De Proft MP, Sauter M, Nicolai BM, Geeraerd AH (2012) Targeted systems biology profiling of tomato fruit reveals coordination of the Yang cycle and a distinct regulation of ethylene biosynthesis during postclimacteric ripening. Plant Physiol 160:1498–1514. doi:10.1104/pp.112.206086
Wilkinson JQ, Lanahan MB, Clark DG, Bleecker AB, Chang C, Meyerowitz EM, Klee HJ (1997) A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nat Biotechnol 15:444–447
Yap KL, Yuan T, Mal TK, Vogel HJ, Ikura M (2003) Structural basis for simultaneous binding of two carboxyl-terminal peptides of plant glutamate decarboxylase to calmodulin. J Mol Biol 328:193–204. doi:10.1016/s0022-2836(03)00271-7
Yin YG, Tominaga T, Iijima Y, Aoki K, Shibata D, Ashihara H, Nishimura S, Ezura H, Matsukura C (2010) Metabolic alterations in organic acids and γ-aminobutyric acid in developing tomato (Solanum lycopersicum L.) fruits. Plant Cell Physiol 51:1300–1314. doi:10.1093/pcp/pcq090
Acknowledgments
We thank all of our laboratory members for their helpful discussions. This study was supported by the “Japan-France Joint Laboratory Project”, the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, and the “Research and Development Program for New Bio-industry Initiative”, Bio-oriented Technology Research Advancement Institution (BRAIN). ‘Micro-Tom’ tomato seeds (Accession No. TOMJPF00001) were obtained from the Gene Research Center, University of Tsukuba, through the National Bioresource Project (NBRP) of MEXT, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by K. Toriyama.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takayama, M., Matsukura, C., Ariizumi, T. et al. Activating glutamate decarboxylase activity by removing the autoinhibitory domain leads to hyper γ-aminobutyric acid (GABA) accumulation in tomato fruit. Plant Cell Rep 36, 103–116 (2017). https://doi.org/10.1007/s00299-016-2061-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-016-2061-4