Abstract
Key message
An algae-based vaccine model against atherosclerosis was developed with positive findings in terms of antigen yield and immunogenicity in mouse.
Abstract
Several immunotherapies against atherosclerosis have been evaluated at the preclinical level thus far, with some of them currently under evaluation in clinical trials. In particular, the p210 epitope from ApoB100 is known to elicit atheroprotective responses. Considering that Chlamydomonas reinhardtii is an attractive host for the production and delivery of subunit vaccines, in this study a chimeric protein consisting of the B subunit of the cholera toxin and the p210 epitope from ApoB100 (CTB:p210) has been expressed in C. reinhardtii chloroplast as an attempt to establish an oral vaccine candidate against atherosclerosis. The Chlamydomonas-made CTB:p210 protein was successfully expressed at levels of up to 60 µg per g of fresh weight biomass. The antigenic activity of the CTB and the p210 moiety was preserved in the CTB:p210 chimera. Moreover the algae-made CTB:p210 showed an immunogenic activity, when orally administered to BALB/c mice, as evidenced the presence of anti-p210 serum antibodies in mice treated with the algae-derived CTB:p210. The antibody response lasts for at least 80 days after the last boost. This experimental model is proposed as a convenient tool in the development of low cost atherosclerosis vaccines of easy compliance and friendly delivery. Further studies will determine the therapeutic potential of this algae-made vaccine in atherosclerosis animal models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular diseases are a group of heart and blood vessels disorders causing 17.3 million deaths each year, which represents the 30 % of deaths due to non-communicable diseases (NCD) worldwide (WHO 2015). In fact, atherosclerosis is the main cause associated to cardiovascular events. This disease has chronic autoimmune and inflammatory components proven through both transgenic mouse models and clinical studies (Sakakura et al. 2013). Atherosclerosis etiology has as primary event the accumulation and oxidation of lipoproteins, which leads to inflammation of the vascular wall with a subsequent formation of an atheromatous or fibro-fatty plaque that causes several diseases (Conti and Shaik-Dasthagirisaeb 2015). The development of therapies for chronic diseases faces the challenges imposed by high costs, low compliance and in some cases significant adverse effects (Patel and Blazing 2013). Immunotherapeutic approaches against atherosclerosis have gained interest in the biomedical field as they theoretically constitute a convenient intervention requiring few doses for the induction of long lasting therapeutic effects (Keijzer et al. 2013). Among the therapeutic targets identified to address atherosclerosis progression, there is a prominent interest in ApoB100 since it is the main Apolipoprotein present in the low density lipoproteins (LDL), which are associated with the pathology initiation when present at sustained elevated levels (Leake 1993). Among the epitopes present in the ApoB100 sequence p210 and p143 gained particular attention as these sequences were identified as targets of antibodies present in sera from healthy subjects (Fredrikson et al. 2003a). In a pioneered study, Fredrikson et al. (2003b) evaluated their immunotherapeutic potential, observing that apoE null mice immunized with these peptides by a parenteral route presented disease attenuation. This effect was associated with the induction of antibodies against p210 and p143. In a further study, the intranasal administration of a chimeric protein consisting of the mucosal adjuvant B subunit of the cholera toxin (CTB) and the p210 sequence induced an atheroprotective effect in the apoE null mice (Klingenberg et al. 2010). Interestingly, anti-p210 IgG levels have been associated with atherosclerosis protection as healthy subjects showed increased antibody levels in comparison with post-infarction patients. This observation indicates that antibody levels are inversely related to the severity of coronary atherosclerosis and individuals with high levels have a 45 % lower risk of developing myocardial infarction (Sjögren et al. 2008). However, p210 therapy has been recently associated with the activation of other arms of the immune system, comprising the expansion of CD4+ regulatory cells (Tregs) (Wigren et al. 2011). Therefore, p210 epitope is a key tool on the development of atherosclerosis vaccines.
In this context, cost is a crucial factor to exploit the benefits derived from vaccination strategies at a global scale. Low cost production platforms are generating substantial expectations in the vaccinology field; among these, Chlamydomonas reinhardtii is a unicellular alga that has been used for the production of a number of vaccine candidates tested in animal models with promising findings (Rosales-Mendoza 2013). Genetic engineering strategies for C. reinhardtii comprise expression of the transgene in the context of the nuclear or the chloroplast genomes. Chloroplast-based expression provides several advantages, including the following: higher yields that those reached by nuclear expression, which is a result of the high genome copy number in chloroplasts and the lack of silencing mechanisms and position effects; the possibility of achieving the expression of multiple genes by polycistronic arrangements; efficient genetic transformation mediated by homologous recombination that allows for a site directed insertion of heterologous DNA; and the ability to assemble multimeric proteins properly (Chen and Melis 2013).
Considering that most immunotherapies explored thus far are based on the use of purified proteins produced under complex and expensive downstream processing or costly synthetic peptides, this study aimed at the production of the CTB:p210 immunogen in C. reinhardtii as a low cost production system, intended to favor the design of new affordable and friendly atherosclerosis immunotherapies. The CTB:p210 immunogen was produced in C. reinhardtii and the immunogenic potential was assessed in mice following an oral immunization scheme.
Materials and methods
Gene design and Chlamydomonas reinhardtii transformation
A chimeric protein named CTB:p210 was designed, consisting of the p210 epitope fused to the C terminus of the cholera toxin B subunit, which serves as an adjuvant carrier. A synthetic gene, called CTB:p210, was defined and synthesized by Genscript (USA) following an optimization procedure in order to favor codon usage in Chlamydomonas chloroplast. The CTB:p210 gene was sub-cloned into the p464 vector trough the NcoI restriction site to obtain the p464-CTB:p210 vector. This expression vector allows for a site-directed insertion of heterologous DNA sequences downstream of the tscA gene. Expression cassette consists of a bicistronic arrangement where the atpA promoter drives the expression of both the gene of interest and the aadA marker gene, which confers spectinomycin resistance. Sub-cloning was verified by restriction analysis and sequencing following standard molecular cloning procedures. Once confirmed, the expression vector was propagated in E. coli cultures and isolated using the GeneGET™ plasmid miniprep kit (Fermentas) to conduct transformation experiments.
Chloroplast transformation of the CC-137 (mt+) C. reinhardtii strain was carried out following the particle bombardment protocol described by Daniell et al. (2005). A single C. reinhardtii colony was inoculated in a 250 ml flask containing 150 ml of TAP medium (Gorman and Levine 1965), and incubated at 25 °C with shaking at 150 rpm under continuous light (100 µmol m−2 s−1). Once the culture reached OD values of about 0.7 (3–5 days), cells were harvested by centrifugation at 1750×g for 5 min. Pellet was resuspended in 3 ml of TAP medium, and 1 ml aliquots were spread onto individual Petri dishes containing TAP agar and plates were subjected to particle bombardment. This procedure was performed with 0.6 µm gold particles in which the target DNA (10 µg) was previously adsorbed following the protocol described by Daniell et al. (2005). Five cultures in individual Petri dishes were bombarded at 1100 Psi with a 9 cm target distance. Plates were incubated at 25 °C during 2 days under a 16 h photoperiod (100 µmol m−2 s−1). Cells were subsequently harvested by resuspending them through pipette up and down 2 ml of TAP medium, and transferred onto selection agar TAP plates supplemented with 100 mg/l spectinomycin and maintained at 25 °C under a 16 h photoperiod (100 µmol m−2 s−1). Individual spectinomycin resistant colonies, described as CT1–40 clones, were subsequently sub-cultured with a 2-week periodicity.
Transgene detection
To investigate the presence of foreign DNA in the candidate clones, total DNA preparations were obtained as follows. Cells were packed by centrifugation at 4000 rpm for 5 min and pellets resuspended in 700 µl of extraction buffer [100 mM Tris–HCl, pH 8; 50 mM EDTA, pH 8; 500 mM NaCl; 0.9 µl/ml β-mercaptoethanol]. After shaking for 10 min, 700 µl of phenol:chloroform were added and the samples were mixed again. The samples were centrifuged at 13,000 rpm during 8 min and aqueous phase transferred to a new tube and 700 µl of isopropanol and 40 µl of 3 M sodium acetate (pH 5) were added. Samples were incubated at −20 °C for 30 min and subsequently centrifuged at 13,000 rpm for 8 min. DNA pellets were washed with 800 µl of 70 % ethanol, air-dried and dissolved in 30 µl of sterile water. PCR analysis was performed with specific oligonucleotides targeting the region comprising atpA promoter and the CTB:p210 gene (forward, 5´ACAAGTGATCTTACCACTCAC 3´; reverse, 5´TACGAGCTTGAGTACAAGCTAGC 3´) and the tubulin gene as a control (forward, 5´ACCGAGGGCGCTGAGCTGATTGAC 3´; reverse, 5´ TTGGGCGAGGGCACGACCGAGAAG 3´) using a standard PCR reaction mix. Cycling conditions consisted of initial denaturation at 94 °C for 5 min; 35 cycles comprising the following steps: 94 °C for 30 s, 55 °C for 40 s, 72 °C for 120 s; and a final extension at 72 °C for 5 min. PCR products were analysed by electrophoresis on a 1 % agarose gel stained with ethidium bromide (1 µg/ml).
GM1-ELISA analysis
GM1 binding activity of the algae-made CTB:p210 was determined using the ganglioside-dependent ELISA assay (GM1–ELISA) as previously described (Rosales-Mendoza et al. 2007). Approximately 100 mg of fresh algae biomass were resuspended in 300 µl of protein extraction buffer [50 mM Tris–HCl, pH 8; 40 mM NaCl; 0.1 % Triton X-100 (v/v); and 1 mM PMSF], subjected to sonication (four pulses of 4 s duration with a 4 s delay in between) using an ultrasonic processor equipment at a 24 % amplitude (model GEX130 PB) and further centrifuged at 8000 rpm for 15 min at 4 °C. Supernatants were transferred to new micro tubes. During ELISA assay, three washes with PBS-Tween 20 (0.05 %) were performed between steps. Assay plates were coated overnight at 4 °C with 1.5 µg of Type III GM1 ganglioside per well, diluted in 0.2 M carbonate buffer (pH 9.6). A subsequent blocking step was conducted with 5 % fat-free dry milk for 2 h at 25 °C. Plates were then incubated with protein extracts overnight at 4 °C. A further incubation was conducted overnight at 4 °C with an anti-CTB serum (1:200 dilution) obtained as previously described (Orellana-Escobedo et al. 2015). The secondary antibody, a goat horseradish peroxidase-conjugated anti-mouse IgG, was added (Sigma, 1:2000 dilution) and plates were incubated for 2 h at 25 °C. An ABTS substrate solution [0.6 mM 2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid); 0.1 M citric acid, pH 4.35; 1 mM of H2O2] was added. After incubating 30 min at 25 °C, OD values at 405 nm were measured.
Anti-CTB:p210 western blot analysis
The integrity of the C. reinhardtii-made CTB:p210 protein was assessed by Western blot analysis. Protein extracts were obtained by resuspending 50 mg of fresh biomass in 200 µl of extraction buffer [750 mM Tris–HCl, pH 8; 15 % (w/v) sucrose; 100 mM β-Mercaptoethanol; 1 mM PMSF (Franklin et al. 2002)] and subjecting samples to sonication as described above. Samples were further centrifuged at 8000 rpm for 3 min and supernatants were transferred to new microtubes. Fifty µl of protein extracts were mixed with the same volume of 2X reducing loading buffer, denatured by boiling for 5 min at 95 °C, and subsequently subjected to SDS-PAGE analysis. Gels were blotted onto BioTrace PVDF membranes (Pall Corporation, www.pall.com) and, after a subsequent blocking step in PBST plus 5 % fat-free milk, blots were incubated with mouse anti-sera against either CTB (1:200 dilution) or p210 (1:100 dilution) obtained as previously described (Orellana-Escobedo et al. 2015). A goat horseradish peroxidase-conjugated secondary anti-IgG antibody (1:2000 dilution, Sigma) was further added and an incubation for 2 h at room temperature was performed. Antigen detection was revealed by incubating blots with the SuperSignal West Dura solution following manufacturer’s instructions. Signal detection was conducted by exposing an X-ray film, which was then treated with standard developer and fixer solutions. A standard curve was constructed using pure CTB to estimate CTB:p210 expression levels in microalgae clones using a Biosens SC805 documentation system and the myImageAnalysis™ 2.0 Software.
Immunogenicity assessment
Immunogenicity of the algae-made CTB:p210 was assessed in 13-week old female BALB/c mice with a body weight of 25 g. Test animals were maintained under standard laboratory conditions with free access to food and water following the procedures indicated by the Federal Regulations for Animal Experimentation and Care (SAGARPA, NOM-062-ZOO-1999, México). The protocol was approved by the Institutional Animal Care and Use Committee.
Five BALB/c mice groups were established (n = 5). The following groups received orally a volume of 200 µl containing: PBS (PBS p.o. group), 20 mg of fresh biomass from a WT clone (WT p.o. group), 20 mg of fresh biomass from the CT10 clone containing 1.2 µg of CTB:p210 (CT10 p.o. group) or 10 µg of p210 synthetic peptide (p210 p.o. group). Another group used as positive control was subcutaneously immunized with 10 µg of p210 synthetic peptide along with 1 µg of pure CTB as adjuvant (p210 s.c. group). Immunization scheme comprised three weekly doses orally administrated at days 1, 8 and 15. Mice were bled at day 0, 5, 12, 19, 47, and 100; serum samples were collected and stored at −70 °C until antibody content analysis. ELISA assays were performed to determine the presence of anti-p210 antibodies in mice sera. Assay plates were coated overnight at 4 °C with the p210 synthetic peptide (1 µg per well; GenScript, sequence: KTTKQSFDLSVKAQYKKNKH) and subsequently blocked with 5 % fat-free dry milk for 2 h at 25 °C. Plates were further incubated overnight at 4 °C with serial dilutions of mice sera (1:20–1:80 dilutions). The secondary antibody, a goat horseradish peroxidase-conjugated anti-mouse IgG, was added (Sigma, 1:2000 dilution) and plates were incubated for 2 h at 25 °C. Signals were developed and measured as described above.
Statistical analysis
ELISA data was analyzed by one-way ANOVA using Minitab 15 software. A P value of less than 0.05 was considered statistically significant.
Results
Rescue of C. reinhardtii clones carrying the CTB:p210 gene
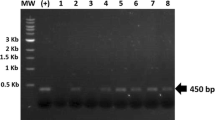
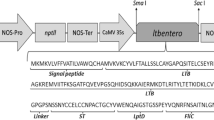
Correct construction of the expression vector p464-CTB:p210 was confirmed by restriction profiles and sequencing (data not shown). The chimeric protein CTB:p210 coded by this vector consists of the sequence of the mature B subunit of the cholera toxin, a linker of six amino acids (DPRVPS) and the p210 epitope from ApoB100 (KTTKQSFDLSVKAQYKKNKH) (Fig. 1). Expression is driven by the constitutive promoter of the atpA gene cluster from C. reinhardtii. The aadA gene serves as a spectinomycin resistance marker. C. reinhardtii was subsequently subjected to transformation by particle bombardment. Two weeks post-bombardment, 40 spectinomycin resistant clones were rescued on selective media and called CT1–40. Candidate clones were subjected to at least four selection rounds, and seven clones showing a stable spectinomycin resistance phenotype were selected for further analyses (clones CT3, CT9, CT10, CT12, CT16, CT17, and CT19). To investigate the transplastomic state of the putative transformed clones, total DNA preparations from the selected clones were assessed by PCR in order to detect the atpA promoter/CTB:p210 transgene sequences. All of these clones showed the expected 600 bp amplicon, which was also present in the positive control, whereas no amplicon was evident in the negative control (Fig. 2).
a Amino acid sequence of the CTB:p210 chimeric protein. The amino acid sequence is represented as follows: mature sequence of the cholera toxin B subunit in italics; linker in gray highlighted letters; and the p210 epitope in underlined letters. b Map of the p464-CTB:p210 vector. This expression vector allows for a site-directed insertion of heterologous DNA sequences downstream of the chloroplast tscA gene from C. reinhardtii. A bicistronic arrangement under control of the promoter from the atpA gen cluster from C. reinhardtii drives the expression of the CTB:p210 gene and the aadA marker gene coding for the aminoglycoside-3′-adenyltransferase that confers spectinomycin resistance. Vector integrity was verified by restriction analysis and conventional sequencing
Detection of the CTB:p210 transgene by PCR analysis. DNA from spectinomycin resistant C. reinhardtii clones were analyzed by PCR with oligonucleotides targeting the CTB:p210 transgene (a) or the β-tubuline as endogenous gene (b). Lane 1, molecular weight marker (1 kb); Lanes 2–8, putative transformed clones (CT3, CT9, CT10, CT12, CT16, CT17, and CT19, respectively); Lane 9, Chlamydomonas reinhardtii WT as a negative control; Lane 10, p464-vector (10 ng) as a positive control. The single 600 bp band showed the presence of the the atpA promoter/CTB:p210 transgene sequences
The CTB:p210 immunogen is expressed into the Chlamydomonas chloroplast
To investigate the expression of the pentameric form of CTB:p210 in algae chloroplasts, a GM1-ELISA assay was performed observing significant higher OD readings for protein samples from transplastomic lines than those from the WT strain, indicating a positive pentamer assembly as only this form binds to the GM1 (Fig. 3). Further protein analysis by means of Western blot confirmed the presence of a signal at 14 kDa, whereas no bands were observed for the WT strain protein extract (Fig. 4). This finding confirms the integrity of the chimeric protein since the theoretical molecular weight for CTB:p210 is 15 kDa. According to the standards made with distinct amounts of pure CTB, expression levels were estimated up to 60 µg CTB:p210 per g of fresh algae biomass (Fig. 5; clone CT10). To further detect the p210 moiety in the chimeric protein, an anti-p210 Western blot was conducted. The presence of a signal at 14 kDa and the absence of bands for the WT strain protein extract confirms the antigenic activity of p210 in the context of the CTB:p210 chimera (Fig. 6). These assays confirmed the potential of these algae clones to conduct further research on the immunogenic activity of the algae-made CTB:p210 antigen in test animals.
GM1 binding activity of the algae-made CTB:p210 protein. Total soluble protein extracts were analyzed in an ELISA assay using an anti-CTB serum. The results indicate a positive GM1 binding activity (P < 0.05) for the transplastomic clones suggesting proper oligomerization of the algae-made CTB:p210. WT, extract from a wild-type clone; CTB(+), 2.5 ng of pure CTB as positive control; CT3, CT9, CT10, CT12, CT16, and CT19, transplastomic clones
Detection of the algae-made CTB:p210 protein by western blot analysis. Total soluble protein extracts were resolved by SDS-PAGE and subjected to blotting and immunodetection by an anti-CTB serum. Lanes 1–4, CTB standards (500, 300, 200, and 100 ng of pure CTB, Sigma); 5–8, transplastomic clones (CT3, CT16, CT19, and CT10, respectively); 9, negative control (Chlamydomonas reinhardtii WT)
CTB:p210 yields in C. reinhardtii clones. Quantification of the CTB:p210 antigen was conducted by a densitometry of Western blots labelled with an anti-CTB-serum. Standards consisted of different amounts of pure CTB (500, 300, 200, and 100 ng). The clone with a higher yield was CT10 with 60 µg CTB:p210 g−1 FW
Detection of the p210 moiety in the algae-made CTB:p210 protein. Total soluble protein extracts were resolved by SDS-PAGE and subjected to blotting and immunodetection by an anti-p210 serum. Lanes 1–6, CT9, CT10, CT13, CT16, CT17, and CT19 transplastomic clones, respectively; 7, negative control (extracts from WT C. reinhardtii strain)
CTB:p210 is orally immunogenic in BALB/c mice
Test mice groups were immunized with either a WT clone or the CT10 clone expressing CTB:p210 following an immunization scheme that consisted of three weekly doses. Sera were screened for the presence of anti-p210 antibodies by means of ELISA assays. As shows Fig. 7 significantly higher OD readings were observed for the CTB:p210 group immunized with the CT10 clone in comparison to those observed for the WT group (P < 0.05). Antibody levels in the group immunized s.c. with p210 were higher than those present in the CTB:p210 group at the time points 3, 4, and 5 (P < 0.05). These findings indicate that oral immunization with the algae-derived CTB:p210 successfully elicited systemic immune responses against the target p210. Interestingly, the humoral responses lasts up to 80 days from the last boost.
Anti-p210 IgG serum responses induced in mice by the algae-made CTB:p210. Test mice were immunized on days 1, 8, and 15 with one of the following treatments: algae containing CTB:p210 (orally), WT algae (orally), PBS (orally), pure p210 peptide (orally) and pure p210 peptide plus CTB as adjuvant (subcutaneously). Blood samples taken on days 0, 5, 12, 19, 47, and 100 (T0 to T5, respectively). ELISA assays were performed to determine the presence of anti-p210 antibodies in mice sera. Statistic differences (P < 0.05) within each point time are indicated by an asterisk (versus WT group) or a cross (versus p210 s.c. group). Significantly higher OD readings were observed for the CTB:p210 group orally immunized with the CT10 clone in comparison to those observed for the WT algae-treated group (P < 0.05). The humoral responses last up to 80 days from the last boost (T5)
Discussion
Several target antigens have been studied in the atherosclerosis vaccination topic with promising findings, representing new potential atherosclerosis therapies (Yamashita et al. 2015). This type of therapies is intended to achieve atheroprotection through modulating the immune responses involved in atherosclerosis pathogenesis (Pierides et al. 2013). In the present study, an oral immunogen targeting the epitope p210 from ApoB100 apolipoprotein was expressed in C. reinhardtii as a tool in the development of inexpensive atherosclerosis vaccines. The use of p210 in immunotherapies against atherosclerosis is well documented (Salazar-González and Rosales-Mendoza 2013). The B subunit of the cholera toxin (CTB) was used as carrier of the target epitope p210 from ApoB100 since CTB is a strong mucosal adjuvant intended to serve as an effective carrier for the elicitation of anti-p210 humoral responses through mucosal immunization (Gloudemans et al. 2013; Holmgren et al. 1993). This concept has been proven by Klingenberg et al. (2010), who developed an E. coli-made immunogen consisting of CTB and p210. Remarkably, intranasal immunization with the CTB:p210 immunogen induced both mucosal and systemic antibody responses as well as cellular immune responses. Interestingly, this vaccine decreased atherosclerotic lesions at a 35 % rate in mice (Klingenberg et al. 2010).
Despite these outstanding advances, a challenge for vaccinology is the implementation of platforms for the production of low cost and easy to administer vaccines. WHO estimates that the reuse of injection equipment may cause 20 million infections with hepatitis B virus (HBV), 2 million infections with hepatitis C virus (HCV), and 250,000 infections with human immunodeficiency virus (HIV) worldwide (Hauri et al. 2003; Hutin et al. 2003). Other limitations of conventional vaccines are imposed by the requirement of cold chain for distribution as well as trained personnel for parenteral administration. Therefore, new vaccine production platforms are required, especially for developing countries where vaccination coverage is limited (Pagliusi et al. 2015). Platforms based on recombinant E. coli strains can reach high expression yields, but extensive downstream processing is required to eliminate endotoxins present in the host and obtain a properly folded protein.
Since oral administration is a friendly route that does not require trained personnel or sterile devices for application, one attractive alternative consists on the development of oral vaccine formulations. Interestingly, several oral vaccine formulations have showed to be promising for immunomodulation of atherosclerosis progression (Maron et al. 2002; Harats et al. 2002; George et al. 2004; van Puijvelde et al. 2006; Mestecky et al. 2008). In addition, the use of GRAS organisms, such as plant cells or algae, has been documented as a convenient strategy to orally deliver biopharmaceuticals using minimally processed biomass since no inherent host toxic compounds exist (Dreesen et al. 2010; www.protalix.com). Herein, an algae-based atherosclerosis vaccine prototype targeting p210 is proposed as an advantageous system in terms of production cost and mode of delivery versus conventional parenteral vaccines that require extensive purification steps. Downstream processing of algae-based vaccines comprises only biomass separation, freeze-drying process and dosage (Rosales-Mendoza 2013). Chloroplast-based expression is an approach that has led to high yields in Chlamydomonas and thus using this technology ensures maximal yields as observed for the obtained clones producing CTB:p210 (up to 60 µg per g of fresh weight). Yields were estimated by Western blot analysis since this technique allows for the detection of the total (oligomeric and monomeric forms) of the recombinant protein. The observed yield is considered sufficient for oral vaccine formulation as previous reports has consisted of 40–160 µg doses of a CTB-based immunogen contained in a reasonable biomass amount (25–100 mg dry weight per mouse), which were able to achieve immunoprotection in mice (Dreesen et al. 2010; Cardi et al. 2010).
The CT10 transplastomic clone, generated in this study, was evaluated as an oral delivery vehicle of the CTB:p210 immunogen. As evidenced in the test animal evaluation, the algae biomass containing the CTB:p210 protein was capable to elicit specific humoral responses against p210. The higher responses observed in the group immunized subcutaneously with p210 is in accordance with the general behavior that s.c. immunization leads to stronger systemic responses than oral immunization (Herzog 2014). However given the enormous advantages of oral immunization in terms of cost and easy administration, the achieved humoral response that lasts for up to 80 days after the last boost is considered promising. All these findings accounts for the potential of this immunization model on exerting a therapeutic effect. This candidate vaccine will be further assessed in the apoE –/– transgenic mice model and rabbits subjected to a high cholesterol diet.
In conclusion, this study provides evidence on a new immunogen produced in a convenient host that may support a vaccine produced in a simplified bioprocess and will account for the development of new therapies of low cost, easy compliance and better efficacy in the fight against atherosclerosis.
Author contribution statement
S.R.-M. designed and supervised the study. B.B.-H. designed and constructed the binary vector. J.I.B.-L., A.R.-M. generated and characterized the algae clones. E.M.-E. determined protein expression levels. A.R.-M. evaluated immunogenicity. S.R.-M. and L.M.T.P.-M. wrote the manuscript with the help of J.I.B.-L., A.R.-M. and E.M.-E. All authors discussed the results, read and approved the final version of the manuscript.
References
Cardi T, Lenzi P, Maliga P (2010) Chloroplasts as expression platforms for plant-produced vaccines. Expert Rev Vaccin 9(8):893–911
Chen HC, Melis A (2013) Marker-free genetic engineering of the chloroplast in the green microalga Chlamydomonas reinhardtii. Plant Biotechnol J 11:818–828
Conti P, Shaik-Dasthagirisaeb Y (2015) Atherosclerosis: a chronic inflammatory disease mediated by mast cells. Cent Eur J Immunol 40(3):380–386
Daniell H, Ruiz ON, Dhingra A (2005) Chloroplast genetic engineering to improve agronomic traits. Methods Mol Biol 286:111–138
Dreesen IA, Charpin-El Hamri G, Fussenegger M (2010) Heat-stable oral alga-based vaccine protects mice from Staphylococcus aureus infection. J Biotechnol 145:273–280
Franklin S, Ngo B, Efuet E, Mayfield SP (2002) Development of a GFP reporter gene for Chlamydomonas reinhardtii chloroplast. Plant J 30(6):733–744
Fredrikson GN, Hedblad B, Berglund G, Alm R, Ares M, Cercek B, Chyu KY, Shah PK, Nilsson J (2003a) Identification of immune responses against aldehyde-modified peptide sequences in apoB associated with cardiovascular disease. Arterioscler Thromb Vasc Biol 23(5):872–878
Fredrikson GN, Söderberg I, Lindholm M, Dimayuga P, Chyu KY, Shah PK, Nilsson J (2003b) Inhibition of atherosclerosis in apoE-null mice by immunization with apoB-100 peptide sequences. Arterioscler Thromb Vasc Biol 23:879–884
George J, Yacov N, Breitbart E, Bangio L, Shaish A, Gilburd B, Shoenfeld Y, Harats D (2004) Suppression of early atherosclerosis in LDL receptor deficient mice by oral tolerance with beta 2-glycoprotein I. Cardiovasc Res 62:603–609
Gloudemans AK, Plantinga M, Guilliams M, Willart MA, Ozir-Fazalalikhan A, van der Ham A, Boon L, Harris NL, Hammad H, Hoogsteden HC, Yazdanbakhsh M, Hendriks RW, Lambrecht BN, Smits HH (2013) The mucosal adjuvant cholera toxin B instructs non-mucosal dendritic cells to promote IgA production via retinoic acid and TGF-β. PLoS One 8(3):e59822
Gorman DS, Levine RP (1965) Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 54:1665–1669
Harats D, Yacov N, Gilburd B, Shoenfeld Y, George J (2002) Oral tolerance with heat shock protein 65 attenuates mycobacterium tuberculosis induced and high-fat-diet-driven atherosclerotic lesions. J Am Coll Cardiol 40:1333–1338
Hauri AM, Armstrong GL, Hutin YJF (2003) Contaminated injections in health care settings. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL (eds) Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. World Health Organization, Geneva
Herzog C (2014) Influence of parenteral administration routes and additional factors on vaccine safety and immunogenicity: a review of recent literature. Expert Rev Vaccin 13(3):399–415
Holmgren J, Lycke N, Czerkinsky C (1993) Cholera toxin and cholera B subunit as oral-mucosal adjuvant and antigen vector systems. Vaccine 11(12):1179–1184
Hutin Y, Hauri A, Chiarello L, Catlin M, Stilwell B, Ghebrehiwet T, Garner J (2003) Injection Safety Best Practices Development Group. Best infection control practices for intradermal, subcutaneous, and intramuscular needle injections. Bull World Health Organ 81(7):491–500
Keijzer C, van der Zee R, van Eden W, Broere F (2013) Treg inducing adjuvants for therapeutic vaccination against chronic inflammatory diseases. Front Immunol 4:245
Klingenberg R, Lebens M, Hermansson A, Fredrikson GN, Strodthoff D, Rudling M, Ketelhuth DF, Gerdes N, Holmgren J, Nilsson J, Hansson GK (2010) Intranasal immunization with an apolipoprotein B-100 fusion protein induces antigen-specific regulatory T cells and reduces atherosclerosis. Arterioscler Thromb Vasc Biol 30(5):946–952
Leake DS (1993) Oxidised low density lipoproteins and atherogenesis. Br Heart J 69:476–478
Maron R, Sukhova G, Faria AM, Hoffmann E, Mach F, Libby P, Weiner HL (2002) Mucosal administration of heat shock protein-65 decreases atherosclerosis and inflammation in aortic arch of low-density lipoprotein receptor-deficient mice. Circulation 106:1708–1715
Mestecky J, Nguyen H, Czerkinsky C, Kiyono H (2008) Oral immunization: an update. Curr Opin Gastroenterol 24(6):713–719
Orellana-Escobedo L, Rosales-Mendoza S, Romero-Maldonado A, Parsons J, Decker EL, Monreal-Escalante E, Moreno-Fierros L, Reski R (2015) An Env-derived multi-epitope HIV chimeric protein produced in the moss Physcomitrella patens is immunogenic in mice. Plant Cell Rep 34(3):425–433
Pagliusi S, Jain R, Suri RK (2015) DCVMN Executive Committee Group. Vaccines, our shared responsibility. Vaccine 33:2197–2202
Patel MJ, Blazing MA (2013) Inflammation and Atherosclerosis: disease modulating Therapies. Curr Treat Options Cardiovasc Med 15(6):681–695
Pierides C, Bermudez-Fajardo A, Fredrikson GN, Nilsson J, Oviedo-Orta E (2013) Immune responses elicited by apoB-100-derived peptides in mice. Immunol Res 56(1):96–108
Rosales-Mendoza S (2013) Future directions for the development of Chlamydomonas-based vaccines. Expert Rev Vaccin 12:1011–1019
Rosales-Mendoza S, Soria-Guerra RE, de Jesús Olivera-Flores MT, López-Revilla R, Argüello-Astorga GR, Jiménez-Bremont JF, García-de la Cruz RF, Loyola-Rodríguez JP, Alpuche-Solís AG (2007) Expression of Escherichia coli heat-labile enterotoxin b subunit (LTB) in carrot (Daucus carota L.). Plant Cell Rep 26(7):969–976
Sakakura K, Nakano M, Otsuka F, Ladich E, Kolodgie FD, Virmani R (2013) Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ 22:399–411
Salazar-González JA, Rosales-Mendoza S (2013) A perspective for atherosclerosis vaccination: is there a place for plant-based vaccines? Vaccine 31:1364–1369
Sjögren P, Fredrikson GN, Samnegard A, Ericsson CG, Ohrvik J, Fisher RM, Nilsson J, Hamsten A (2008) High plasma concentrations of autoantibodies against native peptide 210 of apoB-100 are related to less coronary atherosclerosis and lower risk of myocardial infarction. Eur Heart J 29(18):2218–2226
van Puijvelde GH, Hauer AD, de Vos P, van den Heuvel R, van Herwijnen MJ, van der Zee R, van Eden W, van Berkel TJ, Kuiper J (2006) Induction of oral tolerance to oxidized low-density lipoprotein ameliorates atherosclerosis. Circulation 114:1968–1976
Wigren M, Kolbus D, Dunér P, Ljungcrantz I, Söderberg I, Björkbacka H, Fredrikson GN, Nilsson J (2011) Evidence for a role of regulatory T cells in mediating the atheroprotective effect of apolipoprotein B peptide vaccine. J Intern Med 269(5):546–556
World Health Organization (2015) Descriptive note 317 Ene/2015, http://www.who.int/mediacentre/factsheets/fs317/en/. Accessed on 22 Sep 2015
Yamashita T, Sasaki N, Kasahara K, Hirata KI (2015) Anti-inflammatory and immune-modulatory therapies for preventing atherosclerotic cardiovascular disease. J Cardiol 66(1):1–8
Acknowledgments
This project was funded by CONACYT/México (Grant CB-2008-01, 102109 to SRM) and FAI/UASLP/2015 to SRM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Communicated by A. Dhingra.
J. I. Beltrán-López and A. Romero-Maldonado have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Beltrán-López, J.I., Romero-Maldonado, A., Monreal-Escalante, E. et al. Chlamydomonas reinhardtii chloroplasts express an orally immunogenic protein targeting the p210 epitope implicated in atherosclerosis immunotherapies. Plant Cell Rep 35, 1133–1141 (2016). https://doi.org/10.1007/s00299-016-1946-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-016-1946-6