Abstract
Key message
Highly tolerant herbicide-resistant transgenic rice was developed by expressing codon-modified synthetic CP4 - EPSPS . The transformants could tolerate up to 1 % commercial glyphosate and has the potential to be used for DSR (direct-seeded rice).
Abstract
Weed infestation is one of the major biotic stress factors that is responsible for yield loss in direct-seeded rice (DSR). Herbicide-resistant rice has potential to improve the efficiency of weed management under DSR. Hence, the popular indica rice cultivar IR64, was genetically modified using Agrobacterium-mediated transformation with a codon-optimized CP4-EPSPS (5-enolpyruvylshikimate-3-phosphate synthase) gene, with N-terminal chloroplast targeting peptide from Petunia hybrida. Integration of the transgenes in the selected rice plants was confirmed by Southern hybridization and expression by Northern and herbicide tolerance assays. Transgenic plants showed EPSPS enzyme activity even at high concentrations of glyphosate, compared to untransformed control plants. T 0, T 1 and T 2 lines were tested by herbicide bioassay and it was confirmed that the transgenic rice could tolerate up to 1 % of commercial Roundup, which is five times more in dose used to kill weeds under field condition. All together, the transgenic rice plants developed in the present study could be used efficiently to overcome weed menace.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa L.) is one of the most important food crops and a primary food source for more than a third of the world’s population. At the present trend, rate of global production of rice is not improving and is far below what is needed to meet the projected demands in 2050 (Ray et al. 2013). Apart from numerous biotic and abiotic factors which adversely affect rice production, water is a major limitation because of decreasing ground water level (Wada et al. 2010) and inadequate rain fall globally, creating a water scarcity that could affect 15 million hectares of irrigated rice by 2025 (Bouman et al. 2007). Direct-seeded rice (DSR) technology is a viable alternative for puddle rice and avoids repeated puddling, preventing soil degradation and plow-pan formation (Joshi et al. 2013; IRRI 2006). Moreover, DSR can address the imminent water crisis, emerging issues of labor-shortages and production costs associated with transplanted rice (TPR) cultivation (Farooq et al. 2011). Weed control is the largest single constraint in DSR and is critical to maintain high yields, high quality harvest free of weed seeds. In uncontrolled weed conditions it may lead to yield reductions from 70 to 76 % in DSR (Singh et al. 2005). Therefore, it is imperative to combat weeds to enhance the rice production under DSR.
Glyphosate (N-[phosphonomethyl]-glycine) is a powerful and most widely used broad-spectrum herbicide promises which interferes with the shikimate metabolic pathway by inhibiting 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) (Steinrücken and Amrhein 1980). Its unique target site, high capability to sink into plant tissues, non selective nature and low toxicity risks to humans and environment has rendered it to its present status (Funke et al. 2006). One of the major areas of crop improvement programmes has also been to make crops mitigate the effect of herbicides. Great impact in herbicide resistance was through transgenic technology that promised to offer farmers, an efficient method to control weeds. Various genes from different sources with same or different mode of action have been identified which help the plants combat glyphosate (Comai et al. 1985; Stalker et al. 1985; Kishore et al. 1986; Padgette et al. 1991; Berry et al. 1992; Zhou et al. 1995, 2006; Ye et al. 2001; Kahrizi et al. 2007). Among this, the CP4-EPSPS gene, derived from the common soil bacterium Agrobacterium strain CP4, is widely used as it is naturally tolerant to inhibition by glyphosate and currently exploited in commercial transgenic crops (Berry et al. 1992; Padgette et al. 1996). With the advent of transgenic crops harboring glyphosate tolerant CP4-EPSPS gene, glyphosate can be now efficiently used to control a wide spectrum of weeds, without affecting the crops of interest. In the recent times, reports are available, where transgenic rice plants were developed using a range of modified EPSPS genes (Charng et al. 2008; Zhao et al. 2011; Chandrasekhar et al. 2014).
Considering that, weed control in rice fields could lead to increase in crop production thereby ensuring food security, we have generated the transgenic glyphosate tolerant rice. In the present study, a codon-optimized mCP4-EPSPS gene (modified CP4-EPSPS) with chloroplast targeting peptide was transferred to an elite indica rice cultivar IR64 to confer glyphosate resistance. Transgenic lines were confirmed by molecular, expression and efficacy analysis. The results showed that the transgenic rice plants expressing mCP4-EPSPS could tolerate 1 % active content of commercial glyphosate-based broad-spectrum herbicide Roundup.
Materials and methods
Codon optimization and construction of plant transformation binary vector
The Agrobacterium gene encoding 5-enolpyruvylshikimate-3-phosphate synthase is a bacterial gene with prokaryotic codon usage (Berry et al. 1992; Kishore et al. 1992). The mCP4-EPSPS gene was assembled with varying GC content and plant codon usage. The sequence was codon optimized (GenBank Accession KJ787649) and fused with N-terminal Petunia hybrida chloroplast targeting signal peptide. The codon-optimized mCP4-EPSPS gene was synthesized by the GenScript Corporation (USA). The synthetic mCP4-EPSPS gene in PUC57 vector was digested with EcoRI and SalI and cloned in the respective sites of the binary vector, pCAMBIA1301. The gene was placed under the control of maize ubiquitin (Ubi-1) promoter cloned upstream of the gene at HindIII and EcoRI site. Hygromycin phosphotransferase (HPTII) gene present in pCAMBIA1301 binary vector was used as the selectable marker. The recombinant vector was named as pCAMBIA-ubi::mCP4-EPSPS and was further mobilized into Agrobacterium tumefaciens strain EHA105 by freeze thaw method (Hofgen and Willmitzer 1988).
Agrobacterium-mediated rice transformation
The seeds of rice cultivar IR64 (O. sativa L.) were obtained from Indian Agricultural Research Institute (IARI). Rice was transformed with the binary vector pCAMBIA-ubi-mCP4-EPSPS by Agrobacterium-mediated transformation. The protocol for transforming the rice calli as described by Hiei and Komari (2008) was followed with a few modifications. Mature seeds were manually dehusked and sterilized with 70 % (v/v) alcohol for 3 min followed by 3 washes with autoclaved double distilled water. Further, the seeds were surface sterilized with 0.1 % (w/v) HgCl2 for 4 min followed by three washes with autoclaved distilled water. Embryogenic calli were initiated from scutellar tissues of mature seed embryo on callus induction medium [MS medium (Murashige and Skoog 1962) containing B5 vitamins (Gamborg et al. 1976), 30 g−l sucrose, 500 mg−l proline, 500 mg−l casein hydrolysate, 2.5 mg−l 2,4-dichlorophenoxyacetic acid (2,4-d) and 4 g−l phytagel, pH 5.8].
After 3 weeks, white hard embryogenic calli were infected with over-night grown A. tumefaciens EHA105 culture harboring recombinant binary vector and were co-cultivated for 3 days at 28 °C on MS co-cultivation media (Callus induction medium, 10 g−l glucose, 200 µM acetosyringone, and pH 5.2). After 3 days, the calli were transferred to MS selection medium (MS callus induction medium containing 50 mg−l hygromycin, 250 mg−l cefotaxime, and 250 mg−l carbenicillin). Healthy, proliferating calli were sub-cultured on to fresh selection medium every 15 days. After three rigorous rounds of selections the actively growing calli were transferred to MS regeneration medium (MS callus induction medium, 3 mg−l 6-benzylaminopurine (BAP), 1 mg−l α-naphthaleneacetic acid (NAA), 50 mg−l hygromycin, 250 mg−l cefotaxime, 250 mg−l carbenicillin) and incubated in dark for 7 days and shifted to light. The emerging shoots were then transferred to plant rooting medium (1/2 MS salt containing 15 g−l sucrose, 30 mg−l hygromycin, 250 mg−l cefotaxime, and 4 g−l phytagel) after which the plantlets were hardened and transferred to controlled glasshouse conditions and allowed to set seeds.
Polymerase chain reaction analysis
The genomic DNA was isolated from the transgenic and non-transgenic control rice plants using a modified cetyl-trimethyl-ammonium bromide (CTAB) method (Porebski et al. 1997). PCR analysis was performed to amplify the 1.6 kb mCP4-EPSPS gene using gene-specific primers (forward primer 5′-GGATCCCATGGCCCAGAT-3′ and reverse primer 5′-CTCGACTTACGCTGCTT-3′) and 900 bp HPTII gene using gene-specific primers (forward primer 5′-GGCGAGTACTTCTACACAGC-3′ and reverse primer 5′-TATGTCCTGCGGGTAATAG-3′). PCR amplification was carried out in a thermal cycler (Eppendorf, Germany) programmed with a hot start at 94 °C for 5 min, followed by 30 cycles of 94 °C for 1 min, an annealing step at 55 °C for 1 min and 72 °C for 1.5 min and a final extension step at 72 °C for 10 min. The amplified products were analyzed by electrophoresis on 0.8 % agarose gel.
mCP4-EPSPS protein detection by Immuno strip test analysis
To assay the expression of mCP4-EPSPS protein in the transgenic and non-transgenic control lines, an Immuno strip test (QuickStix Envirologix, Portland, USA) was used. The crude protein was extracted using extraction buffer supplied by manufacturer and QuickStix Immuno strip were dipped in the sample and allowed to develop bands for 10 min before making a final interpretation of the results.
Genomic southern hybridization analysis
About 10 µg of genomic DNA was extracted from the transgenic and non-transgenic control rice plants and digested with the restriction enzyme having a single site in the T-DNA. DNA electrophoresis was performed as described (Sambrook and Russell 2001), blotted overnight onto a Hybond-N+-membrane (Amersham Biosciences, USA), and fixed at 22 J/cm for 2 min using Stratalinker UV crosslinker (Stratagene, UK). Immobilized nucleic acids were hybridized with α [32P]-dCTP labeled genomic fragment using mega prime DNA labeling system (Amersham Biosciences, UK) and hybridized in ULTRAhyb buffer (Ambion, Austin, USA). Subsequent to the hybridization, stringent washes were performed according to the manufacturer’s instructions. The membrane was exposed to the X-ray film and was placed in an intensifying cassette under dark conditions and was stored at −70 °C for 48–72 h and later the exposed X-ray film (Fuzifilm, Kodak) was developed to visualize the results.
Northern analysis
Northern analysis was carried in lines E1 and E2 to co-relate the expression of EPSPS to differences in responses to glyphosate in enzyme activity and bioassays. Total RNA was isolated from T 2 transgenic and non-transgenic control rice plants using Spectrum Plant Total RNA Extraction kit (Sigma, USA) as per manufacturer’s protocol. About 15 μg of total RNA was resolved on a 1.2 % formaldehyde denaturing gel and transferred to the nitrocellulose membrane (Hybond-N+, Amersham Biosciences) using diethylpyrocarbonate (DEPC) treated 20X SSC. Pre-hybridization and hybridization were performed using ULTRAhyb hybridization buffer (Ambion, USA) according to the manufacturer’s protocol. The blot was hybridized at 42 °C with α [32P]-dCTP labeled 1.3 kb mCP4-EPSPS fragment using mega prime DNA labeling system (Amersham Biosciences). The hybridized membrane was washed at 42 °C and was exposed to X-ray film (Fuzifilm, Kodak) in an intensifying cassette under dark conditions. The cassette was placed at −70 °C for 24–48 h and the exposed X-ray film was developed to visualize the results.
Real-time PCR
Real-time PCR was carried out in all of the lines of T 0 to find the spectrum of expression of the transgenes and select high expressing lines that are pivotal to confer glyphosate resistance. Total RNA was extracted from T 0 transgenic and non-transgenic control rice plants using Spectrum Plant Total RNA Extraction kit (Sigma, USA) according to manufacturer’s protocol. The RNA was visualized in 1.2 % formaldehyde denaturing agarose gel to confirm its quality and was quantified using Nanodrop Spectrophotometer (Thermo Scientific, USA). RNA (1 μg) was aliquoted and reverse transcribed using AffinityScript QPCR cDNA Synthesis Kit (Stratagene, USA). Real-time PCR was performed in a 96-well optical reaction plate using Stratagene Mx3005P detection unit, using VeriQuest SYBR Green qPCR Master Mix (Affymetrix, Santa Clara, USA). The primers used for qRT-PCR analysis were, forward primer 5′-ACCTTTATCGGTGACGCCAGCTTA-3′ and reverse primer 5′-TCGCCGTCTTCGCTCTTTACTTGA-3′. Results were analyzed and calculated using the comparative threshold cycle (Ct) method according to the manufacturers’ instructions. For data normalization, housekeeping gene Ubiquitin (GenBank ID Os06g0681400) was used as an internal control. The primers used for amplification of reference gene were, forward primer 5′-GGGTTCACAAGTCTGCCTATTTG-3′ and reverse primer 5′-ACGGGACACGACCAAGGA-3′.
Glyphosate tolerance assay
Putative transgenic (T 0 generation) rice plantlets were hardened and transferred to pots containing soil and shifted to the glasshouse with controlled conditions, suitable for rice development. Once the plants were confirmed by Southern hybridization, they were subjected to glyphosate tolerance assay, wherein the 45-day-old mCP4-EPSPS transgenic rice and non-transgenic control plants grown under similar conditions, were sprayed with 1 % glyphosate (equivalent to 2.5 % (v/v) Roundup, active ingredient isopropylamine salt of glyphosate, 41 %), Monsanto and were observed for a week. In T 2 generation, the transgenic (Lines E1 and E2) seeds and non-transgenic control seeds were germinated on soil-rite medium for 11 days, and were then sprayed with 1 % glyphosate and scored for tolerance after 1 week.
Analysis of mCP4-EPSPS activity
Inorganic phosphate release was measured to determine EPSPS activity, using the malachite green dye-based assay (Lanzetta et al. 1979) with few modifications. Change in optical density positively corresponds to inorganic phosphate released from EPSP synthase activity and measured in the forward direction. The reaction mixture contained, 1 mM shikimate-3-phosphate (S3P), 1 mM Phosphoenolpyruvate (PEP), 100 mM HEPES, 1.5 mg of total crude protein from transgenic lines E1, E2 along with non-transgenic control plant and varying concentrations of glyphosate (0–10 mM) made up to a final volume of 0.1 ml. After an incubation period of 20 min, the reaction was stopped by the addition of 1 ml of colorimetric solution (9.2 mM malachite green and 8.5 mM (NH4)6MO7O24·4H2O in 1 M HCl, with 2 g CHAPS/l added to stabilize color development) and 0.1 ml of 34 % (w/v) Sodium citrate solution after a minute. After 10 min incubation at room temperature, absorbance was measured at A660 using spectrophotometer.
Results and discussion
Biotechnology for herbicide tolerance has come a long way and has had a positive impact on both the scientific and farming community (Carpenter 2010; James 2013). Various strategies have been used to confer herbicide tolerance such as over expression of a target protein, expression of a gene encoding herbicide-detoxifying enzyme and modification of the target enzyme to an insensitive form (Quinn 1990). Among them, insensitive CP4-EPSPS has attracted considerable attention because of its high catalytic activity and very low affinity for glyphosate (Berry et al. 1992). The present study is a novel attempt to demonstrate the utility of a codon-optimized and -modified mCP4-EPSPS and its use in tolerating very high levels of glyphosate. The paper presents a meticulous analysis and usefulness of the modified EPSPS gene and fool-proof demonstration of the integration-inheritance of the gene in transgenic rice.
The observations described herein pertain to the codon optimization of prokaryotic CP4-EPSPS gene and its over expression in an elite indica rice cultivar IR64. The IR64 genotype was selected for plant transformation because of its superior agronomical characters such as wide cultivation and high yield. The presence and expression of transgene (mCP4-EPSPS) in the rice plants has been demonstrated through various molecular and herbicide bioassay analyses. The mCP4-EPSPS expressing rice lines furnished marked tolerance against glyphosate.
Codon optimization of mCP4-EPSPS gene for efficient expression in rice
To achieve high expression levels of heterologous genes introduced into plants, it is often essential to optimize the codon usage to imitate highly expressed plant genes (Murray et al. 1989; Kusnadi et al. 1997). In addition it is necessary to modify the coding sequence, such as the removal of potential RNA processing sequences and polyadenylation signals (Murray et al. 1991; Perlak et al. 1991). Therefore, a synthetic gene encoding the EPSP synthase was constructed based on the codon usage of highly expressed plant nuclear encoded genes especially for rice plant. Furthermore, the ratio of G/C is kept at 52 %, because a high A/U ratio has been previously associated with low mRNA stability and thus low levels of protein expression in higher plants (De Rocher et al. 1998). The GC content of the native CP4-EPSPS is close to 67 % and the GC content is highly skewed at GC3 showing as high as 84 %. Interestingly, despite this high GC content, the native CP4-EPSPS showed a codon adaptive index (CAI) of 0.847. Therefore, the synthetic gene used in the present study was designed as a compromise between GC content (~55 % in rice) and CAI, but care was taken not to use any rare codons. The modified gene has a CAI of 0.751. As the shikimate pathway takes place in chloroplast, the chloroplast transit peptide (CTP) from Petunia hybrida was fused at 5′ end of the mCP4-EPSPS gene, to target the transgene to chloroplast. Later on, the transit peptide is cleaved off while crossing the chloroplast membranes and only the functional enzyme enters the chloroplast (Della-Cioppa et al. 1986). Furthermore, this synthetic codon-optimized mCP4-EPSPS was used to develop glyphosate tolerant transgenic rice plants.
Genetic transformation and production of transgenic plants
For developing herbicide tolerant transgenic plants, embryogenic calli of rice were co-cultivated with the A. tumefaciens strain EHA105 harboring Ti-plasmid pCAMBIA1301-Ubi-mCP4-EPSPS. A total number of 26 putative transformants were obtained from 887 calli of IR64 resulting in 2.91 % transformation efficiency. Amongst these, the plants that showed a normal phenotype and produced seeds were advanced for further studies.
Molecular analysis of primary (T 0) transgenic plants
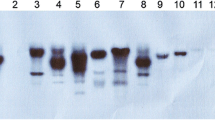
The genomic DNA was isolated from the putative glyphosate tolerant transgenic rice plants as well as from the non-transgenic control plants. PCR analysis of transgenic rice plants showed amplification of 1.6 kb product, representing mCP4-EPSPS coding sequence, while control plants failed to show such amplification (Supplementary Fig. 1). Southern blot analysis was carried out with PCR positive plants. The genomic DNA from 13 transgenic plants along with untransformed control plant was digested with HindIII, and southern blot analysis was performed with 1.3 kb mCP4-EPSPS as a probe to confirm the integration of transgene and to determine their copy number (Fig. 1). Upon integration of the complete T-DNA, junction fragment longer than 3.7 kb are expected to hybridize with mCP4-EPSPS probe (flanking left border). Southern analysis revealed the presence and confirmation of five independent lines viz., E1, E2, E3, E15 and E16. Before performing herbicide bioassay, mCP4-EPSPS protein expression in T 0 transgenic plants was confirmed by the Roundup Ready Immuno strip assay (Supplementary Fig. 2). As expected, it was found that a control band appeared in the non-transgenic control and transgenic plant samples, but EPSPS test band appeared only in the protein extracts of transgenic plants confirming the expression of mCP4-EPSPS protein in samples.
Molecular analysis of T 0 transgenic rice plants. Southern hybridization analysis of T 0 mCP4-EPSPS rice transformants. Genomic DNA (10 μg) was digested with HindIII (restriction site is not present in the probe region) and resolved on 0.8 % agarose gel electrophoresis. The processed gel was transferred on to N+ nylon membrane. The blot was probed with 1.3 kb fragment of mCP4-EPSPS labeled with α [32P]-dCTP. Five independent T 0 transgenic plants are shown in lanes 1, 2, 3, 8 and 9 represents lines E1, E2, E3, E15 and E16, respectively. UC untransformed control plant, PC linearized binary plasmid used as positive control, 1, 3, 8, 10, 11, 12 and 13 shows single copy integration, 2, 4, 6, 7 and 9 shows double copy integration. M λ DNA/HindIII ladder (Thermo Scientific). The left border (LB) junction fragment of the T-DNA is greater than 3.4 kb in pCAMBIA1301-ZmUBI::CP4-EPSPS binary vector (the distance between HindIII and LB)
Further analysis of mCP4-EPSPS expression in different lines of transgenic rice was carried out by quantitative real-time PCR (qRT-PCR) (Fig. 2) and was observed that all the lines showed transcript accumulation of transgene. However, as expected there was variation amongst the transgenic lines and the average expression was higher in the line E3 and E15 compared with the other transgenic lines. Moreover, from the five independent transgenic lines two of the lines (E3 and E15) did not set the seeds, probably due to the high expression of transgene in these lines as inferred from the qRT analysis (Maqbool and Christou 1999).
qRT-PCR analysis of T 0 plants. Expression analysis of mCP4-EPSPS T 0 transgenic rice plants. Ubiquitin gene was used as internal control and ΔCt values were calculated using difference in the Ct mean of the target gene and reference gene. X axis different transgenic lines, Y axis normalized expression of transgene
To check the glyphosate tolerance in transformed T 0 plants, three independent Southern positive fertile lines (E1, E2 and E16) were selected. The mature seedlings of the three lines along with the control untransformed rice plants were sprayed with 1 % (10 g−l glyphosate) glyphosate. After 7 days, the untransformed control rice plants showed severe chlorosis of leaves and meristem tissue, which ultimately lead to stunting and death of the plant, whereas no visible damage was observed in the transgenic lines (Fig. 3). In contrast to this, 0.2 % (2 g−l) glyphosate spray was sufficient to kill the conventional rice at the same rate which is used for weed control (Zhao et al. 2011). However, the variation in expression of the transgenic lines in response to glyphosate spray was observed, as the lines E1 and E2 were found to be very healthy and green than E16 which senesced. Therefore, for advanced generation analysis the line E16 was not included and only the lines E1 and E2 were carried forward.
Inheritance pattern of mCP4-EPSPS gene in T 1 generation
To investigate the inheritance pattern of the transgenes, the primary (T 0) transformants were selfed and seeds were collected. The T 1 progenies were grown to maturity in the glass house. The genomic DNA from these plants was isolated and PCR was performed with mCP4-EPSPS and HPTII primers (Fig. 4a, b). The Immuno strip test was performed (Fig. 4c) for the same plants to check the expression of mCP4-EPSPS gene (data not shown for the line E2). The T 1 plants of the line E1 segregated in a normal Mendelian ratio and showed a monogenic ratio of 3:1 (mCP4EPSPS+/mCP4EPSPS−, hpt+/hpt−, protein strip+/protein strip−) ratio, suggesting a single locus for the integrated T-DNA while, the line E2 which had two copies of T-DNA, segregated in 15:1 ratio (Table 1).
Segregation analysis of T 1 mCP4-EPSPS rice plants. Randomly collected leaf samples from different plants of line E1 have been used to test the segregation by PCR and Immuno strip test. a mCP4-EPSPS gene-specific PCR and b HPTII gene-specific PCR. M 1 kb ladder, W water control, UC untransformed control plant, lane 1–20 T 1 transgenic plants and P binary vector control. c Immunostrip analysis of T 1 transgenic rice UC untransformed control plant; lane 1–20 T 1 transgenic plants
Northern analysis of the T 2 transgenic plants
Northern analysis performed to observe the expression of mCP4-EPSPS gene in the transgenic plants demonstrated the transcript accumulation of mCP4-EPSPS in the T 2 plants of line E1 and E2 when hybridized with mCP4-EPSPS probe. The 1.3 kb mCP4-EPSPS mRNA accumulated to high levels in the transgenic plants, while it was not detected in the untransformed control plants (Fig. 5a). It was observed that the line E2 was a low expresser for mCP4-EPSPS compared to the E1, which showed higher accumulation of the transcripts. For low level of transgene expression in case of line E2, there could be involvement of RNA silencing phenomenon. It is widely known that multiple integration of T-DNA may change their interaction with the host genome which could result in inversion, deletion, truncation, and other chromosomal rearrangements. Moreover, these multiple copy insertions are often characterized by complex integration patterns due to sequence homology, including tandem repeat or inverted repeat may affect the stability of transgene expression by silencing mechanisms (Jorgensen et al. 1996; Depicker and Van Montagu 1997; Hobbs et al. 1993, Reddy et al. 2003).
Expression analysis of mCP4-EPSPS transgenic rice plants. a Northern hybridization analysis of T 2 transgenic rice plant. Total RNA (10 μg) from untransformed control and transgenic lines (E1 and E2) was resolved on 1.2 % denatured formaldehyde gel and transferred the gel on N+ nylon membrane and hybridized with α[32P]-dCTP labeled 1.3 kb mCP4-EPSPS fragment. UC RNA from untransformed control plant, line E1 and E2 represents RNA from single and double copy of mCP4-EPSPS transgenics, respectively. Lower panel shows ethidium bromide-stained agarose gel of total RNA used for northern blot analysis. b mCP4-EPSPS enzyme assay of T 2 transgenic rice plants. EPSPS enzyme assay was performed with crude protein extract from transgenic lines (E1 and E2) along with untransformed control plant by measuring inorganic phosphate release using the malachite green dye method and absorbance was measured at OD660 and graph was plotted. c, d Glyphosate tolerance and of T 2 transgenic rice plants. T 2 rice seeds were germinated on soilroite and grown for 11 days. 1 % commercial Roundup (Monsanto) was sprayed on the transgenic lines (E1 and E2) along with untransformed control plant and photograph was taken 1 week after spray
mCP4-EPSPS enzyme assay
The enzyme assay was performed to analyze the activity of mCP4-EPSPS enzyme in the transgenic lines and untransformed control plants. The assay was based on the amount of inorganic phosphate released during the formation of 5-enolpyruvyl shikimate-3-phosphate from phosphoenol pyruvate and shikimate-3-phosphate. The inorganic phosphate was measured using malachite green dye-based method and after completion of reaction the optical density was measured. For the assay, the crude protein enzyme extract was prepared from the lines E1 and E2 along with untransformed control plant. The enzyme assay was performed with varying concentrations of glyphosate (inhibitor of reaction) viz., 0–10 mM for each of the crude enzyme sample and the respective optical density for each of the sample was measured and plotted on graph (Fig. 5b). As expected, it was found that in the case of untransformed control plant as the glyphosate concentration increases, the rate of inhibition of EPSPS also increased and at 6 mM glyphosate concentration enzyme activity was completely inhibited. While, in the case of transgenic line E2, the enzyme showed complete inhibition at 10 mM concentration, the line E1 was found to be highly tolerant to glyphosate compared to E2 and even at 10 mM glyphosate concentration the enzyme was not inhibited. The observation shows that the line E1 had highest level of glyphosate tolerance when compared to that of E2. This is in line with the observations of mRNA expression analysis carried out by Northern analysis where line E1 showed higher expression of the transgene. Taken together, the activity of the enzyme showed strict co-relation to the expression of the transgene.
Glyphosate tolerance analysis of the T 2 transgenic plants
The transgenic plants of lines E1 and E2 along with untransformed control plants were grown in a tray under controlled conditions for 11 days. All the individual transgenic plants were analyzed by PCR to determine if they were transgenic or null segregants and further, only transgenic plants were used for further analysis (data not shown). All the plants were then sprayed with 1 % glyphosate. After 7 days, the transgenic plants were found to be healthy when compared to non-transgenic plants which died due to senescence (Fig. 5c, d).
The glyphosate (1 %) solution used in this study for spraying is equivalent to 10 g−l or 10,000 ppm or 10 mM (175 ml), which is remarkably high as compared to other reports (Charng et al. 2008; Zhao et al. 2011; Chandrasekhar et al. 2014). Charng et al. (2008) used 5,000 ppm glyphosate for spraying on rice seedlings generated through insertion of modified rice EPSPS gene, which was sufficient to kill the non-transgenic seedlings, as with same dose the transgenic plants were found healthy, green and alive. In another study, Zhao et al. (2011) developed transgenic rice expressing G6 gene from Pseudomonas putida encoding EPSPS, which showed the survival of transgenic rice after spraying of glyphosate at the rate of 8 g−l whereas the non-transgenic control plants died. Apart from this, Chandrasekhar et al. (2014) developed transgenic rice through modified rice EPSPS and ASAL and observed that, when the transgenic plants (along with control plants) grown in 4 mM glyphosate solution for 24 h and subsequently transferred to normal Hoagland solution, remained healthy but non-transgenic control plants senesced and later died. We have generated the mCP4-EPSPS expressing transgenic rice which can tolerate 1 % glyphosate concentration, five times more than the dose used to kill weeds under field condition. In the present study, the use of 1 % glyphosate solution for spraying proves the higher tolerance of rice against glyphosate herbicide due to high expression of codon-optimized mCP4-EPSPS gene driven by Zea mays Ubiqutin1 promoter.
Southern hybridization analysis of the T 2 transgenic plants
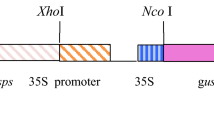
The Northern analysis, enzyme assay and herbicide bioassay revealed the effectiveness of the line E1 compared to E2. Hence, transgenic line E1 was chosen for T-DNA insertion analysis and carried forward. The genomic DNA was isolated from two representative plants of the line E1. The DNA was digested with EcoRI and Southern hybridization analysis was performed with 1.3 kb fragment of mCP4-EPSPS probe to confirm the integration of complete T-DNA and segregation of the gene in the subsequent generation. Upon integration of the complete T-DNA, junction fragments longer than 4.3 kb are expected to hybridize with mCP4-EPSPS (flanking left border [LB]) (Fig. 6a). For right border (RB) junction fragment analysis, the genomic DNA was digested with HindIII and was expected to hybridize with 1 kb fragment of uidA gene generating larger than 3.2 kb long fragment (Fig. 6b). Thus, Southern hybridization analysis with the LB and RB probes enabled us to identify the stable integration and segregation of complete T-DNA in T 2 generation, showing the single copy of the transgene in line E1. Furthermore, there was no yield penalty or any other aberrations of phenotype observed in the transgenic plants as compared with non-transgenic control plants. This was also proved by the measurement of several yield parameters in the T 1 transgenic rice plants of both the lines E1 and E2 (Table 2).
Southern hybridization analysis of T 2 rice plants. a Genomic DNA (10 μg) of untransformed control plant and transgenics were digested with EcoRI and probed with 1.3 kb fragment of mCP4-EPSPS for left border (LB) junction fragment analysis (>4.3 kb). b For right border (RB) junction fragment analysis (>3.2 kb) genomic DNA was digested with HindIII and probed with 1 kb fragment of uidA. UC untransformed control plant, E1 transgenic plant, PC linearized binary plasmid control digested with EcoRI (Fig. 6a) and HindIII (Fig. 6b). M λ DNA/HindIII ladder (Thermo Scientific). c T-DNA region of the binary vector pCAMBIA1301 harboring codon-optimized mCP4-EPSPS under maize ubiquitin promoter and 35S terminator. The RB and LB junction fragment of the T-DNA is >4.3 kb (the distance between EcoRI and LB) and >3.2 kb (the distance between HindIII and RB) with mCP4-EPSPS and uidA probe, respectively
Our results demonstrate the efficient performance of the transgenic plants in the presence of high levels of glyphosate. The gene not only has the potential to be utilized in the development of commercially viable transgenics, but also can be used as an effective selectable marker in the binary vectors.
To meet an ever growing demand for food to feed the increasing world population, there is an urgent need to increase the crop production with the help of new technologies without adversely affecting the environment or human health. In such a scenario, herbicide tolerant crops with newer genes will complement the existing herbicide technologies and thus be a principle component of integrated weed management ultimately leading to enhanced crop yield ensuring food security.
Author contribution statement
PAK, MLVP, SC and SR conceived and designed the experiments. SC and GP performed the experiments. SC, VKS and GD analyzed the data. SC and SR wrote the manuscript. GP, RC and VKS contributed reagents/materials/analysis tools. SC, RS and PAK read and corrected the manuscript. SC, RS and PAK approved the final manuscript. All authors read and approved the manuscript.
References
Berry G, Kishore G, Padgette S, Taylor M, Kolacz K, Weldon M, Re D, Eichholtz D, Fincher D, Hallas L (1992) Inhibitors of amino acid biosynthesis: strategies for imparting glyphosate tolerance to crop plants. In: Sinch BK, Flores HE, Shannon JC (eds) Biosynthesis and molecular regulation of amino acids in plants. American Society of Plant Physiologists, Rockville, pp 139–145
Bouman BAM, Lampayan RM, Tuong TP (2007) Water management in irrigated rice: coping with water scarcity. International Rice Research Institute, Los Baños
Carpenter JE (2010) Peer-reviewed surveys indicate positive impact of commercialized GM crops. Nat Biotechnol 28:319–321
Chandrasekhar K, Reddy GM, Singh J, Vani K, Vijayalakshmi M, Kaul T, Reddy MK (2014) Development of transgenic rice harbouring mutated rice 5-enolpyruvylshikimate 3-phosphate synthase (Os-mEPSPS) and Allium sativum Leaf Agglutinin (ASAL) genes conferring tolerance to herbicides and sap-sucking insects. Plant Mol Biol Rep 32:1146–1157
Charng YC, Li KT, Tai HK, Lin NS, Tu J (2008) An inducible transposon system to terminate the function of a selectable marker in transgenic plants. Mol Breeding 21:359–368
Comai LD, Facciotti D, Hiatt WR, Thompson G, Rose RE, Stalker DM (1985) Expression in plants of a mutant aroA gene from Salmonella typhimurium confers tolerance to glyphosate. Nature 317:741–744
De Rocher EJ, Vargo-Gogola TC, Diehn SH, Green PJ (1998) Direct evidence for rapid degradation of Bacillus thuringiensis toxin mRNA as a cause of poor expression in plants. Plant Physiol 117:1445–1461
Della-Cioppa G, Bauer SC, Klein BK, Shah DM, Fraley RT, Kishore GM (1986) Translocation of the precursor of 5-enolpyruvylshikimate 3-phosphate synthase into chloroplasts of higher plants in vitro. Proc Natl Acad Sci USA 83:6873–6877
Depicker A, Van Montagu M (1997) Post-transcriptional gene silencing in plants. Curr Opin Cell Biol 9:373–382
Farooq M, Siddique KHM, Rehman H, Aziz T, Lee D, Wahid A (2011) Rice direct seeding: experiences, challenges and opportunities. Soil Tillage Res 111:87–98
Funke T, Han H, Healy-Fried ML, Fischer M, Schönbrunn E (2006) Molecular basis for the herbicide resistance of roundup ready crops. Proc Natl Acad Sci USA 103:13010–13015
Gamborg OL, Murashige T, Thorpe TA, Vasil IK (1976) Plant tissue culture media. In Vitr Cell Dev Biol Plant 12:473–478
Hiei Y, Komari T (2008) Agrobacterium-mediated transformation of rice using immature embryos or calli induced from mature seed. Nat Protoc 3:824–834
Hobbs SLA, Warketin TD, Delong CMO (1993) Transgene copy number can be positively or negatively associated with transgene expression. Plant Mol Biol 21:17–26
Hofgen R, Willmitzer L (1988) Storage of competent cells for Agrobacterium transformation. Nucl Acids Res 16:9877
IRRI (International Rice Research Institute) (2006) Direct seeded rice: a low cost establishment technology. IRRI Rice factsheet. Available at http://www.narc.org.np/rice_knowledge_bank/factsheet/rice.pdf
James C (2013) Global status of commercialized biotech, GM crops: 2013. ISAAA Brief No 35. International Service for the Acquisition of Agri-Biotech Applications, Ithaca
Jorgensen RA, Cluster PD, English J, Que Q, Napoli CA (1996) Chalcone synthase co suppression phenotypes in petunia flowers: comparison of sense vs. antisense constructs and single-copy vs. complex T-DNA sequences. Plant Mol Biol 31:957–973
Joshi E, Kumar D, Lal B, Nepalia V, Gautam P, Vyas AK (2013) Management of direct seeded rice for enhanced resource-use efficiency. Plant Knowledge Journal 2:119–134
Kahrizi D, Salmanian AH, Afshari A, Moieni A, Mousavi A (2007) Simultaneous substitution of Gly96 to Ala and Ala183 to Thr in 5-enolpyruvylshikimate-3-phosphate synthase gene of E. coli (k12) and transformation of rapeseed (Brassica napus L.) in order to make tolerance to glyphosate. Plant Cell Rep 26:95–104
Kishore GM, Brundage L, Kolk K, Padgette SR, Rochester D, Huynh QK, Della-Cioppa G (1986) Isolation, purification and characterization of a glyphosate tolerant mutant E. coli EPSP synthase. Proc Fed Am Soc Exp Biol 45:1506
Kishore GM, Padgette SR, Fraley RT (1992) History of herbicide tolerant crops, methods of development and current state of the art-emphasis on glyphosate tolerance. Weed Technol 6:626–634
Kusnadi AR, Nikolov ZL, Howard JA (1997) Production of recombinant proteins in transgenic plants: practical considerations. Biotechnol Bioeng 56:473–484
Lanzetta PA, Alvarez LJ, Reinach PS, Candia OA (1979) An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem 100:95–97
Maqbool SB, Christou P (1999) Multiple traits of agronomic importance in transgenic indica rice plants: analysis of transgene integration patterns, expression levels and stability. Mol Breeding 5:471–480
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant 15:473–497
Murray EE, Lotzer J, Eberle M (1989) Codon usage in plant genes. Nucleic Acids Res 17:477–498
Murray EE, Rocheleau T, Eberle M, Stock C, Sekar V, Adang M (1991) Analysis of unstable RNA transcripts of insecticidal crystal protein genes of Bacillus thuringiensis in transgenic plants and electroporated protoplasts. Plant Mol Biol 16:1035–1050
Padgette SR, Re DB, Gasser CS, Eichholtz DA, Frazier RB, Hironaka CM, Levine EB, Shah DM, Fraley RT, Kishore GM (1991) Site-directed mutagenesis of a conserved region of the 5-enolpyruvylshikimic acid-3-phosphate synthase active site. J Biol Chem 266:22361–22369
Padgette SR, Re DB, Berry GF, Eichholtz DE, Delannay X, Fuchs RL, Kishore GM, Fraley RT (1996) New weed control opportunities: development of soybeans with a Roundup Ready gene. In: Duke SO (ed) Herbicide-resistant crops: agricultural, economic, environmental, regulatory and technological aspects. CRC Press, Boca Raton, pp 53–84
Perlak FJ, Fuchs RL, Dean DA, McPherson SL, Fischhoff DA (1991) Modification of the coding sequence enhances plant expression of insect control genes. Proc Natl Acad Sci USA 88:3324–3328
Porebski S, Bailey G, Baum BR (1997) Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol Biol Rep 15:8–15
Quinn JP (1990) Evolving strategies for the genetic engineering of herbicide resistance in plants. Biotechnol Adv 8:321–333
Ray DK, Mueller ND, West PC, Foley JA (2013) Yield trends are insufficient to double total crop production by 2050. PLoS ONE. doi:10.1371/journal.pone.0066428
Reddy MS, Dinkins RD, Collins GB (2003) Gene silencing in transgenic soybean plants transformed via particle bombardment. Plant Cell Rep 21:676–683
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Singh S, Singh G, Singh VP, Singh AP (2005) Effect of establishment methods and weed management practices on weeds and rice in rice-wheat cropping system. Indian J Weed Sci 37:51–57
Stalker DM, Hiatt WR, Comai LD (1985) A single amino acid substitution in the enzyme 5-enolpyruvylshikimic acid-3-phosphate synthase confers resistance to herbicide glyphosate. J Biol Chem 260:4724–4728
Steinrücken HC, Amrhein N (1980) The herbicide glyphosate is a potent inhibitor of 5-enolpyruvyl-shikimic acid-3-phosphate synthase. Biochem Biophys Res Commun 94:1207–1212
Wada Y, Van Beek LPH, Van Kempen CM, Reckman JWTM, Vasak S, Bierkens MFP (2010) Global depletion of groundwater resources. Geophys Res Lett 37:L20402
Ye GN, Hajdukiewicz PT, Broyles D, Rodriguez D, Xu CW, Nehra N, Staub JM (2001) Plastid-expressed 5-enolpyruvylshikimate-3-phosphate synthase genes provide high level glyphosate tolerance in tobacco. Plant J 25:261–270
Zhao T, Lin CH, Shen ZC (2011) Development of transgenic glyphosate resistant rice with G6 gene encoding 5-enolpyruvylshikimate-3-phosphate synthase. Agr Sci China 10(9):1307–1312
Zhou H, Arrowsmith JW, Fromm ME, Hironaka CM, Taylor ML, Rodriguez D, Pajeau ME, Brown SM, Santino CG, Fry JE (1995) Glyphosate-tolerant CP4 and GOX genes as a selectable marker in wheat transformation. Plant Cell Rep 15:159–163
Zhou M, Xu H, Wei X, Ye Z, Wei L, Gong W, Wang Y, Zhu Z (2006) Identification of a glyphosate-resistant mutant of rice 5-enolpyruvylshikimate 3-phosphate synthase using a directed evolution strategy. Plant Physiol 140:184–195
Acknowledgments
We are grateful to Nuziveedu Seeds Limited (NSL), Hyderabad for providing financial assistance. We acknowledge the help of Dr Vikrant Nain for codon optimization of mCP4-EPSPS gene. We are also thankful to our colleagues Hari Kishore CM and Shruti Yadava for help.
Conflict of interest
The authors do not have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Manoj Prasad.
Electronic supplementary material
Below is the link to the electronic supplementary material.

299_2014_1732_MOESM1_ESM.jpg
Supplementary Fig. 1: mCP4-EPSPS gene-specific PCR analysis of putative T 0 transgenic rice plant. M: 1-kb ladder, W: Water control; UC: Untransformed control plant; Lanes 1-15: Putative transgenic plants; PC: Binary plasmid control. (JPEG 13 kb)

299_2014_1732_MOESM2_ESM.jpg
Supplementary Fig. 2: Immunostrip analysis of T 0 CP4-EPSPS transgenic rice. UC; Untransformed control plant, Lane 1 to 15; T 0 putative transgenic plants (JPEG 44 kb)
Rights and permissions
About this article
Cite this article
Chhapekar, S., Raghavendrarao, S., Pavan, G. et al. Transgenic rice expressing a codon-modified synthetic CP4-EPSPS confers tolerance to broad-spectrum herbicide, glyphosate. Plant Cell Rep 34, 721–731 (2015). https://doi.org/10.1007/s00299-014-1732-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-014-1732-2