Abstract
Sedentary plant endoparasitic nematodes can cause detrimental yield losses in crop plants making the study of detailed cellular, molecular, and whole plant responses to them a subject of importance. In response to invading nematodes and nematode-secreted effectors, plant susceptibility/resistance is mainly determined by the coordination of different signaling pathways including specific plant resistance genes or proteins, plant hormone synthesis and signaling pathways, as well as reactive oxygen signals that are generated in response to nematode attack. Crosstalk between various nematode resistance-related elements can be seen as an integrated signaling network regulated by transcription factors and small RNAs at the transcriptional, posttranscriptional, and/or translational levels. Ultimately, the outcome of this highly controlled signaling network determines the host plant susceptibility/resistance to nematodes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants are often exposed to biotic stresses derived from viruses, bacteria, fungi, nematodes, and insects. Interactions between host plants and their pathogens determine the degree of pathogenesis observed. Successful pathogens attach to a host plant, penetrate through the physical barriers of the cell wall, and override host plant defenses. Once inside the plant, pathogens can either kill plant cells (necrotrophic pathogens), or live within host tissues without causing plant cell death (biotrophic pathogens). In response, resistant plants have evolved the ability to recognize pathogens and make timely defensive responses.
The interactions between plants and pathogens are summarized as a so called ‘zigzag’ model where plants are able to recognize pathogen-associated molecular patterns (PAMPs) derived from the pathogen utilizing pattern recognition receptors (PRRs) leading to pattern-triggered immunity (PTI) (Jones and Dangl 2006). PAMPs are invariant epitopes derived from pathogens that are: fundamental to the fitness of pathogens, absent in the host, and recognized by a wide array of potential hosts (He et al. 2007; Schwessinger and Zipfel 2008). PRRs are cell-surface-localized receptors, usually harboring an extracellular leucine-rich repeat (LRR) domain, that recognize conserved pathogen elements (Schwessinger and Zipfel 2008).
Pathogens that successfully suppress PTI responses can release pathogenic effectors into host plants, altering host-cell structures and suppressing defense responses leading to effector-triggered susceptibility (Jones and Dangl 2006). Specific resistance proteins (R-proteins) have also evolved in response to such effectors, to sense pathogen effectors yielding effector-triggered immunity (ETI) (Jones and Dangl 2006).
Canonical R-proteins are intracellular and often referred to as NBS-LRR proteins because they typically contain nucleotide-binding site (NBS) and leucine-rich repeat (LRR) domains (Hogenhout et al. 2009; Shirasu 2009). While the LRR domain is believed to be responsible for interaction between plant receptors and pathogen effectors, the NBS domain is characterized by NTPase activity and functions as a molecular switch activating subsequent downstream signal transduction during contact with pathogen-derived effectors (Glowacki et al. 2011). R-proteins can directly sense pathogen effectors, or they can detect pathogens through other cofactors, which are direct host targets of pathogens (Glowacki et al. 2011).
Sedentary plant endoparasitic nematodes (SPENs) are biotrophic pathogens that can cause significant yield loss in crop plants. The most well studied and crop-impactful SPENs are root-knot nematodes (RKN, Meloidogyne spp.) and cyst nematodes (CN, Globodera and Heterodera spp.), while reniform nematodes (RN, Rotylenchulus reniformis Linford & Oliveira.), a kind of plant semi-endoparasite, are also known to affect important crops such as cotton and soybean (Robinson 2007).
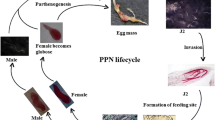
The life cycle of the different SPENs, i.e. RKN, CN, and RN, typically requires around 3 weeks under favorable environmental conditions of optimal soil moisture and temperatures. Eggs generated by female nematodes are deposited into a gelatinous matrix on the host root surface, which protects eggs from dehydration (Williamson and Gleason 2003). There are four juvenile stages, separated by molts, needed for eggs to mature into adults. While the second-stage juvenile of RKN and CN penetrates into the host plant root, for RN it is the female young adult that is the infective stage.
Once inside the host plant, successful nematode parasitism is contingent upon establishment of a nematode feeding site (NFS), which serves as the sole nutrient source on which the nematode lives. The NFS are hypertrophied, multinucleate root cells with enlarged nuclei and dense cytoplasm, which resulted from nuclear division without cytokinesis of infected host cells (giant cells, in the case of RKN) (Williamson 1999), or break down of cell walls between initial feeding cells and neighboring cells (syncytia, in the case of CN and RN) (Williamson and Gleason 2003; Robinson 2007). These sedentary endoparasites ingest nutrient from the NFS through their stylet, a specialized hollow needle-like structure mouthpart. Female nematodes feeding on the host root enlarge and start to produce eggs.
Nematode effectors secreted through the stylet are essential in NFS initiation and maintenance. These effector proteins act as PAMPs or pathogenic effectors aiding nematode parasitism, conditioning host defense responses, and/or modifying host plant physiology (Hewezi and Baum 2013; Mitchum et al. 2013). These nematode effectors are directly secreted into the cytoplasm of host cells to interact with components of the cell cycle, cytoskeleton, and cellular metabolism, or alternatively, they accumulate in host extracellular spaces to degrade plant cell walls and change cell wall architecture (Mitchum et al. 2013).
In this review, signaling and signal transduction involved in plant general biotic defense mechanisms will be reviewed for plant susceptibility/resistance (S/R) to SPENs, and the nature of the integrated signaling network that determines plant responses to nematodes will be defined. Specifically, different R-genes mediating nematode resistance and their upstream signaling will be reviewed. The roles of different classes of phytohormones, their synthesis and signaling, and the role(s) of reactive oxygen and nitrogen species (ROS and RNS) generation and signaling in plant responses to SPENs will be reviewed. The emerging and integrative regulatory role of small RNAs in plant S/R to SPENs will be considered then in summary.
R-proteins and their upstream signaling in plant resistance to SPENs
Initial sensing of nematode infestation can occur either extra- or intracellularly and typically involves interaction with an R-protein receptor (typically identified as NBS-LRR proteins) (Fig. 1). Many distinct loci involved in initiating resistance responses to SPENs have been mapped to plant genomes (Table S1). The first cloned locus was Hs1 pro−1, predicted to encode an extracellular leucine-rich region containing protein. This was followed by the cloning of a series of other R-genes including: Mi-1, Gpa2, Gro1-4, “Hero A”, CaMi, and Ma, that have all been shown to encode canonical intracellular NBS-LRR type R-protein receptors (Table 1).
The integrated signaling network in plant responses to SPENs Sedentary endoparasitic nematodes attack host plants and secrete various effectors functioning as PAMPs or pathogenic effectors. Upon recognition of invading nematodes with plant transmembrane extracellular R proteins or intracellular NBS-LRR proteins, ROS and various hormone-signaling pathways are activated. Different transcription factors and small RNAs regulate plant defense related factors at transcriptional, post-transcriptional, and/or translational levels leading to plant S/R to nematodes
These cloned NBS-LRR proteins can also be further classified as TIR-NBS-LRR or CC-NBS-LRR based on their amino-terminus motif (Table 1). Both TIR (toll-interleukin receptor) and CC (coiled-coil) domains are thought to be crucial in the signal transduction of innate immunity (Glowacki et al. 2011). Further investigating how different domains contribute to pathogen sensing and signal transduction will be helpful to understand R-gene-mediated signaling in nematode resistance. Specifically, a WRKY-like domain on the carboxyl terminus of the Ma gene encoded protein suggests a direct role of the Ma protein in downstream regulation of defense gene expression and plant immunity (Table 1). More discussion and comparison of these R-protein structures can be found in detailed reviews (Fuller et al. 2008; Goverse and Smant 2013; Williamson and Kumar 2006).
Rhg1 and Rhg4 are two unlinked quantitative trait loci (QTLs) in soybean that appear to condition S/R to SCN and are unique when compared to the canonical R-gene loci mentioned above (Hauge et al. 2001). Since the discovery of these QTLs it has been hypothesized that extracellular LRR kinase-type R-proteins found in the coding regions of these QTLs conditioned resistance to SCN (Hauge et al. 2001).
However, transgenic soybean plants with over-expressed or silenced LRR-kinase genes from the Rhg1 locus showed little change in S/R to SCN (Melito et al. 2010), contradicting the original disclosure. Subsequently, it was shown that Rhg1-conditioned SCN resistance was determined by three genes encoding an amino acid transporter, an α-SNAP protein, and a wound-inducible domain protein (Cook et al. 2012). The copy number of a 31 kb repeat sequence containing these 3 genes appears to determine SCN resistance: multiple copies of the repeat produced resistance, while a single copy produced a susceptible phenotype (Cook et al. 2012). While, the precise role of the 31 kb gene repeat remains unclear at this time, it appears that the Rhg1 locus plays a regulatory role that may involve the expression of some type of as yet undefined R-gene. In addition, differentially methylated regions within Rhg1 correlated with SCN resistance. This fact along with the observation of copy number of the 31 kb repeat-region mentioned above suggest the possibility that some type of epigenetically mediated phenomenon may play a role in Rhg1-mediated host plant resistance (Cook et al. 2014) the details of which remain to be elucidated.
Rhg4-mediated SCN resistance or susceptibility is linked to two nucleotide polymorphisms in a single copy gene encoding a serine hydroxymethyltransferase (SHMT) (Liu et al. 2012) in contradiction to the earlier report (Hauge et al. 2001) that the LRR-gene near the Rhg4 QTL locus conditioned SCN resistance. SHMT may affect plant S/R to nematodes through regulation of folate one-carbon metabolism, since folate deficiency may cause parasitizing nematode death and degradation of nematode induced syncytia.
Thus, none of the gene products from Rhg1 and Rhg4 resemble either extracellular or intracellular canonical R-proteins, and further work is required to elucidate the detailed roles of Rhg1 and Rhg4 in SCN resistance.
The best-studied example of an R-protein involvement in nematode resistance is tomato Mi-1. RKN resistance mediated by the Mi-1 gene is dependent on pathogen recognition and resistance signal transmission mediated by an LRR domain internal to the Mi-1 protein (Hwang et al. 2000; Hwang and Williamson 2003) and by an ATPase activity associated with its NBS domain (Tameling et al. 2002). Subsequently, it has been shown that the extended N-terminus of the Mi-1 protein has both negative and positive regulatory roles in the activation of Mi-1 protein (Lukasik-Shreepaathy et al. 2012).
Upstream of Mi-1, a gene product of the Rme1 locus is required for RKN resistance, although the Rme1 sequence has not been cloned as yet, and thus its exact biological function is unknown (Martinez de Ilarduya et al. 2004). In tomato, orthologs of Arabidopsis HSP90-1 and SGT1 were also required for Mi-1-mediated RKN resistance, as demonstrated by virus-induced gene silencing (Bhattarai et al. 2007). Based on a proposed model of R-protein-mediated signaling (Glowacki et al. 2011), the Mi-1 encoded NBS-LRR protein, an HSP90-1 protein, and an SGT1 protein form an R-protein signaling complex that can activate downstream signaling pathways by detection of nematode effector-induced conformational changes in the protein coded by the Rme1 gene that may be an interacting partner of the Mi-1 protein as well as the direct target of nematode effectors (Bhattarai et al. 2007). Similarly, PCN resistance mediated by Gpa2 also requires the RAN GTP activating protein 2 that functions as a cofactor for Gpa2 (Sacco et al. 2009).
As pathogen receptors, NBS-LRR proteins detect pathogen effectors and trigger ETI type resistance characterized by hypersensitive responses (HR). In accordance, several nematode effectors that may interact with plant NBS-LRR proteins that are coupled with HR have been identified. These include a PCN SPRY domain-containing protein, RBP-1, that interacts with the Gpa2 protein (Sacco et al. 2009), an RKN protein coded by the MAP-1 gene that may interact with the tomato Mi-1 protein (Semblat et al. 2001), and another RKN encoded protein product of the Cg-1 gene that may interact with Mi-1 protein (Gleason et al. 2008). It also could be concluded that there are other unknown nematode effectors interacting with host plant R-proteins, because HR type cell death is frequently identified in plant NBS-LRR genes mediating nematode resistance (Chen et al. 2007; Khallouk et al. 2011; Sacco et al. 2009; Sobczak et al. 2005; Williamson 1999).
Moreover, recent findings involving small regulatory RNAs (sRNAs) add a new layer of complexity to the regulation of NBS-LRR genes during plant defense responses (Li et al. 2012a, b; Shivaprasad et al. 2012; Zhai et al. 2011), which is discussed in greater detail below in consideration of the role of sRNAs in nematode pathogenesis.
Hormone signaling in plant S/R to SPENs
Downstream of PTI and ETI activation, plant hormone signaling induces or suppresses defense responses to nematodes through regulation of different transcription factors (Fig. 1). Salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) function in plant stress responses (Table 2). Hormones are also involved in plant growth and development related to nematode infection, such as Auxin (AX) and Cytokinin (CK). AX plays an essential role in modulating plant cell morphology regulating NFS development (Table 2). CK also function as modulators in NFS development (Table 2).
Salicylic acid (SA)
SA signaling is required in R-gene mediated defense responses to SPENs (Branch et al. 2004; Kandoth et al. 2011; Uehara et al. 2010). Specifically, SA signaling and response genes were strongly induced in tomato plants harboring the Mi-1 R-gene (Molinari et al. 2013), the “Hero A” gene (Uehara et al. 2010), and the resistance QTL, Rhg1 (Kandoth et al. 2011). Transgenic tomato plants containing dominant Mi-1 or “Hero A” alleles expressing NahG (encoding SA hydroxylase) exhibited reduced resistance to RKN or CN, respectively (Branch et al. 2004; Uehara et al. 2010). Furthermore, the SA analog benzothiadiazole (BTH), completely restored RKN resistance in NahG transformed tomato roots harboring an active Mi-1 gene, but resistance was not established in susceptible plants lacking a functional Mi-1 gene (Branch et al. 2004).
Downstream of SA signaling, Arabidopsis WRKY70 is required for both basal defense and R-gene mediated resistance (Eulgem and Somssich 2007). In tomato plants containing Mi-1 alleles, orthologs of Arabidopsis WRKY70 were induced after exogenous application of SA. Similarly, attenuated Mi-1-mediated resistance against RKN was observed when tomato WRKY70 was silenced (Bhattarai et al. 2010). However, another WRKY transcription factor, WRKY72, was found to control Mi-1 mediated defense responses and basal resistance to RKN, independent of SA signaling (Atamian et al. 2012). While different WRKYs are differentially regulated in roots after nematode infestation (Barcala et al. 2010; Klink et al. 2007; Portillo et al. 2013; Uehara et al. 2010), their specific roles remain elusive. Yet, it is known that WRKY transcription factors regulate the expression of defense related genes in both PTI and ETI (Eulgem and Somssich 2007). Specifically, the down regulation of WRKY6, WRKY11, WRKY17, and WRKY33 in Arabidopsis roots in response to BCN infestation has been demonstrated to favor nematode and NFS development (Ali et al. 2014).
In addition to R-gene-mediated resistance, genes involved in SA biosynthesis, SA signaling, and SA responses also contribute to nematode basal resistance (Table 2). The requirement of endogenous SA accumulation for resistance to SCN was demonstrated in Arabidopsis iso-chorismate synthase gene (ICS) mutants and NahG transgenic lines where each demonstrates increased SCN susceptibility (Wubben et al. 2008). ICS is a key enzyme in the SA biosynthesis pathway (Vlot et al. 2009). Higher levels of ICS expression were detected in SAMT-overexpressing (SA methyltransferase) soybean roots, where these plants exhibited resistance to SCN (Lin et al. 2013). SAMT modulates SA levels by converting SA to methyl salicylic acid (MeSA) (Vlot et al. 2009), and MeSA can function as a mobile signal, mediating systemic acquired resistance (SAR) in some plants (Park et al. 2007).
The Atpad4 mutant at the PHYTOALEXIN DEFICIENT locus which is involved in SA signaling showed increased SCN susceptibility; overexpressing wild type AtPAD4 in soybean showed increased resistance to RKN (Wubben et al. 2008; Youssef et al. 2013). PAD4 acts upstream of SA in pathogen responses via interaction with ENHANCED DISEASE SUSCEPTIBILITY 1 (EDS1) that has a similar sequence to PAD4 (Vlot et al. 2009). In soybean roots, EDS1 transcript levels were induced after infestation by both compatible and incompatible populations of SCN (Klink et al. 2007). These findings together demonstrated that SA-upstream signaling is required in nematode resistance.
Signaling downstream of SA is largely regulated via the NON-EXPRESSOR of PATHOGENESIS RELATED 1 gene (NPR1) (Vlot et al. 2009) recently identified as an SA receptor (Wu et al. 2012). In the nucleus, NPR1 interacts with TGA transcription factors which can bind to a cis-element required for SA responsiveness (Vlot et al. 2009). Arabidopsis NPR1-deficient mutants showed increased susceptibility to SCN, and SUPPRESSOR OF npr1-1 INDUCIBLE (SNI1) deficient mutants exhibited increased resistance to SCN (Wubben et al. 2008). Similarly, transgenic expression of AtNPR1 conferred resistance to RN, RKN, and SCN in cotton, tobacco, and soybean, respectively (Parkhi et al. 2010; Priya et al. 2011). The same effect of SCN parasitism suppression was also observed in soybean root over expressing AtTGA2 (Matthews et al. 2014).
The best-studied SA responsive defense gene is PR-1 (pathogenesis related 1) although induction of PR-2 and PR-5 are also used as indicators of SA-mediated activation in resistance responses (Vlot et al. 2009). Suppression of PR-1, PR-2, and PR-5 gene expression in 10A06 SCN effector gene transformed Arabidopsis increased susceptibility to SCN (Hewezi et al. 2010). The disruption of SA-responsive defenses may be critical in at least SCN parasitism. Consistent with these findings, the suppression of PR-1 and PR-5 in roots where an NFS was successfully established and decrease of SCN parasitism in AtPR-5 over-expressed soybean roots, support the SA-responsive defenses in nematode resistance (Barcala et al. 2010; Hewezi et al. 2010; Matthews et al. 2014; Portillo et al. 2013).
In contrast, PR-1 was highly induced in the RKN more susceptible lox3 mutant of maize (Gao et al. 2008). LOX3 encodes a 9-lipoxygenase that oxidizes fatty acids to oxylipins, including JA (Mosblech et al. 2009) and lox3 mutant also shows increased levels of JA and ET responsive and biosynthetic genes (Gao et al. 2008). Exogenous foliar application of the SA analog, BTH in rice only slightly induced RKN resistance compared to the MeJA or the ET-generating compound ethephon (Nahar et al. 2011). Thus, while SA plays a critical role in nematode S/R there are other hormones and interacting signaling pathways involved in plant responses to nematode infestation.
Jasmonic acid (JA)
Blocking JA perception via the COI receptor in Mi-1 resistant tomato plants did not compromise resistance to RKN (Bhattarai et al. 2008; Mantelin et al. 2013). However, negative crosstalk between the JA- and SA-signaling pathways in Mi-1-mediated resistance was consistent with the fact that SA-induced WRKY70 was suppressed after treatment with the JA derivative MeJA (Atamian et al. 2012).
JA signaling, unlike SA production and signaling appears to be required for susceptibility to SPENs, since it was shown that a mutant JA receptor (coi-1) led to significantly lower numbers of RKN egg masses on RKN-susceptible tomato roots (Bhattarai et al. 2008). In addition, JA biosynthesis increased in nematode susceptible tomato genotypes (Bhattarai et al. 2008; Gao et al. 2008; Ozalvo et al. 2013).
Induction of JA biosynthesis genes (Table 2) in the Arabidopsis lox4 mutant makes plants more susceptible to RKN infestation implicating a link between JA accumulation and nematode susceptibility (Ozalvo et al. 2013). A similar result was found in the RKN susceptible lox3 maize mutant where JA biosynthesis genes (Table 2) were also induced (Gao et al. 2008). Both LOX4 and ZmLOX3 are induced in response to RKN infestation (Gao et al. 2008; Ozalvo et al. 2013). Taken together, it appears that JA biosynthesis may play a positive role in plant susceptibility to nematode (Gao et al. 2008; Ozalvo et al. 2013).
Direct application of JA induces RKN resistance responses in tomato (Cooper et al. 2005) in a dose dependent manner (Fujimoto et al. 2011), and some studies have shown that protease inhibitors may be down-stream regulators of JA-induced nematode resistance. High expression of a multicystatin-type gene and protease inhibitor encoding genes in tomato roots was observed when RKN infection was repressed (Fujimoto et al. 2011). Similarly, JA-responsive protease inhibitor (Pin2) and γ–thionin-coding genes were repressed in tomato plants overexpressing the Mj-FAR-1 gene (Iberkleid et al. 2013). Mj-FAR-1 is a member of RKN-specific fatty acid and retinol binding family protein, and Mj-FAR-1 may play a positive role in plant susceptibility to RKN (Iberkleid et al. 2013).
JA and ET appear to play a greater role in rice resistance to RKN than does SA. Both MeJA and ET treatment induce strong resistance to RKN correlated with strong induction of resistance genes (Nahar et al. 2011). Foliar treatment with JA or ET biosynthesis inhibitors increase rice susceptibility to RKN (Nahar et al. 2011), and genes involved in JA and ET biosynthesis and signaling were mainly suppressed in rice roots and shoots after RKN infestation (Kyndt et al. 2012). Furthermore, JA was found to be an indispensable signal in rice, mediating resistance to RKN, and ET-mediated RKN resistance is dependent on JA biosynthesis (Nahar et al. 2011). ET foliar treatment had no effect on the response to RKN infestation in rice mutants with impaired JA biosynthesis, but JA-induced defense was still functional when ET-signaling was impaired (Nahar et al. 2011).
Ethylene (ET)
ET signaling is not involved in Mi-1-mediated RKN resistance (Fujimoto et al. 2011), but ET and ET-signaling affect basal resistance to both RKN and CN (Table 2). ET-treated soybean roots exhibited increased SCN susceptibility (Tucker et al. 2010) while the inhibitors of ET action, 1-methycyclopropene (MCP) and 2,5-norbornadiene (NBD), reduced SCN colonization in soybean roots consistent with ET playing a positive role in nematode susceptibility. Arabidopsis ET-overproducing mutants (eto1, eto2, and eto3, Table 2) demonstrate hyper-susceptibility to CN (Wubben et al. 2001), but the same ET overproducing mutants showed reduced susceptibility (increased resistance) to RKN (Table 2, Fudali et al. 2013). Such opposing results have also been reported with ET signaling mutants in CN and RKN resistance (Table 2). For example, Arabidopsis ET receptor mutant (etr1) and ET signaling mutants (ein2 and ein3) showed decreased susceptibility to CN (Wubben et al. 2001); and a gene encoding UDP-glucose-4-epimerase that contributes to CN resistance was negatively regulated by the intermediate ET-signaling genes, EIN2 and EIN3 (Wubben et al. 2004). ET-insensitive mutants (etr1, ers2, ein4) and tomato (Nr) and mutant genes positively regulating ET signaling (ein2, ein3, ein5, and ein7) resulted in higher levels of RKN infestation, while the negatively regulating ET signaling mutant (ctr1) attracted fewer RKN (Table 2). Thus, it can be concluded that the ET biosynthesis and signaling pathways positively regulate susceptibility to CN, whereas they contribute to RKN resistance (Table 2) although the mechanistic basis of such opposing effects is unclear at this time.
Ethylene Response Factors (ERFs or EREBPs) that specifically bind to a GCC box cis-element sequences have been found in many PR-protein coding gene promoters (Wang et al. 2002). EREBP transcription factor was induced in soybean resistant reactions but suppressed in susceptible reactions to SCN (Mazarei et al. 2011). One soybean EREBP (GmEREBP1) appears to be involved in the induction of different classes of PR-genes in roots of both GmEREBP1-overexpressing soybean and Arabidopsis plants (Mazarei et al. 2002), although this transgenic overexpression did not confer increased resistance to SCN in Arabidopsis (Mazarei et al. 2007). In addition to ET-induced PR-protein coding genes GmPR2, GmPR3, and AtPDF1.2, SA-responsive PR-protein coding genes (AtPR1, GmPR1, and AtPR2) and JA-responsive PR-genes (GmPR3 and AtPDF1.2) were also induced in GmEREBP1 overexpressing plants (Mazarei et al. 2007).
These studies demonstrate widely varying roles of ET biosynthesis and signaling in S/R to SPENs that vary according to the specific nematode investigated. ET may have pleiotropic effects in plant resistance to nematodes because: (1) unique mechanisms are required for different nematode species in host attraction, as see where CN and RKN responded differently in ET biosynthesis and signaling mutants; (2) complex unknown crosstalk between ET and JA or SA signaling pathways occur during nematode infestation, as suggested by induction of different classes of PR proteins in GmEREBP1 transgenic plants. Thus, it is currently difficult to determine a precise role for ET in nematode infestation, but it is clear that ET does play important roles in specific nematode pathogenesis and possibly indirectly in resistance.
Auxin (AX)
AX insensitive mutants appear to be resistant to CN compared to their wild type counterparts (Goverse et al. 2000), and AX levels increase transiently in the expanding NFS and cells surrounding the NFS (Goverse et al. 2000; Karczmarek et al. 2004; Absmanner et al. 2013). In particular, AX responsive elements were found in the cis-element of NtCel7 gene (Wang et al. 2007). NtCel7, a tobacco endo-β-1,4-glucanase gene, functions in cell-wall degradation and is strongly induced in both RKN and CN feeding cells (Wang et al. 2007). Taken together, these studies suggest that a local and transient accumulation of AX in feeding cells upon nematode infection support NFS establishment and nematode parasitism.
Polar AX transport manipulates AX distribution in feeding cells during nematode infestation (Table 2). At the beginning stages of CN infection, AX accumulates in the infection site by induced LAX3/AUX1-mediated AX import and reduced PIN1-mediated AX export, whereas when a syncytium is expanding, PIN3 and PIN4 facilitate the lateral transport of AX to the cells surrounding the initial syncytium (Grunewald et al. 2009; Lee et al. 2011). Specifically, LAX3 was demonstrated to be a direct target of the nematode secreted protein Hs19C07 (Lee et al. 2011). Binding to Hs19C07 can activate LAX3, leading to subsequent syncytia development (Lee et al. 2011).
AX effects on nematode S/R are also mediated through AX response factors (ARF) by activating or repressing AX-responsive genes (Matthews et al. 2013). Members of the ARF gene family are distinctly and dynamically regulated in host Arabidopsis plants in response to BCN infestation compared to uninfected plants (Hewezi et al. 2014). AX accumulation and ARFs appear to play a transient role in NFS initiation and early development (Goverse et al. 2000; Karczmarek et al. 2004; Absmanner et al. 2013). However, continued high expression of ARFs in fully developed syncytia (Hewezi et al. 2014) and AX-responsive mature root galls supports a functional role of AX and ARFs in mature NFS as well (Cabrera et al. 2014). It should be noted that ARFs are targets of several microRNAs and small interfering RNAs (see sRNA regulation section below, and Table 3). The detailed role of such sRNA regulation of ARFs in plant responses to SPENs has not been extensively investigated to date, but given the role of ARFs in NFS growth and development this area is likely to yield significant information on plant/nematode interactions in the future.
Downstream of ARFs, the AX responsive gene LATERAL ORGAN BOUNDARIES-DOMAIN 16 (LBD16), which showed activation upon RKN infestation, was implicated in the induction of both root galls and lateral roots (Table 2, Cabrera et al. 2014). This study established the first molecular link between root gall induction and lateral root formation (Cabrera et al. 2014).
In contrast to the above studies that suggested a dependence of NFS initiation and morphogenesis on AX, AtWRKY23, which acts downstream of ARFs was induced in early syncytium development during BCN infection independent of AX (Grunewald et al. 2008). This result suggests the involvement of other pathways in the regulation of early plant responses to nematode infestation. AX is known to interact synergistically with ET in general, but ET-mediated nematode susceptibility was independent of AX (Wubben et al. 2004; Fudali et al. 2013). This inconsistency of AX dependence in uninfected and infected plant signaling pathways suggests the possibility of nematode secreted effectors bypassing plant AX signaling to modulate plant responses (Lee et al. 2011). It is also possible that AX-like compounds found in nematode secretions can manipulate plant S/R to nematodes (Hewezi and Baum 2013; Mitchum et al. 2013).
Cytokinin (CK)
CK signaling components are also differentially regulated during SCN infestation (Table 2). It was found that in RKN infested L. japonicus roots, the CK-inducible gene ARR5 was strongly induced in rapidly dividing small cells around the giant cell but absent in mature galls (Lohar et al. 2004). Similarly, significantly fewer and smaller galls were formed on transgenic L. japonicus roots, when CK was degraded by overexpressed CK oxidase (Lohar et al. 2004).
In addition to functioning downstream of R-gene mediated nematode resistance, different hormones are also important players in plant basal defense responses. Stress hormones SA, JA, and ET regulate plant S/R to SPENs mainly through PR genes or other resistance related factors; growth hormones AX and CK primarily affect nematode parasitism though manipulation of NFS initiation and development. The specific role of each hormone in plant responses to nematodes is a bit more complex showing both plant and nematode species specificity as well as differences that depend on infestation timing. In addition, using different gene/proteins for studying hormone effects on plant-nematode interactions could lead to different conclusions because there are complex interactions between various hormone-signaling pathways. Moreover, nematode secretions should also be considered when interpreting these plant responses, since nematode secreted effectors are known to manipulate plant development and defense responses by interacting with or mimicking plant genes/proteins (Hewezi and Baum 2013; Mitchum et al. 2013).
ROS generation and signaling in plant S/R to SPENs
ROS are chemically reactive molecules containing oxygen, including singlet oxygen, superoxide, hydrogen peroxide (H2O2), and hydroxyl radical (Ray et al. 2012). Plants constantly produce ROS as a byproduct of metabolic processes such as photosynthesis and respiration (Tripathy and Oelmuller 2012). Steady-state levels of ROS are tightly regulated by competing ROS generation and scavenging mechanisms. ROS over-accumulation usually causes oxidation of lipids, proteins, and DNA as well as other components often leading to cell death (Tripathy and Oelmuller 2012). When challenged by pathogens, ROS production increases rapidly in what is often called the oxidative burst, which can lead to local cell death (Tripathy and Oelmuller 2012). The local burst of ROS in response to pathogen infection could also be transferred systemically in a cell-to-cell auto-propagating manner, integrating with other signaling pathways generating a SAR response (Baxter et al. 2014; Tripathy and Oelmuller 2012).
In response to RKN infection, both RKN susceptible and resistant tomato plants showed nematode penetration into their roots (Melillo et al. 2006, 2011). However, 48 h post infection (hpi), significantly fewer RKN were observed in Mi-1-mediated incompatible infested roots than in compatible infested roots (Melillo et al. 2006). In accordance, an oxidative burst was observed at the root infection site as soon as 12 hpi for both compatible and incompatible responses, but this was only prolonged in incompatible responses (Mi-1 tomato responses to avirulent RKN) until 48 hpi when cell death became evident (Melillo et al. 2006, 2011).
NADPH oxidases were the main source of ROS production in plant incompatible responses and subcellular localization of H2O2 production for incompatible responses followed a pattern consistently observed in HR (Melillo et al. 2006). Collectively, these observations show that, as one of the most sensitive signals monitoring cellular metabolic changes, ROS accumulation occurs rapidly in response to RKN infection, and temporal and spatial differences in ROS (particularly H2O2) accumulation are crucial in determining the extent of RKN pathogenesis in host plants.
Rapid apoplastic generation of ROS has been mainly associated with pathogen resistance (Baxter et al. 2014; Tripathy and Oelmuller 2012). Consistent with this, RKN genes encoding ROS scavenging enzymes (clade B peroxiredoxins) were more actively transcribed in parasitic stages to protect RKN from the oxidative responses of the host, and knockdown of these genes resulted in reduced RKN parasitism (Dubreuil et al. 2011).
Conversely, a recent study demonstrated a negative role for ROS in cell death and BCN resistance in Arabidopsis (Siddique et al. 2014). Loss of two specific NADPH oxidase genes, RbohD and/or RbohF, required for ROS production at 24 hpi after BCN infestation, resulted in reduced BCN parasitism, smaller syncytium size, and enhanced cell death (Siddique et al. 2014). In addition, the suppression of cell death in RbohD and/or RbohF mutants was demonstrated to be independent of SA accumulation, and an antagonistic relationship between SA and ROS was suggested because SA responsive genes were induced in RbohD/F double mutants but were suppressed in RbohD overexpressing Arabidopsis (Siddique et al. 2014).
ROS also work in concert with nitric oxide (NO), the main reactive nitrogen species (RNS) found in biological systems, to control plant responses to SPENs (Melillo et al. 2011; Yu et al. 2012). NO is a gaseous nitrogen-containing free radical, endogenously produced by plants serving as an important mediator of defense responses (Bellin et al. 2013). In tomato plants demonstrating an incompatible reaction to RKN, the peak generation of ROS was preceded by production of NO (Melillo et al. 2011). Similarly, in P. thunbergii responding to pine wood nematode, endogenous NO levels increased coincident with a rapid increase in H2O2 levels, whereas H2O2 levels decreased when pretreated with an NO scavenger (Yu et al. 2012).
ROS generation is always associated with plant defense responses, and rapid apoplastic generation of ROS often leads to an HR-type cell death, thus restricting the spread of the infection leading to pathogen resistance (Baxter et al. 2014). However, newly identified ROS suppression of cell death and support of BCN parasitism suggests an activation of HR-type cell death can occur through other unknown signaling mechanisms (Feng and Shan 2014). This new finding is also consistent with ROS having distinct roles in plant S/R to SPENs.
Small RNAs may be important regulators in plant S/R to SPENs
Small regulatory RNAs (20–24 nucleotides in length) are emerging as important aspects of plant defense responses resulting from epigenetic, transcriptional, posttranscriptional, and/or translational gene regulation (Shukla et al. 2008; Ruiz-Ferrer and Voinnet 2009; Katiyar-Agarwal and Jin 2010). The primary classes of plant sRNAs are the short interfering RNAs (siRNAs) and the micro RNAs (miRNAs) although there are other emerging classes of sRNA that have not yet been extensively investigated in the context of pathogenesis (Axtell 2013).
miRNAs are the best studied class of sRNAs. miRNAs are derived from single stranded RNA transcripts, which form hairpin loop structures (Axtell 2013). Both miRNAs and siRNAs are processed from their double-stranded RNA precursors by DICER-like proteins (DCLs) and the resulting miRNAs and siRNAs are loaded into Argonaute (AGO) proteins to form an RNA-induced silencing complex (RISC) that can bind to target RNAs or DNAs (Axtell 2013).
Plant miRNAs and siRNAs play important roles in plant biotic stress responses by regulating genes involved in plant PTI and ETI (Katiyar-Agarwal and Jin 2010), hormone signaling (Liu and Chen 2009), ROS generation and signaling (Shukla et al. 2008), and various other types of signaling (Ruiz-Ferrer and Voinnet 2009). Thus, sRNAs are positioned to integrate various aspects of the pathogenesis responses into regulatory networks. Current studies further suggest that miRNAs and siRNAs are playing such regulatory roles during nematode pathogenesis in host plants (Fig. 1; Tables 3, S2).
Genes encoding proteins associated with miRNA or siRNA biogenesis and/or function including DCLs, AGOs, RDRs, and genes encoding DNA methylase proteins, as well as histone methylation and deacetylation-related genes, are regulated in RKN-induced tomato root galls and RKN-infected rice roots (Ji et al. 2013; Portillo et al. 2013). DNA and histone methylation and histone acetylation are important mechanisms mediating epigenetic gene regulation in plants (Sahu et al. 2013). Taken together, these results are consistent with miRNA and siRNA biogenesis and function playing an important role in plant responses to SPENs.
The biogenesis and functioning of miRNAs and siRNAs were also demonstrated to be required in plant S/R to SCN (Hewezi et al. 2008). Responses to SCN were examined in several single, double, and triple mutants of Arabidopsis. The genes examined included genes coding for the DCLs and RDRs, various isoforms of which are involved in the production of specific miRNAs and siRNAs. Mutation in these sRNA-producing genes all displayed decreased SCN susceptibility compared to wild type (Hewezi et al. 2008).
Predicted targets of differentially expressed miRNAs and siRNAs indicated specific roles of sRNAs in nematode pathogenesis in host plants (Tables 3, S2). Genes encoding R-proteins, ARFs, Heat Shock Proteins, ROS scavenger Cu/Zn superoxide dismutases, and various transcription factors are all predicted to be the targets of one or more differentially expressed miRNAs or siRNAs (Tables 3, S2). Among the differentially expressed sRNAs in response to nematode infestation, Arabidopsis miR396 was down-regulated 4 days post SCN infestation and up-regulated 7 days post infestation (Hewezi et al. 2008). Targets of miR396, including Arabidopsis GRF (Growth Regulating Factors) exhibited the opposite expression trends to miR396 post SCN infestation (Hewezi et al. 2008).
To investigate the role of miR396/GRF in plant responses to SCN, Arabidopsis mutants deficient in GRF genes or overexpressing miR396 were examined, and overexpression of miR396 and/or reduced GRF gene expression resulted in reduced SCN susceptibility (Hewezi et al. 2012). Furthermore, miR396-overexpressing Arabidopsis roots produced smaller syncytia with fewer SCN infections. Similar characteristics were also observed in miR396 binding site-deficient mutants (Hewezi et al. 2012).
Since the GRF gene family positively controls cell proliferation and size, the coordinated expression of miR396 and its target GRF genes are critical in syncytia development during SCN infection. Moreover, almost half of the genes differentially expressed in syncytia overlapped with genes differentially regulated in GRF deficient and miR396 resistant Arabidopsis mutants, indicating that miR396/GRF is an essential regulatory system in reprogramming of gene expression in SCN-induced syncytia (Hewezi et al. 2012).
sRNA expression changes in response to RN infestation have been investigated in cotton (Li and Locy, unpublished result). It was found that specific miRNAs and siRNA sequences (including cotton miR396 and miR482 among others) exhibit distinct expression patterns in response to RN infestation in cotton genotypes differing in RN resistance and susceptibility. The spectrum of sRNA target genes derived from differentially expressed sRNAs include genes previously implicated in plant innate immunity, hormone signaling, ROS generation and signaling, as well as sRNA biogenesis and function, and in epigenetic regulation. This analysis supports the idea that sRNAs serve to integrate a signaling network that regulates most, if not all, of the various signaling pathways discussed above.
Viral (Shivaprasad et al. 2012), bacterial (Shivaprasad et al. 2012), or fungal (Zhu et al. 2013) infection of tomato or diploid cotton (G. raimondii) all resulted in suppression of specific miRNAs and induction of their target R-genes (NBS-LRR genes). Co-expression of miRNAs and their NBS-LRR targets in tobacco caused decreased resistance to TMV (Li et al. 2012a). Based on bioinformatics and experimental data generated from different plant species, some NBS-LRR genes can produce clustered secondary siRNAs from their mRNA transcripts in a phased manner, and miRNA targeting is required for the production of secondary siRNAs (Shivaprasad et al. 2012; Zhu et al. 2013; Zhai et al. 2011; Li et al. 2012a). Furthermore, some secondary siRNAs (i.e. trans-acting siRNAs) can also target other defense related genes (Shivaprasad et al. 2012) while others have shown involvement in AX signaling regulation of root development (Fahlgren et al. 2006; Marin et al. 2010; Yoon et al. 2010). Since NBS-LRR proteins and AX signaling clearly have a role in endoparasitic nematode S/R, it is reasonable to presume that miRNA/siRNA signaling plays important roles in integrating nematode signaling systems.
Support for such an hypothesis comes from a bioinformatic analysis of sRNA regulatory networks involved in RN signaling in cotton. A RN-responsive miRNA (miR482 family) was predicted to target an NBS-LRR protein-coding mRNA that could be cleaved into a cluster of secondary phased siRNAs (Li and Locy, unpublished results). These siRNAs also target a series of transcription factors and other proteins many of which are known pathogenesis-related genes involved in signaling pathways. This provides preliminary support for the involvement of sRNA regulatory network in cotton-RN interactions through miRNAs and NBS-LRR protein coding genes producing secondary siRNAs that have been implicated in plant innate immunity as described for other pathogens ( Zhai et al. 2011; Li et al. 2012a; Shivaprasad et al. 2012; Fei et al. 2013).
Overall, these studies suggested that the miRNA regulation of NBS-LRR gene expression via the production of secondary siRNAs is playing an important role in nematode pathogenesis in host plants, although the exact nature of such regulatory network remains to be defined. It is possible that host plants can defend themselves by down regulating specific miRNAs leading to the expression critical R-genes involved in nematode resistance. The suppression of NBS-LRR gene expression via the sRNA pathway serves as a protective mechanism for plants since large increases in NBS-LRR transcripts and protein levels could trigger cell death and/or plant HR (Qiao et al. 2013). It is also possible that infecting nematodes secrete specific effectors that induce sRNA regulatory network suppressing the expression of NBS-LRR genes and thus promoting nematode parasitism. Since the delivery of sRNAs between host plants and infecting nematodes can suppress the expression of genes essential for nematode pathogenesis and development (Fairbairn et al. 2007; Klink et al. 2009; Charlton et al. 2010; Dalzell et al. 2010; Li et al. 2010), it is possible that nematode effector RNAs can act as the initiators of the plant sRNA regulatory networks, although this remains to be established.
Conclusions
In conclusion, SPENs resistance in host plants appears to be initiated when plant R-proteins sense nematode secreted effectors. Detection of nematode effectors leads to massive downstream reprogramming of gene expression through various hormones, ROS, and NO signaling pathways. However, each hormone’s role in particular plant-nematode interaction is unique. Detection of nematode by R-proteins also leads to a localized cell necrosis at the nematode infection site, and a local burst of ROS and NO are causally correlated with HR observed in R-gene-mediated nematode resistance, although ROS play a supportive role in parasitism when incompatible R-gene interactions are involved.
It is clear that, instead of functioning independently, different signaling factors work in concert with each other in a highly controlled regulatory network. The specific nature of nematode produced factors interacting with plant factors also plays an important role in mediating the behavior of the regulatory network developing plant responses. sRNAs are emerging as important regulators of pathogen resistance that are implicated in the regulation of crucial regulatory nodes in plant defense responses, such as NBS-LRR genes. Based on these findings, it could be concluded that sRNA might be the hub of plant S/R to nematodes, but additional, continuing studies are required to reveal and demonstrate the specific roles of sRNAs in various plant-nematode interactions.
The application of emergent next generation sequencing technologies and network analysis strategies will not only support the implication of canonical signaling pathways in plant S/R to nematodes, but will also play a key role in implicating pathway cross talk and integration as we move forward.
References
Absmanner B, Stadler R, Hammes UZ (2013) Phloem development in nematode-induced feeding sites: the implications of auxin and cytokinin. Front Plant Sci 4:241
Ali MA, Wieczorek K, Kreil DP, Bohlmann H (2014) The beet cyst nematode heterodera schachtii modulates the expression of WRKY transcription factors in syncytia to favour its development in arabidopsis roots. PLoS One 9:e102360
Ammiraju JSS, Veremis JC, Huang X, Roberts PA, Kaloshian I (2003) The heat-stable root-knot nematode resistance gene Mi-9 from Lycopersicon peruvianum is localized on the short arm of chromosome 6. Theor Appl Genet 106:478–484
Atamian HS, Eulgem T, Kaloshian I (2012) SlWRKY70 is required for Mi-1-mediated resistance to aphids and nematodes in tomato. Planta 235:299–309
Axtell MJ (2013) Classification and comparison of small RNAs from plants. Annu Rev Plant Biol 64:137–159
Bakker E, Achenbach U, Bakker J, van Vliet J, Peleman J, Segers B, van der Heijden S, van der Linde P, Graveland R, Hutten R, van Eck H, Coppoolse E, van der Vossen E, Bakker J, Goverse A (2004) A high-resolution map of the H1 locus harbouring resistance to the potato cyst nematode Globodera rostochiensis. Theor Appl Genet 109:146–152
Barcala M, Garcia A, Cabrera J, Casson S, Lindsey K, Favery B, Garcia-Casado G, Solano R, Fenoll C, Escobar C (2010) Early transcriptomic events in microdissected Arabidopsis nematode-induced giant cells. Plant J Cell Mol Biol 61:698–712
Baxter A, Mittler R, Suzuki N (2014) ROS as key players in plant stress signalling. J Exp Bot 65:1229–1240
Bellin D, Asai S, Delledonne M, Yoshioka H (2013) Nitric oxide as a mediator for defense responses. Mol Plant-Microbe Interact MPMI 26:271–277
Bhattarai KK, Li Q, Liu Y, Dinesh-Kumar SP, Kaloshian I (2007) The MI-1-mediated pest resistance requires Hsp90 and Sgt1. Plant Physiol 144:312–323
Bhattarai KK, Xie QG, Mantelin S, Bishnoi U, Girke T, Navarre DA, Kaloshian I (2008) Tomato susceptibility to root-knot nematodes requires an intact jasmonic acid signaling pathway. Mol Plant-Microbe InteractMPMI 21:1205–1214
Bhattarai KK, Atamian HS, Kaloshian I, Eulgem T (2010) WRKY72-type transcription factors contribute to basal immunity in tomato and Arabidopsis as well as gene-for-gene resistance mediated by the tomato R gene Mi-1. Plant J Cell Mol Biol 63:229–240
Bleve-Zacheo T, Bongiovanni M, Melillo MT, Castagnone-Sereno P (1998) The pepper resistance genes Me1 and Me3 induce differential penetration rates and temporal sequences of root cell ultrastructural changes upon nematode infection. Plant Sci 133:79–90
Branch C, Hwang CF, Navarre DA, Williamson VM (2004) Salicylic acid is part of the Mi-1-mediated defense response to root-knot nematode in tomato. Mol Plant-Microbe Interact MPMI 17:351–356
Budahn H, Peterka H, Mousa MAA, Ding YH, Zhang SS, Li JB (2009) Molecular mapping in oil radish (Raphanus sativus L.) and QTL analysis of resistance against beet cyst nematode (Heterodera schachtii). Theor Appl Genet 118:775–782
Cabrera J, Diaz-Manzano FE, Sanchez M, Rosso MN, Melillo T, Goh T, Fukaki H, Cabello S, Hofmann J, Fenoll C, Escobar C (2014) A role for LATERAL ORGAN BOUNDARIES-DOMAIN 16 during the interaction Arabidopsis-Meloidogyne spp. provides a molecular link between lateral root and root-knot nematode feeding site development. New Phytol 203:632–645
Cai D (1997) Positional cloning of a gene for nematode resistance in sugar beet. Science 275:832–834
Charlton WL, Harel HY, Bakhetia M, Hibbard JK, Atkinson HJ, McPherson MJ (2010) Additive effects of plant expressed double-stranded RNAs on root-knot nematode development. Int J Parasitol 40:855–864
Chen R, Li H, Zhang L, Zhang J, Xiao J, Ye Z (2007) CaMi, a root-knot nematode resistance gene from hot pepper (Capsium annuum L.) confers nematode resistance in tomato. Plant Cell Rep 26:895–905
Claverie M, Dirlewanger E, Bosselut N, Van Ghelder C, Voisin R, Kleinhentz M, Lafargue B, Abad P, Rosso MN, Chalhoub B, Esmenjaud D (2011) The Ma gene for complete-spectrum resistance to Meloidogyne species in Prunus is a TNL with a huge repeated C-terminal post-LRR region. Plant Physiol 156:779–792
Cook DE, Lee TG, Guo X, Melito S, Wang K, Bayless AM, Wang J, Hughes TJ, Willis DK, Clemente TE, Diers BW, Jiang J, Hudson ME, Bent AF (2012) Copy number variation of multiple genes at Rhg1 mediates nematode resistance in soybean. Science 338:1206–1209
Cook DE, Bayless AM, Wang K, Guo X, Song Q, Jiang J, Bent AF (2014) Distinct copy number, coding sequence, and locus methylation patterns underlie Rhg1-mediated soybean resistance to soybean cyst nematode. Plant Physiol 165:630–647
Cooper WR, Jia L, Goggin L (2005) Effects of jasmonate-induced defenses on root-knot nematode infection of resistant and susceptible tomato cultivars. J Chem Ecol 31:1953–1967
Dalzell JJ, Warnock ND, Stevenson MA, Mousley A, Fleming CC, Maule AG (2010) Short interfering RNA-mediated knockdown of drosha and pasha in undifferentiated Meloidogyne incognita eggs leads to irregular growth and embryonic lethality. Int J Parasitol 40:1303–1310
de Majnik J, Ogbonnaya FC, Moullet O, Lagudah ES (2003) The Cre1 and Cre3 nematode resistance genes are located at homeologous loci in the wheat genome. Mol Plant Microbe Interact 16:1129–1134
Dighe ND, Robinson AF, Bell AA, Menz MA, Cantrell RG, Stelly DM (2009) Linkage mapping of resistance to reniform nematode in cotton following introgression from gossypium longicalyx (Hutch. & Lee). Crop Sci 49:1151–1164
Djian-Caporalino C, Pijarowski L, Fazari A, Samson M, Gaveau L, O’Byrne C, Lefebvre V, Caranta C, Palloix A, Abad P (2001) High-resolution genetic mapping of the pepper (Capsicum annuum L.) resistance loci Me-3 and Me-4 conferring heat-stable resistance to root-knot nematodes (Meloidogyne spp.). Theor Appl Genet 103:592–600
Djian-Caporalino C, Fazari A, Arguel MJ, Vernie T, VandeCasteele C, Faure I, Brunoud G, Pijarowski L, Palloix A, Lefebvre V, Abad P (2007) Root-knot nematode (Meloidogyne spp.) Me resistance genes in pepper (Capsicum annuum L.) are clustered on the P9 chromosome. Theor Appl Genet 114:473–486
Dubreuil G, Deleury E, Magliano M, Jaouannet M, Abad P, Rosso MN (2011) Peroxiredoxins from the plant parasitic root-knot nematode, Meloidogyne incognita, are required for successful development within the host. Int J Parasitol 41:385–396
Ernst K, Kumar A, Kriseleit D, Kloos DU, Phillips MS, Ganal MW (2002) The broad-spectrum potato cyst nematode resistance gene (Hero) from tomato is the only member of a large gene family of NBS-LRR genes with an unusual amino acid repeat in the LRR region. Plant J Cell Mol Biol 31:127–136
Eulgem T, Somssich IE (2007) Networks of WRKY transcription factors in defense signaling. Curr Opin Plant Biol 10:366–371
Fahlgren N, Montgomery TA, Howell MD, Allen E, Dvorak SK, Alexander AL, Carrington JC (2006) Regulation of AUXIN RESPONSE FACTOR3 by TAS3 ta-siRNA affects developmental timing and patterning in Arabidopsis. Curr Biol CB 16:939–944
Fairbairn DJ, Cavallaro AS, Bernard M, Mahalinga-Iyer J, Graham MW, Botella JR (2007) Host-delivered RNAi: an effective strategy to silence genes in plant parasitic nematodes. Planta 226:1525–1533
Fei Q, Xia R, Meyers BC (2013) Phased, secondary, small interfering RNAs in posttranscriptional regulatory networks. Plant Cell 25:2400–2415
Feng B, Shan L (2014) ROS open roads to roundworm infection. Sci Signal 7:pe10
Finkers-Tomczak A, Danan S, van Dijk T, Beyene A, Bouwman L, Overmars H, van Eck H, Goverse A, Bakker J, Bakker E (2009) A high-resolution map of the Grp1 locus on chromosome V of potato harbouring broad-spectrum resistance to the cyst nematode species Globodera pallida and Globodera rostochiensis. Theor Appl Genet 119:165–173
Fudali SL, Wang C, Williamson VM (2013) Ethylene signaling pathway modulates attractiveness of host roots to the root-knot nematode Meloidogyne hapla. Mol Plant-Microbe Interact MPMI 26:75–86
Fujimoto T, Tomitaka Y, Abe H, Tsuda S, Futai K, Mizukubo T (2011) Expression profile of jasmonic acid-induced genes and the induced resistance against the root-knot nematode (Meloidogyne incognita) in tomato plants (Solanum lycopersicum) after foliar treatment with methyl jasmonate. J Plant Physiol 168:1084–1097
Fuller VL, Lilley CJ, Urwin PE (2008) Nematode resistance. New Phytol 180:27–44
Gao X, Starr J, Gobel C, Engelberth J, Feussner I, Tumlinson J, Kolomiets M (2008) Maize 9-lipoxygenase ZmLOX3 controls development, root-specific expression of defense genes, and resistance to root-knot nematodes. Mol Plant-Microbe Interact MPMI 21:98–109
Gleason CA, Liu QL, Williamson VM (2008) Silencing a candidate nematode effector gene corresponding to the tomato resistance gene Mi-1 leads to acquisition of virulence. Mol Plant-Microbe Interact MPMI 21:576–585
Glowacki S, Macioszek VK, Kononowicz AK (2011) R proteins as fundamentals of plant innate immunity. Cell Mol Biol Lett 16:1–24
Goverse A, Smant G (2013) The activation and suppression of plant innate immunity by parasitic nematodes. Annu Rev Phytopathol 52:243–265
Goverse A, Overmars H, Engelbertink J, Schots A, Bakker J, Helder J (2000) Both induction and morphogenesis of cyst nematode feeding cells are mediated by auxin. Mol Plant-Microbe Interact MPMI 13:1121–1129
Grunewald W, Karimi M, Wieczorek K, Van de Cappelle E, Wischnitzki E, Grundler F, Inze D, Beeckman T, Gheysen G (2008) A role for AtWRKY23 in feeding site establishment of plant-parasitic nematodes. Plant Physiol 148:358–368
Grunewald W, Cannoot B, Friml J, Gheysen G (2009) Parasitic nematodes modulate PIN-mediated auxin transport to facilitate infection. PLoS Pathog 5:e1000266
Gutierrez OA, Robinson AF, Jenkins JN, McCarty JC, Wubben MJ, Callahan FE, Nichols RL (2011) Identification of QTL regions and SSR markers associated with resistance to reniform nematode in Gossypium barbadense L. accession GB713. Theor Appl Genet 122:271–280
Hauge BM, Wang Ming L, Parsons Jeremy D, Parnell Laurence D (2001) Soybean cyst nematode (SCN) resistance loci RHG1 AND RHG4. In. Monsanto Technology LLC, St. Louis
He P, Shan L, Sheen J (2007) Elicitation and suppression of microbe-associated molecular pattern-triggered immunity in plant-microbe interactions. Cell Microbiol 9:1385–1396
Hewezi T, Baum TJ (2013) Manipulation of plant cells by cyst and root-knot nematode effectors. Mol Plant-Microbe Interact MPMI 26:9–16
Hewezi T, Howe P, Maier TR, Baum TJ (2008) Arabidopsis small RNAs and their targets during cyst nematode parasitism. Mol Plant-Microbe Interact MPMI 21:1622–1634
Hewezi T, Howe PJ, Maier TR, Hussey RS, Mitchum MG, Davis EL, Baum TJ (2010) Arabidopsis spermidine synthase is targeted by an effector protein of the cyst nematode Heterodera schachtii. Plant Physiol 152:968–984
Hewezi T, Maier TR, Nettleton D, Baum TJ (2012) The Arabidopsis microRNA396-GRF1/GRF3 regulatory module acts as a developmental regulator in the reprogramming of root cells during cyst nematode infection. Plant Physiol 159:321–335
Hewezi T, Piya S, Richard G, Rice JH (2014) Spatial and temporal expression patterns of auxin response transcription factors in the syncytium induced by the beet cyst nematode Heterodera schachtii in Arabidopsis. Molecular plant pathology
Hogenhout SA, Van der Hoorn RA, Terauchi R, Kamoun S (2009) Emerging concepts in effector biology of plant-associated organisms. Molecular plant-microbe interactions : MPMI 22:115–122
Hwang CF, Williamson VM (2003) Leucine-rich repeat-mediated intramolecular interactions in nematode recognition and cell death signaling by the tomato resistance protein Mi. The Plant journal : for cell and molecular biology 34:585–593
Hwang CF, Bhakta AV, Truesdell GM, Pudlo WM, Williamson VM (2000) Evidence for a role of the N terminus and leucine-rich repeat region of the Mi gene product in regulation of localized cell death. Plant Cell 12:1319–1329
Iberkleid I, Vieira P, de Almeida Engler J, Firester K, Spiegel Y, Horowitz SB (2013) Fatty acid-and retinol-binding protein, Mj-FAR-1 induces tomato host susceptibility to root-knot nematodes. PLoS ONE 8:e64586
Ithal N, Recknor J, Nettleton D, Maier T, Baum TJ, Mitchum MG (2007) Developmental transcript profiling of cyst nematode feeding cells in soybean roots. Molecular plant-microbe interactions : MPMI 20:510–525
Ji H, Gheysen G, Denil S, Lindsey K, Topping JF, Nahar K, Haegeman A, De Vos WH, Trooskens G, Van Criekinge W, De Meyer T, Kyndt T (2013) Transcriptional analysis through RNA sequencing of giant cells induced by Meloidogyne graminicola in rice roots. J Exp Bot 64:3885–3898
Jones JD, Dangl JL (2006) The plant immune system. Nature 444:323–329
Kandoth PK, Ithal N, Recknor J, Maier T, Nettleton D, Baum TJ, Mitchum MG (2011) The Soybean Rhg1 locus for resistance to the soybean cyst nematode Heterodera glycines regulates the expression of a large number of stress- and defense-related genes in degenerating feeding cells. Plant Physiol 155:1960–1975
Karczmarek A, Overmars H, Helder J, Goverse A (2004) Feeding cell development by cyst and root-knot nematodes involves a similar early, local and transient activation of a specific auxin-inducible promoter element. Molecular plant pathology 5:343–346
Katiyar-Agarwal S, Jin H (2010) Role of small RNAs in host-microbe interactions. Annu Rev Phytopathol 48:225–246
Khallouk S, Voisin R, Van Ghelder C, Engler G, Amiri S, Esmenjaud D (2011) Histological mechanisms of the resistance conferred by the Ma gene against Meloidogyne incognita in Prunus spp. Phytopathology 101:945–951
Klink VP, Overall CC, Alkharouf NW, MacDonald MH, Matthews BF (2007) Laser capture microdissection (LCM) and comparative microarray expression analysis of syncytial cells isolated from incompatible and compatible soybean (Glycine max) roots infected by the soybean cyst nematode (Heterodera glycines). Planta 226:1389–1409
Klink VP, Kim KH, Martins V, Macdonald MH, Beard HS, Alkharouf NW, Lee SK, Park SC, Matthews BF (2009) A correlation between host-mediated expression of parasite genes as tandem inverted repeats and abrogation of development of female Heterodera glycines cyst formation during infection of Glycine max. Planta 230:53–71
Kyndt T, Nahar K, Haegeman A, De Vleesschauwer D, Hofte M, Gheysen G (2012) Comparing systemic defence-related gene expression changes upon migratory and sedentary nematode attack in rice. Plant biology 14(Suppl 1):73–82
Lee C, Chronis D, Kenning C, Peret B, Hewezi T, Davis EL, Baum TJ, Hussey R, Bennett M, Mitchum MG (2011) The novel cyst nematode effector protein 19C07 interacts with the Arabidopsis auxin influx transporter LAX3 to control feeding site development. Plant Physiol 155:866–880
Li J, Todd TC, Oakley TR, Lee J, Trick HN (2010) Host-derived suppression of nematode reproductive and fitness genes decreases fecundity of Heterodera glycines Ichinohe. Planta 232:775–785
Li F, Pignatta D, Bendix C, Brunkard JO, Cohn MM, Tung J, Sun H, Kumar P, Baker B (2012a) MicroRNA regulation of plant innate immune receptors. Proc Natl Acad Sci USA 109:1790–1795
Li X, Wang X, Zhang S, Liu D, Duan Y, Dong W (2012b) Identification of soybean microRNAs involved in soybean cyst nematode infection by deep sequencing. PLoS ONE 7:e39650
Lin J, Mazarei M, Zhao N, Zhu JJ, Zhuang X, Liu W, Pantalone VR, Arelli PR, Stewart CN Jr, Chen F (2013) Overexpression of a soybean salicylic acid methyltransferase gene confers resistance to soybean cyst nematode. Plant Biotechnol J 11:1135–1145
Liu Q, Chen YQ (2009) Insights into the mechanism of plant development: interactions of miRNAs pathway with phytohormone response. Biochemical and biophysical research communications 384:1–5
Liu S, Kandoth PK, Warren SD, Yeckel G, Heinz R, Alden J, Yang C, Jamai A, El-Mellouki T, Juvale PS, Hill J, Baum TJ, Cianzio S, Whitham SA, Korkin D, Mitchum MG, Meksem K (2012) A soybean cyst nematode resistance gene points to a new mechanism of plant resistance to pathogens. Nature 492:256–260
Lohar DP, Schaff JE, Laskey JG, Kieber JJ, Bilyeu KD, Bird DM (2004) Cytokinins play opposite roles in lateral root formation, and nematode and Rhizobial symbioses. The Plant journal : for cell and molecular biology 38:203–214
Lorieux M, Reversat G, Diaz SXG, Denance C, Jouvenet N, Orieux Y, Bourger N, Pando-Bahuon A, Ghesquiere A (2003) Linkage mapping of Hsa-1(Og), a resistance gene of African rice to the cyst nematode, Heterodera sacchari. Theor Appl Genet 107:691–696
Lukasik-Shreepaathy E, Slootweg E, Richter H, Goverse A, Cornelissen BJ, Takken FL (2012) Dual regulatory roles of the extended N terminus for activation of the tomato MI-1.2 resistance protein. Molecular plant-microbe interactionsq: MPMI 25:1045–1057
Luzzi BM, Boerma HR, Hussey RS (1994) A GENE FOR RESISTANCE TO THE SOUTHERN ROOT-KNOT NEMATODE IN SOYBEAN. J Hered 85:484–486
Mantelin S, Bhattarai KK, Jhaveri TZ, Kaloshian I (2013) Mi-1-mediated resistance to Meloidogyne incognita in tomato may not rely on ethylene but hormone perception through ETR3 participates in limiting nematode infection in a susceptible host. PLoS ONE 8:e63281
Marin E, Jouannet V, Herz A, Lokerse AS, Weijers D, Vaucheret H, Nussaume L, Crespi MD, Maizel A (2010) miR390, Arabidopsis TAS3 tasiRNAs, and their AUXIN RESPONSE FACTOR targets define an autoregulatory network quantitatively regulating lateral root growth. Plant Cell 22:1104–1117
Martinez de Ilarduya O, Nombela G, Hwang CF, Williamson VM, Muniz M, Kaloshian I (2004) Rme1 is necessary for Mi-1-mediated resistance and acts early in the resistance pathway. Molecular plant-microbe interactions : MPMI 17:55–61
Matthews BF, Beard H, MacDonald MH, Kabir S, Youssef RM, Hosseini P, Brewer E (2013) Engineered resistance and hypersusceptibility through functional metabolic studies of 100 genes in soybean to its major pathogen, the soybean cyst nematode. Planta 237:1337–1357
Matthews BF, Beard H, Brewer E, Kabir S, MacDonald MH, Youssef RM (2014) Arabidopsis genes, AtNPR1, AtTGA2 and AtPR-5, confer partial resistance to soybean cyst nematode (Heterodera glycines) when overexpressed in transgenic soybean roots. BMC Plant Biol 14:96
Mazarei M, Puthoff DP, Hart JK, Rodermel SR, Baum TJ (2002) Identification and characterization of a soybean ethylene-responsive element-binding protein gene whose mRNA expression changes during soybean cyst nematode infection. Molecular plant-microbe interactions : MPMI 15:577–586
Mazarei M, Lennon KA, Puthoff DP, Rodermel SR, Baum TJ (2003) Expression of an Arabidopsis phosphoglycerate mutase homologue is localized to apical meristems, regulated by hormones, and induced by sedentary plant-parasitic nematodes. Plant Mol Biol 53:513–530
Mazarei M, Elling AA, Maier TR, Puthoff DP, Baum TJ (2007) GmEREBP1 is a transcription factor activating defense genes in soybean and Arabidopsis. Molecular plant-microbe interactions : MPMI 20:107–119
Mazarei M, Liu W, Al-Ahmad H, Arelli PR, Pantalone VR, Stewart CN Jr (2011) Gene expression profiling of resistant and susceptible soybean lines infected with soybean cyst nematode. TAG Theoretical and applied genetics Theoretische und angewandte Genetik 123:1193–1206
Melillo MT, Leonetti P, Bongiovanni M, Castagnone-Sereno P, Bleve-Zacheo T (2006) Modulation of reactive oxygen species activities and H2O2 accumulation during compatible and incompatible tomato-root-knot nematode interactions. The New phytologist 170:501–512
Melillo MT, Leonetti P, Leone A, Veronico P, Bleve-Zacheo T (2011) ROS and NO production in compatible and incompatible tomato-Meloidogyne incognita interactions. Eur J Plant Pathol 130:489–502
Melito S, Heuberger AL, Cook D, Diers BW, MacGuidwin AE, Bent AF (2010) A nematode demographics assay in transgenic roots reveals no significant impacts of the Rhg1 locus LRR-Kinase on soybean cyst nematode resistance. BMC Plant Biol 10:104
Milligan SB, Bodeau J, Yaghoobi J, Kaloshian I, Zabel P, Williamson VM (1998) The root knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell 10:1307–1319
Mitchum MG, Hussey RS, Baum TJ, Wang X, Elling AA, Wubben M, Davis EL (2013) Nematode effector proteins: an emerging paradigm of parasitism. The New phytologist 199:879–894
Molinari S, Fanelli E, Leonetti P (2013) Expression of tomato salicylic acid (SA)-responsive pathogenesis-related genes in Mi-1-mediated and SA-induced resistance to root-knot nematodes. Molecular plant pathology
Mosblech A, Feussner I, Heilmann I (2009) Oxylipins: structurally diverse metabolites from fatty acid oxidation. Plant physiology and biochemistry: PPB/Societe francaise de physiologie vegetale 47:511–517
Nahar K, Kyndt T, De Vleesschauwer D, Hofte M, Gheysen G (2011) The jasmonate pathway is a key player in systemically induced defense against root knot nematodes in rice. Plant Physiol 157:305–316
Nombela G, Williamson VM, Muniz M (2003) The root-knot nematode resistance gene Mi-1.2 of tomato is responsible for resistance against the whitefly Bemisia tabaci. Molecular plant-microbe interactions: MPMI 16:645–649
Ozalvo R, Cabrera J, Escobar C, Christensen SA, Borrego EJ, Kolomiets MV, Castresana C, Iberkleid I, Brown Horowitz S (2013) Two closely related members of Arabidopsis 13-lipoxygenases (13-LOXs), LOX3 and LOX4, reveal distinct functions in response to plant-parasitic nematode infection. Molecular plant pathology
Paal J, Henselewski H, Muth J, Meksem K, Menendez CM, Salamini F, Ballvora A, Gebhardt C (2004) Molecular cloning of the potato Gro1-4 gene conferring resistance to pathotype Ro1 of the root cyst nematode Globodera rostochiensis, based on a candidate gene approach. The Plant journal : for cell and molecular biology 38:285–297
Park SW, Kaimoyo E, Kumar D, Mosher S, Klessig DF (2007) Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318:113–116
Parkhi V, Kumar V, Campbell LM, Bell AA, Shah J, Rathore KS (2010) Resistance against various fungal pathogens and reniform nematode in transgenic cotton plants expressing Arabidopsis NPR1. Transgenic Res 19:959–975
Portillo M, Cabrera J, Lindsey K, Topping J, Andres MF, Emiliozzi M, Oliveros JC, Garcia-Casado G, Solano R, Koltai H, Resnick N, Fenoll C, Escobar C (2013) Distinct and conserved transcriptomic changes during nematode-induced giant cell development in tomato compared with Arabidopsis: a functional role for gene repression. The New phytologist 197:1276–1290
Priya DB, Somasekhar N, Prasad J, Kirti P (2011) Transgenic tobacco plants constitutively expressing Arabidopsis NPR1 show enhanced resistance to root-knot nematode. Meloidogyne incognita. BMC research notes 4:231
Qiao Y, Liu L, Xiong Q, Flores C, Wong J, Shi J, Wang X, Liu X, Xiang Q, Jiang S, Zhang F, Wang Y, Judelson HS, Chen X, Ma W (2013) Oomycete pathogens encode RNA silencing suppressors. Nat Genet 45:330–333
Ray PD, Huang BW, Tsuji Y (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24:981–990
Robinson AF (2007) Reniform in U.S. cotton: when, where, why, and some remedies. Annu Rev Phytopathol 45:263–288
Romano GB, Sacks EJ, Stetina SR, Robinson AF, Fang DD, Gutierrez OA, Scheffler JA (2009) Identification and genomic location of a reniform nematode (Rotylenchulus reniformis) resistance locus (Ren (ari)) introgressed from Gossypium aridum into upland cotton (G. hirsutum). Theor Appl Genet 120:139–150
Ruiz-Ferrer V, Voinnet O (2009) Roles of plant small RNAs in biotic stress responses. Annu Rev Plant Biol 60:485–510
Sacco MA, Koropacka K, Grenier E, Jaubert MJ, Blanchard A, Goverse A, Smant G, Moffett P (2009) The cyst nematode SPRYSEC protein RBP-1 elicits Gpa2- and RanGAP2-dependent plant cell death. PLoS Pathog 5:e1000564
Sahu PP, Pandey G, Sharma N, Puranik S, Muthamilarasan M, Prasad M (2013) Epigenetic mechanisms of plant stress responses and adaptation. Plant Cell Rep 32:1151–1159
Schwessinger B, Zipfel C (2008) News from the frontline: recent insights into PAMP-triggered immunity in plants. Curr Opin Plant Biol 11:389–395
Seifi A, Kaloshian I, Vossen J, Che DD, Bhattarai KK, Fan JM, Naher Z, Goverse A, Tjallingii WF, Lindhout P, Visser RGF, Bai YL (2011) Linked, if Not the Same, Mi-1 Homologues Confer Resistance to Tomato Powdery Mildew and Root-Knot Nematodes. Mol Plant Microbe Interact 24:441–450
Semblat JP, Rosso MN, Hussey RS, Abad P, Castagnone-Sereno P (2001) Molecular cloning of a cDNA encoding an amphid-secreted putative avirulence protein from the root-knot nematode Meloidogyne incognita. Molecular plant-microbe interactions: MPMI 14:72–79
Shen XL, Van Becelaere G, Kumar P, Davis RF, May OL, Chee P (2006) QTL mapping for resistance to root-knot nematodes in the M-120 RNR Upland cotton line (Gossypium hirsutum L.) of the Auburn 623 RNR source. Theor Appl Genet 113:1539–1549
Shirasu K (2009) The HSP90-SGT1 chaperone complex for NLR immune sensors. Annu Rev Plant Biol 60:139–164
Shivaprasad PV, Chen HM, Patel K, Bond DM, Santos BA, Baulcombe DC (2012) A microRNA superfamily regulates nucleotide binding site-leucine-rich repeats and other mRNAs. Plant Cell 24:859–874
Shukla LI, Chinnusamy V, Sunkar R (2008) The role of microRNAs and other endogenous small RNAs in plant stress responses. Biochim Biophys Acta 1779:743–748
Siddique S, Matera C, Radakovic ZS, Hasan MS, Gutbrod P, Rozanska E, Sobczak M, Torres MA, Grundler FM (2014) Parasitic worms stimulate host NADPH oxidases to produce reactive oxygen species that limit plant cell death and promote infection. Science signaling 7:ra33
Sobczak M, Avrova A, Jupowicz J, Phillips MS, Ernst K, Kumar A (2005) Characterization of susceptibility and resistance responses to potato cyst nematode (Globodera spp.) infection of tomato lines in the absence and presence of the broad-spectrum nematode resistance Hero gene. Molecular plant-microbe interactions: MPMI 18:158–168
Tameling WI, Elzinga SD, Darmin PS, Vossen JH, Takken FL, Haring MA, Cornelissen BJ (2002) The tomato R gene products I-2 and MI-1 are functional ATP binding proteins with ATPase activity. Plant Cell 14:2929–2939
Tripathy BC, Oelmuller R (2012) Reactive oxygen species generation and signaling in plants. Plant signaling & behavior 7:1621–1633
Tucker ML, Xue P, Yang R (2010) 1-Aminocyclopropane-1-carboxylic acid (ACC) concentration and ACC synthase expression in soybean roots, root tips, and soybean cyst nematode (Heterodera glycines)-infected roots. J Exp Bot 61:463–472
Uehara T, Sugiyama S, Matsuura H, Arie T, Masuta C (2010) Resistant and susceptible responses in tomato to cyst nematode are differentially regulated by salicylic acid. Plant Cell Physiol 51:1524–1536
van der Voort J, Janssen GJW, Overmars H, van Zandvoort PM, van Norel A, Scholten OE, Janssen R, Bakker J (1999) Development of a PCR-based selection assay for root-knot nematode resistance (Rmc1) by a comparative analysis of the Solanum bulbocastanum and S-tuberosum genome. Euphytica 106:187–195
van der Vossen EA, van der Voort JN, Kanyuka K, Bendahmane A, Sandbrink H, Baulcombe DC, Bakker J, Stiekema WJ, Klein-Lankhorst RM (2000) Homologues of a single resistance-gene cluster in potato confer resistance to distinct pathogens: a virus and a nematode. The Plant journal: for cell and molecular biology 23:567–576
Vlot AC, Dempsey DA, Klessig DF (2009) Salicylic Acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47:177–206
Vos P, Simons G, Jesse T, Wijbrandi J, Heinen L, Hogers R, Frijters A, Groenendijk J, Diergaarde P, Reijans M, Fierens-Onstenk J, de Both M, Peleman J, Liharska T, Hontelez J, Zabeau M (1998) The tomato Mi-1 gene confers resistance to both root-knot nematodes and potato aphids. Nat Biotechnol 16:1365–1369
Wang KL, Li H, Ecker JR (2002) Ethylene biosynthesis and signaling networks. Plant Cell 14(Suppl):S131–S151
Wang C, Ulloa M, Roberts PA (2006) Identification and mapping of microsatellite markers linked to a root-knot nematode resistance gene (rkn1) in Acala NemX cotton (Gossypium hirsutum L.). Theor Appl Genet 112:770–777
Wang X, Replogle A, Davis EL, Mitchum MG (2007) The tobacco Cel7 gene promoter is auxin-responsive and locally induced in nematode feeding sites of heterologous plants. Molecular plant pathology 8:423–436
Williamson VM (1999) Plant nematode resistance genes. Curr Opin Plant Biol 2:327–331
Williamson VM, Gleason CA (2003) Plant–nematode interactions. Curr Opin Plant Biol 6:327–333
Williamson VM, Kumar A (2006) Nematode resistance in plants: the battle underground. Trends in genetics : TIG 22:396–403
Wu Y, Zhang D, Chu JY, Boyle P, Wang Y, Brindle ID, De Luca V, Despres C (2012) The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell reports 1:639–647
Wubben MJ 2nd, Su H, Rodermel SR, Baum TJ (2001) Susceptibility to the sugar beet cyst nematode is modulated by ethylene signal transduction in Arabidopsis thaliana. Molecular plant-microbe interactions: MPMI 14:1206–1212
Wubben MJ 2nd, Rodermel SR, Baum TJ (2004) Mutation of a UDP-glucose-4-epimerase alters nematode susceptibility and ethylene responses in Arabidopsis roots. The Plant journal : for cell and molecular biology 40:712–724
Wubben MJ, Jin J, Baum TJ (2008) Cyst nematode parasitism of Arabidopsis thaliana is inhibited by salicylic acid (SA) and elicits uncoupled SA-independent pathogenesis-related gene expression in roots. Molecular plant-microbe interactions : MPMI 21:424–432
Yaghoobi J, Yates JL, Williamson VM (2005) Fine mapping of the nematode resistance gene Mi-3 in Solanum peruvianum and construction of a S-lycopersicum DNA contig spanning the locus. Mol Genet Genomics 274:60–69
Yoon EK, Yang JH, Lim J, Kim SH, Kim SK, Lee WS (2010) Auxin regulation of the microRNA390-dependent transacting small interfering RNA pathway in Arabidopsis lateral root development. Nucleic Acids Res 38:1382–1391
Youssef RM, MacDonald MH, Brewer EP, Bauchan GR, Kim KH, Matthews BF (2013) Ectopic expression of AtPAD4 broadens resistance of soybean to soybean cyst and root-knot nematodes. BMC Plant Biol 13:67
Yu LZ, Wu XQ, Ye JR, Zhang SN, Wang C (2012) NOS-like-mediated nitric oxide is involved in Pinus thunbergii response to the invasion of Bursaphelenchus xylophilus. Plant Cell Rep 31:1813–1821
Zhai J, Jeong DH, De Paoli E, Park S, Rosen BD, Li Y, Gonzalez AJ, Yan Z, Kitto SL, Grusak MA, Jackson SA, Stacey G, Cook DR, Green PJ, Sherrier DJ, Meyers BC (2011) MicroRNAs as master regulators of the plant NB-LRR defense gene family via the production of phased, trans-acting siRNAs. Genes Dev 25:2540–2553
Zhu QH, Fan L, Liu Y, Xu H, Llewellyn D, Wilson I (2013) miR482 regulation of NBS-LRR defense genes during fungal pathogen infection in cotton. PLoS ONE 8:e84390
Acknowledgments
The authors would like to thank Alabama Cotton Commission, Cotton Inc,, and the Alabama Agricultural Experimental Station for research support for this work. Additionally, the financial support from the Auburn University Department of Biological Sciences and the Auburn University Cellular and Molecular Biosciences Program to Ruijuan Li during her graduate program is gratefully acknowledged.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Neal Stewart.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, R., Rashotte, A.M., Singh, N.K. et al. Integrated signaling networks in plant responses to sedentary endoparasitic nematodes: a perspective. Plant Cell Rep 34, 5–22 (2015). https://doi.org/10.1007/s00299-014-1676-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-014-1676-6