Abstract
Key message
Expression of a truncated form of wheat TdSOS1 in Arabidopsis exhibited an improved salt tolerance. This finding provides new hints about this protein that can be considered as a salt tolerance determinant.
Abstract
The SOS signaling pathway has emerged as a key mechanism in preserving the homeostasis of Na+ and K+ under saline conditions. We have recently identified and functionally characterized, by complementation studies in yeast, the gene encoding the durum wheat plasma membrane Na+/H+ antiporter (TdSOS1). To extend these functional studies to the whole plant level, we complemented Arabidopsis sos1-1 mutant with wild-type TdSOS1 or with the hyperactive form TdSOS1∆972 and compared them to the Arabidopsis AtSOS1 protein. The Arabidopsis sos1-1 mutant is hypersensitive to both Na+ and Li+ ions. Compared with sos1-1 mutant transformed with the empty binary vector, seeds from TdSOS1 or TdSOS1∆972 transgenic plants had better germination under salt stress and more robust seedling growth in agar plates as well as in nutritive solution containing Na+ or Li+ salts. The root elongation of TdSOS1∆972 transgenic lines was higher than that of Arabidopsis sos1-1 mutant transformed with TdSOS1 or with the endogenous AtSOS1 gene. Under salt stress, TdSOS1∆972 transgenic lines showed greater water retention capacity and retained low Na+ and high K+ in their shoots and roots. Our data showed that the hyperactive form TdSOS1∆972 conferred a significant ionic stress tolerance to Arabidopsis plants and suggest that selection of hyperactive alleles of the SOS1 transport protein may pave the way for obtaining salt-tolerant crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salinity is one of the most severe environmental stresses affecting plant productivity worldwide. High concentration of sodium (Na+) in the soil solution impairs cell metabolism and photosynthesis by imposing an osmotic stress on cell–water relations and by the toxicity of Na+ in the cytoplasm. Plants prevent excessive Na+ accumulation in the symplast through three mechanisms that function in a cooperative manner, i.e., restriction of Na+ influx, active Na+ extrusion at the root–soil interface, and vacuolar sequestration of Na+ (Niu et al. 1995; Tester and Davenport 2003). Moreover, transport systems that control net Na+ uptake into the root xylem vessels and regulate Na+ movement to shoots are salt tolerance determinants (Shi et al. 2002; Olías et al. 2009; Munns et al. 2012).
In Arabidopsis thaliana, three SOS genes (SOS1, SOS2 and SOS3) have been found to function in a common pathway (Wu et al. 1996; Zhu 2000). The SOS signal transduction pathway is important for ion homeostasis and salt tolerance in plants (Zhu 2003; Pardo et al. 2006). The biochemical and physiological functions of SOS1 have been demonstrated first in Arabidopsis thaliana and later in other plant species by genetic criteria (Shi et al. 2000, 2002; Oh et al. 2009; Olías et al. 2009). SOS1 encodes for a plasma membrane Na+/H+ antiporter involved in removing Na+ ions from cells (Shi et al. 2000, 2002; Pardo 2010). SOS1 is preferentially expressed in the epidermis of the root tip region and in xylem parenchyma cells (Shi et al. 2002). In salt stress conditions, SOS1 protein controls long-distance Na+ transport since this ion is transported from the root to the shoot via the xylem (Shi et al. 2002). This critical function was demonstrated also in the halophytic Arabidopsis relative Thellungiella salsuginea (Oh et al. 2009) and in tomato (Olías et al. 2009). In Arabidopsis, SOS1 likely works in concert with HKT1 at the plasma membrane of xylem parenchyma cells to finely regulate the amount of Na+ being delivered to the shoot (Sunarpi et al. 2005; Pardo et al. 2006; Pardo 2010). SOS proteins contribute to different responses to salt stress besides ions homeostasis (Ji et al. 2013). SOS1 has been also implicated in oxidative stress tolerance by interaction of its cytoplasmic tail with RCD1 (radical-induced cell death) (Agarwal et al. 2006). The role of SOS1 in K+ acquisition may be indirect and could possibly arise through H+ coupling with H+-K+ co-transporters (Huang et al. 2012).
Sodium efflux by SOS1 exchanger is regulated through protein phosphorylation by the alternative SOS2/SOS3 and SOS2/CBL10 protein kinase complexes (Qiu et al. 2002; Quintero et al. 2002, 2011; Quan et al. 2007). In addition to the SOS2 kinase, SOS1 is also phosphorylated in an as yet uncharacterized site by mitogen-activated protein kinase 6 (MPK6) (Yu et al. 2010). SOS2 is a serine/threonine protein kinase belonging to the SNF1-related kinase 3 (SnRK3) family (Liu et al. 2000; Gong et al. 2002). SOS3 is a myristoylated calcium binding protein capable of sensing Ca2+ oscillations elicited by salt stress (Liu and Zhu 1998). Both N-myristoylation and calcium binding are required for SOS3 function in plant salt tolerance (Ishitani et al. 2000). The C-terminal regulatory domain of SOS2 contains an auto-inhibitory FISL motif that binds to SOS3 or CBL10, thereby releasing SOS2 from auto-inhibition (Liu et al. 2000; Guo et al. 2001; Quan et al. 2007). SOS3 acts primarily in Arabidopsis roots under salt stress, whereas the SOS3 homolog SOS3-LIKE CALCIUM BINDING PROTEIN8 (SCABP8)/CALCINEURIN B-LIKE 10 functions mainly in shoot response to salt toxicity (Halfter et al. 2000; Quan et al. 2007). It has been shown that under salt stress, SOS2 phosphorylates SCaBP8/CBL10 at its C-terminus. This phosphorylation occurs at the membrane, stabilizes the SCaBP8–SOS2 interaction and enhances plasma membrane Na+/H+ exchange activity of SOS1 (Quan et al. 2007; Lin et al. 2009). SOS2 was also shown to interact with type 2C protein phosphatase abcisic acid (ABA)-insensitive 2 (ABI2), suggesting a crosstalk between the ABA pathway and SOS pathway (Ohta et al. 2003). In a recent report, the photoperiodical and circadian clock switch Gigantea (GI) was shown to be a negative regulator of the SOS pathway. By physical interaction, GI effectively sequesters SOS2, thus preventing the activation of SOS1 in the absence of stress (Kim et al. 2013). Recently, it has been demonstrated that SOS3 plays an important role in salt tolerance by mediating calcium-dependent microfilament reorganization (Ye et al. 2013).
The Arabidopsis AtSOS1 is relieved from auto-inhibition upon phosphorylation of the auto-inhibitory domain by SOS2/SOS3 (Quintero et al. 2011). The auto-inhibitory domain of AtSOS1 interacts intra-molecularly with an adjacent domain that is essential for activity. Likewise, the activation mechanism of durum wheat TdSOS1 protein involves the phosphorylation and inactivation of an auto-inhibitory domain located at the C-terminal end of the transporter. This region contains the essential residues for its phosphorylation by the Arabidopsis protein kinase SOS2 (Feki et al. 2011). Deletion of the auto-inhibitory domains of AtSOS1 and TdSOS1 rendered SOS1 proteins that were biochemically hyperactive and independent of the SOS2/SOS3 regulatory complex. These truncated proteins conveyed much greater salt tolerance to the budding yeast, but they have not been assayed for salt tolerance in whole plants. Since the overexpression of Arabidopsis thaliana AtSOS1 gene improved plant salt tolerance in A. thaliana and in tobacco (Shi et al. 2003; Yue et al. 2011), the question addressed here is whether the hyperactive forms of SOS1 could bestow whole plants with enhanced salt tolerance compared to wild-type proteins. In the present study, durum wheat TdSOS1 and its hyperactive form TdSOS1∆972 were expressed under the control of a constitutive promoter and transferred into Arabidopsis sos1-1 mutant genome by Agrobacterium-mediated transformation. The generated transgenic Arabidopsis plants were used to investigate which form of durum wheat SOS1 (the wild-type TdSOS1 and/or the hyperactive form TdSOS1∆972) improved ionic stress tolerance of Arabidopsis plants. Our results demonstrated that the hyperactive form TdSOS1∆972 conferred significant ionic stress tolerance to the transgenic Arabidopsis that was superior to that imparted by wild-type SOS1 protein. Thus, this hyperactive form can be considered as a salt tolerance determinant that could be screened for in a mutagenized plant population as a way to create salt-tolerant crops that are not considered genetically modified organisms (GMO).
Materials and methods
Plasmid constructs and Arabidopsis transformation
The two durum wheat cDNAs encoding for Na+/H+ antiporter (TdSOS1) and its hyperactive form (TdSOS1∆972) were reported in Feki et al. (2011). The open reading frame of TdSOS1 was amplified by PCR, using the recombinant plasmid pTdSOS1-1 (Feki et al. 2011) as a template, and the primers SX (5′-GTGTCTAGAATGGAGACGGAGGAGGCCGGCTCCC-3′, XbaI site underlined) and SE (5′-ATACTAGTTCAGCTGCCTCGCGGTGGGCCGGA-3′, SpeI site underlined). The resulting fragment was cloned downstream of the constitutive cauliflower mosaic virus (CaMV) 35S promoter into the XbaI and SpeI sites of the pBI321 binary vector containing the kanamycin-resistant selectable marker (Martίnez-Atienza et al. 2007). The resultant plasmid was named BSOS. The full length of TdSOS1Δ972 open reading frame was amplified using the primers SX and SHE (5′-TAACTAGTTCAGAGGGTTGAAGACAGCAA-3′, SpeI site underlined) using the recombinant plasmid pTdSOS1-3 (Feki et al. 2011) as a template. The resulting fragment was cloned in the same binary vector using the same restriction enzymes, and the resultant recombinant binary vector was named herein BSOSH. These two recombinant plasmids were introduced separately into Agrobacterium tumefaciens strain GV3101 (Konez and Schell 1986). The Arabidopsis thaliana transformation was performed using the floral dipping technique (Clough and Bent 1998). Transgenic plants harboring TdSOS1 or TdSOS1∆972 were selected on Murashige and Skoog (MS) agar medium (Murashige and Skoog 1962) supplemented with 50 mg L−1 kanamycin. The presence and the integrity of the transgene were further confirmed by PCR amplifications using specific primers for TdSOS1 and TdSOS1∆972 sequences. Transgenic lines were advanced to T3 generation and homozygous lines were used in this study. The Arabidopsis sos1-1 mutant line transformed with 35S-AtSOS1 has been previously described (Shi et al. 2000).
RNA extraction and RT-PCR
Total RNA was extracted from 100 mg of young Arabidopsis transgenic lines using the Trizol method (Invitrogen). Total RNA (10 μg) was treated with RNase-free DNase (Promega) at 37 °C for 15 min and further incubated at 65 °C for 10 min. The reverse transcription was performed at 37 °C for 1 h, using the oligo-dT (18 mer) primer and M-MLV reverse transcriptase (Invitrogen). One microliter of the first strand cDNAs was used as template for PCR amplification with a pair of gene-specific primer KM1 (5′- GCATCTTATTGGAAGGATTTCTGAA-3′) and KM2 (5′-GGAGAGTCCACTCTGACGAT-3′) for TdSOS1 allele and the primers KH1 (5′-AATGCTTGAAGAGGGACGAATAA-3′) and KH2 (5′-CCAGTTAATGCCTCATATAGACC-3′) for TdSOS1∆972 allele. An Arabidopsis thaliana β-tubulin gene fragment, used as an internal control, was amplified with the following primers (5′-GTCCAGTGTCTGTGATATTGCACC-3′) and (5′-GCTTACGAATCCGAGGGTGCC-3′). Samples were denatured for 3 min at 94 °C and then run for 30 cycles of 1 min at 94 °C, 45 s at 55 °C, and 1 min 30 s at 72 °C with a final extension of 5 min at 72 °C. The PCR products were separated by agarose gel electrophoresis.
Growth conditions and stress treatments
Seeds of each transgenic Arabidopsis thaliana line were surface sterilized and stratified at 4 °C for 2–4 days. A germination test was initiated with seeds sown on MS agar medium containing 35 mM or 50 mM NaCl. The seed germination assay was performed by plating 30 seeds of each T3 homozygous seeds of transgenic Arabidopsis lines and sos1-1 mutant expressing AtSOS1 on MS medium containing NaCl.
For ionic-tolerance experiments, T3 homozygous seeds of transgenic Arabidopsis lines were grown on MS agar medium for 1 week under light/dark cycle condition of 16 h light/8 h dark cycle at 22 °C and then transferred to MS medium supplemented with 35, 75 mM NaCl or 15 mM LiCl. Plates were incubated vertically with seedling in the upright position. Plant survival was monitored after 10 days of ionic stress.
For ionic stress treatment under greenhouse conditions, transgenic plants were grown in nutrient solution (Hewitt 1966) for 2 weeks, with a light/dark cycle of 16 h light (23 °C)/8 h dark (18 °C) cycle at 60–70 % relative humidity. Thereafter, the nutrient solution was supplemented with NaCl to a final concentration of 5 mM, or with LiCl to a final concentration of 0.2 mM, and plants were kept under stress for 13 days.
Root elongation test
Seeds of TdSOS1 or TdSOS1∆972 transgenic plants of homozygous T3 generation were surface sterilized and plated on MS agar medium plates with Arabidopsis sos1-1 mutant transformed with the empty binary vector (called pBI line) or with the endogenous AtSOS1 gene (denoted as the AtS line henceforth). Plates were placed vertically in a growth chamber under a 16 h light/8 h dark cycle at 22 °C. To evaluate the growth rate under ionic stress conditions, 7-day-old transgenic seedlings were transferred to MS medium supplemented with different concentrations of NaCl (25, 35 and 75 mM) or LiCl (10 and 15 mM). After 10 days of incubation, root elongation was determined.
Leaf surface determination
Total leaf area of Arabidopsis seedlings in nutritive solution containing 5 mM NaCl or 0.2 mM LiCl was calculated using image processing and analysis program UTHSCSA (http://compdent.uthscsa.edu/dig/itdesc.html).
Determination of fresh weight and ion contents
Fresh weights were determined immediately at the end of salt treatment. To determine Na+ and K+ contents in plant tissues, leaves were carefully separated from roots, rinsed in deionized water, blotted with filter paper and oven dried at 70 °C until constant mass was reached. Dry material was mineralized using 0.5 N HNO3. After centrifugation to remove debris, the supernatants were analyzed by atomic absorption spectrometry.
Determination of relative water content (RWC)
Leaves were harvested and their fresh weight (FW) was determined immediately. They were floated on deionized water overnight at 4 °C and the turgescent weight (TW) was recorded. Finally, leaves were dried at 70 °C and the dry weight (DW) was recorded. Relative water content was calculated using the following formula: RWC (%) = (FW − DW/TW − DW) × 100.
Statistical analysis
Data were analyzed using one-way analysis of variance and treatment mean separations were performed using Duncan’s multiple range tests at the 5 % level of significance.
Results
Molecular characterization of transgenic Arabidopsis lines
Mutants of Arabidopsis lacking SOS1 are extremely salt sensitive and have combined defects in Na+ extrusion and in control of long-distance Na+ transport (Qiu et al. 2002; Shi et al. 2002). The Arabidopsis sos1-1 mutant bears a 14-bp gene deletion that completely abrogates AtSOS1 activity (Shi et al. 2000; Qiu et al. 2002). The durum wheat Na+/H+ antiporter TdSOS1 (GenBank accession no. EU552490) and its hyperactive form TdSOS1Δ972 were previously characterized (Feki et al. 2011). The difference in the protein structure between these two wheat SOS1 forms is that the hyperactive protein has a shorter C-terminal tail from which the auto-inhibitory domain (AID) and SOS2 phosphorylation site (S2P) have been removed (Fig. 1). To investigate whether the truncated form of durum wheat SOS1 protein complemented the Arabidopsis sos1-1 mutant, two binary vectors BSOS and BSOSH containing TdSOS1 or TdSOS1∆972, respectively, were constructed. These vectors contain the NPTII gene conferring resistance to kanamycin as a marker for plant transformation. After Agrobacterium-mediated transformation of Arabidopsis plants and selection with kanamycin, several transformants were produced. For each transformation, four transgenic lines were propagated to the T3 generation from which homozygous plants were isolated for further analyses. The T-DNA region of these two constructs is schematically presented in Fig. 2a. Using TdSOS1- and TdSOS1∆972-specific primers, a fragment of the T-DNA was amplified to verify the presence of the transgene, and the sos1-1 genetic background was verified by diagnostic PCR of the 14-bp deletion that defines this mutant allele (data not shown).
Schematic representation of SOS1 protein structure. TdSOS1 has a pore domain with 12 transmembrane regions, followed by a long hydrophilic tail containing a putative cyclic nucleotide binding domain (CNBD), an auto-inhibitory domain (AID) and the SOS2 phosphorylation site (S2P). The TdSOS1Δ972 protein has a shorter C-terminal domain than the wild-type TdSOS1, which contains only the CNBD domain. Numbers present the position of amino acid residues delimiting each domain
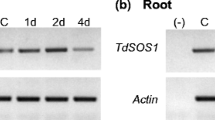
Molecular analysis of TdSOS1 and TdSOS1Δ972 transgenic plants. a Physical map of the T-DNA region in the binary vectors BSOS and BSOSH. The transgene was inserted between the CaMV35S promoter (P35S) and NOS terminator (TNOS); the NPTII marker is flanked by the NOS promoter (PNOS) and terminator (TNOS). KM1, KM2, KH1 and KH2 indicate the primers used for RT-PCR amplification. Expression analysis by RT-PCR of TdSOS1 in TdSOS1 transgenic lines (MW) (b) and of TdSOS1Δ972 in TdSOS1Δ972 transgenic lines (MH) (c). Specific PCR products of 0.6 kb for MW transgenic lines and 0.8 kb for MH transgenic lines were detected, which were absent in non-transformed (NT) Arabidopsis sos1-1 mutant. A 0.5 kb of β-tubulin gene fragment was amplified by RT-PCR as an internal control. (−), negative control without cDNA
The expression level of TdSOS1 and TdSOS1∆972 was analyzed using reverse transcriptase polymerase chain reaction (RT-PCR), performed on young leaves of eight transgenic lines and non-transformed plants. As a control for cDNA amplification, the constitutively expressed β-tubulin gene was amplified. As shown in Fig. 2b, c, specific bands of the expected sizes were obtained for TdSOS1 transgenic lines (MW1, 7, 8 and 9) and for TdSOS1∆972 transgenic lines (MH1, 3, 4 and 6). However, no amplification was obtained in the case of non-transformed plants (NT). The expression level was almost similar for lines expressing TdSOS1 or TdSOS1∆972 except MH3 line (Fig. 2b, c). The higher expression level of TdSOS1∆972 observed in line MH3 was probably due to multiple insertions since the NPTII marker failed to segregate as a single locus; because of this the MH3 line was not used for further analyses. For other transgenic lines, the corresponding mRNA was stably produced through generations T0–T3. For physiological analyses, we selected lines MW1, MW7 and MW8 for transgenic plants harboring TdSOS1 gene, and MH1, MH4 and MH6 for transgenic plants harboring TdSOS1∆972. For these selected lines, genetic segregation data performed using NPTII gene gave rise to a 3:1 ratio confirming that this marker segregates as a single copy gene.
Seed germination under salt stress condition
The effect of NaCl on seed germination on MS medium was investigated in the transgenic MW1, MW7, MH1 and MH6 lines and Arabidopsis sos1-1 mutant transformed with the empty binary vector (pBI line) or with the endogenous AtSOS1 gene (AtS line). The germination rate of these transgenic lines was not affected by 35 mM NaCl concentration. However, while the transgenic lines expressing durum wheat SOS1 proteins kept growing, seedlings of the pBI line stopped the growth after 7 days of germination in this medium. At 50 mM NaCl, the pBI line failed to germinate at all, while TdSOS1∆972 transgenic lines presented a higher germination rate (almost 90 %) than TdSOS1 transgenic lines and AtS lines (≈60 %) (Fig. 3a). These data show that both wheat alleles, TdSOS1 and TdSOS1∆927, are functional in planta and that the hyperactive form TdSOS1∆972 indeed confers a better germination rate than the wild-type TdSOS1 form and than AtSOS1.
Salt tolerance phenotypes of transgenic lines. a Seeds of control lines [Arabidopsis sos1-1 mutant transformed with the empty binary vector pBI321 (pBI), or with the endogenous AtSOS1 gene (AtS)], the TdSOS1Δ972 transgenic lines (MH1 and MH6) and the TdSOS1 transgenic lines (MW1 and MW7) were germinated directly on MS medium and on MS medium supplemented with 35 mM NaCl or 50 mM NaCl, and then grown for 7 days. b Effect of stress treatments on the growth of TdSOS1 transgenic lines (MW1, MW7 and MW8), TdSOS1Δ972 transgenic lines (MH1, MH4 and MH6) and Arabidopsis sos1-1 transformed with the empty binary vector (pBI) or with the endogenous AtSOS1 gene (AtS). Each line was germinated on MS agar medium for 7 days and then transferred to MS medium and to MS medium supplemented with 35 mM NaCl, 75 mM NaCl or with 15 mM LiCl. Photographs were taken 10 days after the transfer. c Root elongation of TdSOS1 transgenic lines (MW1 and MW7), TdSOS1Δ972 transgenic lines (MH1 and MH6), pBI and AtS lines, on MS medium containing different concentrations of NaCl or LiCl. Values are mean ± SE (n = 4). Asterisks indicate statistically significant greater mean values compared to Arabidopsis transformed with the endogenous AtSOS1 gene (AtS line) (P < 0.05)
The hyperactive form of durum wheat SOS1 confers better ionic stress tolerance to Arabidopsis than the wild-type form and AtSOS1
One-week-old seedlings were transferred to MS agar medium containing NaCl or LiCl, and plant survival was monitored after 10 days. The toxic effect of NaCl in Arabidopsis sos1-1 mutant transformed with the empty binary vector (pBI line) was observed by the inhibition of growth. In the presence of NaCl, phenotypic suppression of the Arabidopsis sos1-1 mutant by durum wheat TdSOS1 was partial because of the greater growth of congenic lines transformed with Arabidopsis AtSOS1 (AtS line). By contrast, TdSOS1∆972 transgenic lines (MH lines) showed a significantly better salt tolerance phenotype than AtS line in the presence of high concentration of NaCl (75 mM). This concentration was found to be toxic for Arabidopsis plants transformed with the wild-type TdSOS1 form (MW lines) or with the empty binary vector (Fig. 3b).
The Arabidopsis sos1-1 mutant is hypersensitive to both Na+ and Li+ ions (Wu et al. 1996). Li+ is often used as a Na+ analog with higher toxicity and hence it can be used at lower concentrations than Na+ to inhibit plant growth, thus avoiding the osmotic effect of high salt concentrations. In medium containing 15 mM LiCl, the toxic effect of Li+ was significant for the empty vector pBI line (Fig. 3b). These plants showed chlorotic leaves with clear growth inhibition. The wild-type TdSOS1 protein partially suppressed the Li+ sensitivity of the sos1-1 mutant, albeit less efficiently than the AtSOS1 protein. On the other hand, expression of the TdSOS1∆972 protein imparted maximal Li+ tolerance above the levels achieved by the full-length proteins TdSOS1 and AtSOS1 (Fig. 3b). We quantitated and compared the root elongation of TdSOS1 and TdSOS1∆972 transgenic lines with that of the Arabidopsis sos1-1 mutant transformed with endogenous AtSOS1 gene (AtS line) or with the empty binary vector (pBI line). The root elongation of the pBI line decreased dramatically with the addition of NaCl or LiCl. Roots of TdSOS1 transgenic lines (MW1 and MW7) showed a modest growth improvement compared to pBI line. By contrast, the TdSOS1∆927 transgenic lines (MH1, MH6) displayed the highest growth rate, which was only slightly affected by the NaCl treatment but reduced by about 40-60 % by LiCl (Fig. 3c).
A more physiological salt tolerance test was performed using hydroponic cultures. Plants were grown in control nutrient solution for 2 weeks and then the medium was supplemented with 5 mM NaCl or 0.2 mM LiCl. After 13 days, the transgenic line expressing the wild-type TdSOS1 allele (MW1 and MW7) displayed a partial Na+- and Li+-tolerance phenotype, since it grew better than the pBI line, but AtS transgenic plants were bigger under the same conditions. By contrast, the transgenic lines overexpressing the hyperactive form TdSOS1∆972 (MH1 and MH6) produced the strongest tolerance phenotype in the presence of Na+ or Li+, demonstrating again that the gain of function of the wheat mutant allele is translated into a better salt tolerance phenotype (Fig. 4a). The salt-induced growth inhibition was analyzed by measuring the total leaf area (TLA) on Arabidopsis transgenic lines (MW1 MW7, MH1 and MH6) and the control plants (pBI and AtS) subjected to NaCl and LiCl stress (Fig. 4b). In the absence of stress, similar TLA values were scored for all these lines (≈35 mm2). In the NaCl stress condition, MH1 and MH6 lines showed only a slight reduction of TLA relative to control conditions. Moreover, the TLA values registered in MH1 and MH6 lines were higher than those of other lines, including the control line AtS. At 0.2 mM LiCl, the decrease in TLA values was significant for all lines, albeit in the TdSOS1∆972 transgenic lines, MH1 and MH6, the TLA was reduced only by 25 % as in the control line AtS (Fig. 4b).
Stress tolerance of transgenic lines grown in hydroponic culture. a Seeds of TdSOS1 transgenic lines (MW1 and MW7) and TdSOS1Δ972 transgenic lines (MH1 and MH6) were germinated in distillated water solution for 1 week and then transferred to nutritive solution and nutritive solution supplemented with 5 mM NaCl or 0.2 mM LiCl. The response to ionic stress was evaluated in comparison to Arabidopsis sos1-1 mutant transformed with the empty vector (pBI) or with the endogenous AtSOS1 gene (AtS). Photographs were taken after 13 days in the hydroponic culture. b Total leaf area (TLA) of individual leaves from the control (pBI and AtS) and transgenic (MW1, MW7, MH1 and MH6) Arabidopsis plants. Values are means of five replicates of one fully expanded leaf per plant. c Relative water content (RWC) of transgenic and control plants after 13 days in salt stress (5 mM NaCl) on hydroponic solution. d Shoots and roots fresh weight of Arabidopsis sos1-1 transgenic lines after 13 days in nutritive solution containing 2.5 or 5 mM NaCl (left) or 0.2 mM LiCl (right). Values are mean ± SE (n = 5). Asterisks indicate significantly greater mean values compared to Arabidopsis transformed with the endogenous AtSOS1 gene (AtS line) (P < 0.05)
We also measured shoots and roots weight of Arabidopsis transgenic TdSOS1 and TdSOS1∆972 lines in comparison with the control plants (pBI and AtS lines). In the absence of stress, similar shoots and roots fresh weight were scored for all plants. At 2.5 mM NaCl, a reduction of ≈50 % in shoot and root fresh weight was registered in the pBI line. However, the reduction in root fresh weight for the two MH lines was slight and no difference was detected in fresh shoot weight compared to control conditions without salt. Concerning the two MW line, only root fresh weight was significantly reduced. At 5 mM NaCl, shoot and root fresh weight was reduced significantly for all the transgenic lines. In fact, the reduction of fresh weight of MW lines was about 50 % in shoots and 75 % in roots. These reductions were significantly lower for TdSOS1∆972 transgenic lines compared to the positive control line AtS (Fig. 4c). Addition of LiCl to the hydroponic solution reduced the shoot fresh weight and root to ≈50 % for MH1 and MH6 lines. However, shoot and root fresh weight of the MH1 and MH6 lines was still higher than that of TdSOS1 and AtS transgenic lines (Fig. 4c).
Taken together, these results indicate that the wild-type TdSOS1 form of durum wheat SOS1 is functional, but less efficient than the native AtSOS1 counterpart to complement the Arabidopsis sos1-1 mutant. This complementation is highly improved by the use of the hyperactive form TdSOS1∆972, which produces plants with better performance under salt stress even when compared with the transgenics that overexpress the wild-type Arabidopsis SOS1 protein, suggesting that the truncated wheat SOS1 protein is a superior salt tolerance determinant.
Relative water content of Arabidopsis transgenic lines
The relative water content (RWC) of Arabidopsis transgenic lines (MW1, MW7, MH1 and MH6) and the control plants (pBI and AtS) subjected to salt stress for 13 days was determined. Values of RWC around 85–95 % were found in well-hydrated tissues. A RWC lower than the critical mark of 50 % typically results in plant death (Pardo 2010). After application of salt stress, RWC of TdSOS1 and TdSOS1∆972 transgenic plants was higher than 50 %. As shown in Fig. 4d, in control conditions, the RWC was almost the same for all tested lines. In the presence of 5 mM NaCl, all transgenic plants showed reduction in their RWC. However, a higher water loss was observed in the pBI line compared to the other transgenic lines. The RWC registered in TdSOS1∆972 transgenic lines decreased slightly to a value of ≈0.8 relative to non-stress conditions. This result indicated a greater water retention capacity in TdSOS1∆972 transgenic plants than the other transgenic lines.
TdSOS1∆972 transgenic lines accumulate less Na+ than transgenic lines expressing wild-type SOS1 proteins
The main function of SOS1 is to maintain a favorable Na+/K+ ratio in plants under saline stress (Pardo 2010). It has been shown that sos1-1 mutant plants accumulate Na+, inducing K+ loss, and conversely plants overexpressing SOS1 decrease internal Na+ levels (Wu et al. 1996; Shi et al. 2003). We analyzed Na+ and K+ contents in shoots and roots of transgenic plants (MW1, MW7, MH1 and MH6 lines) and in control plants (pBI and AtS lines), subjected to salt stress (5 mM NaCl) in hydroponic solution for 13 days. The contents were determined on a dry matter basis and hence were independent of the RWC of the samples. As shown in Fig. 5, under control conditions, the Na+ and K+ contents were almost the same in shoots and roots for all the transgenic plants. When grown at 5 mM NaCl, the Na+ content increased in shoots and roots of these plants and especially for Arabidopsis sos1-1 mutant transformed with the empty binary vector (pBI line). As expected, all other transgenic lines accumulated less Na+ in shoot and root than pBI line. However, the Na+ content in MW1 and MW7 lines was higher than MH1 and MH6 lines (Fig. 5a), reflecting the different activity of the durum wheat wild-type protein and the hyperactive one. In the presence of 5 mM NaCl, the K+ content decreased significantly in the shoot and root of the pBI line. The K+ content of TdSOS1 transgenic lines was slightly higher than in the pBI line in both shoot and root. The retention of K+ in the shoot and root of TdSOS1∆972 transgenic line was significantly higher due to the lower level of Na+ accumulation (Fig. 5b). Arabidopsis sos1-1 transformed with AtSOS1 gene showed intermediates values of Na+ and K+ to those observed in TdSOS1 and TdSOS∆972 transgenic lines (Fig. 5).
Ion contents. Sodium (a) and potassium (b) contents in shoots and roots of transgenic and control plants grown in hydroponic culture under salt treatment (5 mM NaCl) for 13 days. Values are mean ± SE (n = 4). Asterisks indicate statistical differences of either smaller sodium contents or greater potassium contents in the transgenic lines compared to the control Arabidopsis line transformed with the endogenous AtSOS1 gene (AtS line) (P < 0.05)
Discussion
Arabidopsis sos1-1 mutant plants have been shown to display sensitivity to both Na+ and Li+ ions (Wu et al. 1996; Shi et al. 2000). Recently, the durum wheat SOS1 has been functionally characterized in a yeast strain lacking endogenous Na+ efflux proteins. The auto-inhibitory domain at the C-terminus of TdSOS1 regulates its activation by the protein kinase SOS2. The truncation of the auto-inhibitory domain in the TdSOS1∆972 form bypasses the need of activation by Arabidopsis thaliana SOS2–SOS3 complex and becomes a constitutively active exchanger (Feki et al. 2011). Overexpression of SOS1 has been shown to enhance the salt tolerance of transgenic Arabidopsis (Shi et al. 2003) and tobacco plants (Yue et al. 2011). In this work, we addressed the question of whether the hyperactive form of TdSOS1 was a superior salt tolerance determinant compared to wild-type TdSOS1 protein. To this end, we generated Arabidopsis sos1-1 mutant transgenic plants expressing TdSOS1 or TdSOS1∆972. Firstly, our data showed that expression of the wild-type protein TdSOS1 slightly complemented the Na+ and Li+ sensitivity of Arabidopsis sos1-1 mutant (Figs. 3, 4). Notwithstanding these results in planta, TdSOS1 behaved as functionally equivalent to the Arabidopsis AtSOS1 protein in the yeast system (Feki et al. 2011). Like AtSOS1 (Qiu et al. 2002; Shi et al. 2002), OsSOS1 (Martίnez-Atienza et al. 2007) and TaSOS1 (Xu et al. 2008), the TdSOS1 protein catalyzed Na+/H+ antiport in yeast (Feki et al. 2011). The functional analysis of the yeast system also showed that the Arabidopsis SOS2–SOS3 complex recognized and activated in vivo wild-type TdSOS1. Similar results were reported with the rice OsSOS1 homolog, which complemented the sos1-1 mutant of Arabidopsis albeit less efficiently than endogenous AtSOS1 (Martínez-Atienza et al. 2007). By contrast, complementation of the sos2 and sos3 mutants with the corresponding OsCIPK24 and OsCBL4 proteins of rice produced near wild-type tolerance to NaCl (Martínez-Atienza et al. 2007). Thus, it appears that activation of heterologous SOS1 proteins expressed in Arabidopsis requires additional event(s) besides phosphorylation by the SOS2–SOS3 complex. One of such regulatory events could be phosphorylation by MPK6 (Yu et al. 2010; Ji et al. 2013). Deletion of the C-terminal auto-inhibitory domain of TdSOS1 either bypasses this requirement or compensates for it owing to the derepressed activity of TdSOS1∆972.
Recently, Feki and colleagues (2011) demonstrated that in yeast the TdSOS1 protein catalyzes efficiently not only Na+ efflux, but also Li+ efflux (Feki et al. 2011). This is in contrast to Arabidopsis, in which SOS1 appears to have a preference for Na+ transport over Li+, while the shorter but highly related protein AtNHX8 catalyzes specific Li+/H+ antiporter. Overexpression of AtNHX8 complemented the sensitivity to Li+, but not to Na+, of the sos1-1 mutant (An et al. 2007). No protein homologous to AtNHX8 has been found in the sequenced genomes of cereals. Here, we confirmed that TdSOS1 is a dual Na+, Li+/H+ antiporter in planta since TdSOS1∆972 transgenic plants showed a tolerance phenotype in medium containing toxic concentration of Na+ or Li+.
Secondly, the tolerance was significantly greater for transgenic plants expressing the hyperactive form TdSOS1∆972 than those that expressed the wild-type form TdSOS1 or AtSOS1. This tolerance was illustrated by both higher seed germination and growth rate in medium containing toxic concentration of Na+ or Li+. It has been demonstrated that the hyperactive form TdSOS1∆972 confers a significant tolerance to the transformed yeast grown in medium containing high concentration of Na+ or Li+. Yeast cells transformed with the hyperactive form TdSOS1∆972 showed a vigorous growth independently of the presence or not of the Arabidopsis thaliana SOS2–SOS3 protein kinase complex (Feki et al. 2011). This finding can explain the significant tolerance of TdSOS1∆972 transgenic Arabidopsis lines.
Under the salinity conditions used in this study, the difference in RWC in the control and transgenic plants suggest the possibility of a higher uptake of water and maintenance of turgor for TdSOS1∆972 transgenic plants. Increased turgor can allow for maintenance of greater stomatal aperture (hence, CO2 supply to the leaf) as well as increased cell division and expansion (hence, leaf growth and development). On the other hand, TdSOS1∆972 transgenic plants retained less Na+ and more K+ than the other transgenic plants (Fig. 5). Maintenance of appropriate intracellular K+/Na+ balance is critical for metabolic function as Na+ cytotoxicity is largely due to competition with K+ for binding sites in enzymes essential for cellular functions (Serrano 1996; Maathuis and Amtmann 1999). K+ is an essential macronutrient that is required for diverse cellular processes such as osmotic regulation, enzyme activity, protein and starch synthesis, respiration and photosynthesis (Hauser and Horie 2010). The capacity to maintain a low Na+ concentration or a high K+/Na+ ratio is considered as an indicator of potential salinity tolerance (Tester and Davenport 2003; Volkov et al. 2004; Hauser and Horie 2010).
Although considerable progress has been made in our current understanding of plant adaptation to environmental stress tolerance, the goal of improving the resistance of crops to abiotic stresses has seen limited success (Pardo 2010). Numerous genes and proteins have been shown to effect tolerance to environmental stress in an array of plant species, but their implementation into improved crops largely relies on genetic modifications that face administrative hurdles and negative public perception. The use of hardy wild relatives or relatively stress-tolerant varieties of crop plants as a source of genetic determinants of stress tolerance is time-consuming and labor intensive. Moreover, classical breeding approaches have revealed that stress tolerance traits are dispersed in various quantitative trait loci (QTLs), which make genetic selection of these traits difficult, while undesirable genes are often transferred in combination with desirable ones. Our results demonstrated that the wild-type or the hyperactive form of durum wheat SOS1 suppressed the ionic sensibility phenotype of Arabidopsis sos1-1 mutant. These two TdSOS1 variants could substitute the endogenous protein AtSOS1 and consequently regulate the Na+ and K+ homeostasis in cells. Unlike the wild-type form, the hyperactive form TdSOS1∆972 conferred a significant ionic stress tolerance to the transgenic Arabidopsis. Thus, this hyperactive SOS1 form can be considered as a salt tolerance determinant and it should be feasible to screen for this or synonymous mutations in a mutagenized population of a crop by TILLING as a way to create salt-tolerant crops that are not considered GMO.
References
Agarwal SK, Zhu J, Kim K, Agarwal M, Fu X, Huang A, Zhu JK (2006) The plasma membrane Na+/H+ antiporter SOS1 interacts with RCD1 and functions in oxidative stress tolerance in Arabidopsis. Proc Natl Acad Sci USA 103:18816–18821. doi:10.1073/pnas.0604711103
An R, Chen QJ, Chai MF, Lu PL, Su Z, Qin ZX, Chen J, Wang XC (2007) AtNHX8, a member of the monovalent cation: proton antiporter 1 family in Arabidopsis thaliana, encodes a putative Li+/H+ antiporter. Plant J 49:718–728. doi:10.1111/j.1365-313X.2006.02990.x
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743. doi:10.1046/j.1365-313x.1998.00343.x
Feki K, Quintero FJ, Pardo JM, Masmoudi K (2011) Regulation of durum wheat Na+/H+ exchanger TdSOS1 by phosphorylation. Plant Mol Biol 76:545–556. doi:10.1007/s11103-011-9787-8
Gong D, Guo Y, Jagendorf AT, Zhu JK (2002) Biochemical characterization of the Arabidopsis protein kinase SOS2 that functions in salt tolerance. Plant Physiol 130:256–264. doi:10.1104/pp.004507
Guo Y, Halfter U, Ishitani M, Zhu JK (2001) Molecular characterization of functional domains in the protein kinase SOS2 that is required for salt tolerance. Plant Cell 13:1383–1399. doi:10.1105/TPC.010021
Halfter U, Ishitani M, Zhu JK (2000) The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc Natl Acad Sci USA 97:3735–3740. doi:10.1073/pnas.97.7.3735
Hauser F, Horie T (2010) A conserved primary salt tolerance mechanism mediated by HKT transporters: a mechanism for sodium exclusion and maintenance of high K+/Na+ ratio in leaves during salinity stress. Plant, Cell Environ 33:552–565. doi:10.1111/j.1365-3040.2009.02056.x
Hewitt EJ (1966) Sand and water culture methods used in the study of plant nutrition. Technical Communication No. 22 (revised 2nd edn). Com Bur of Horticul and Plant Crops East Malling, Maidstore, Kent
Huang GT, Ma SL, Bai LP, Zhang L, Ma H, Jia P, Liu J, Zhong M, Guo ZF (2012) Signal transduction during cold, salt, and drought in plants. Mol Biol Rep 39:969–987. doi:10.1007/s11033-011-0823-1
Ishitani M, Liu J, Halfter U, Kim C-S, Shi W, Zhu J-K (2000) SOS3 function in plant salt tolerance requires N-myristoylation and calcium binding. Plant Cell 12:1667–1677. doi:10.1105/tpc.12.9.1667
Ji H, Pardo JM, Batelli G, Van Oosten MJ, Bressan RA, Li X (2013) The salt overly sensitive (SOS) pathway: established and emerging roles. Mol Plant 6:275–286. doi:10.1093/mp/sst017
Kim WY, Ali Z, Park HJ et al (2013) Release of SOS2 kinase from sequestration with GIGANTEA determines salt tolerance in Arabidopsis. Nat Commun 4:1352. doi:10.1038/ncomms2357
Konez C, Schell J (1986) The promoter of TL-DNA gene 5′ controls the tissue specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet 204:383–396. doi:10.1007/BF00331014
Lin H, Yang Y, Quan R et al (2009) Phosphorylation of SOS3-LIKE CALCIUM BINDING PROTEIN 8 by SOS2 protein kinase stabilizes their protein complex and regulates salt tolerance in Arabidopsis. Plant Cell 21:1607–1619. doi:10.1105/tpc.109.066217
Liu J, Zhu JK (1998) A calcium sensor homolog required for plant salt tolerance. Science 280:1943–1945. doi:10.1126/science.280.5371.1943
Liu J, Ishitani M, Halfter U, Kim SC, Zhu JK (2000) The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc Natl Acad Sci USA 97:3730–3734. doi:10.1073/pnas.97.7.3730
Maathuis FJM, Amtmann A (1999) K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Ann Bot 84:123–133. doi:10.1006/anbo.1999.0912
Martίnez-Atienza J, Jiang X, Garciadeblas B, Mendoza I, Zhu JK, Pardo JM, Quintero FJ (2007) Conservation of salt overly sensitive pathway in Rice. Plant Physiol 143:1001–1012. doi:10.1104/pp.106.092635
Munns R, James RA, Xu B, Athman A, Conn SJ et al (2012) Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nat Biotechnol 30:360–364. doi:10.1038/nbt.2120
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Niu X, Bressan RA, Hasegawa PM, Pardo JM (1995) Ion homeostasis in NaCl stress environments. Plant Physiol 109:735–742. doi:10.1104/pp.109.3.735
Oh DH, Leidi E, Zhang Q, Hwang SM, Li Y, Quintero FJ, Jiang X, D’Urzo MP, Lee SY, Zhao Y, Bahk JD, Bressan RA, Yun DJ, Pardo JM, Bohnert HJ (2009) Loss of halophytism by interference with SOS1 expression. Plant Physiol 151:210–222. doi:10.1104/pp.109.137802
Ohta M, Guo Y, Halfter U, Zhu JK (2003) A novel domain in the protein kinase SOS2 mediates interaction with the protein phosphatase 2C ABI2. Proc Natl Acad Sci USA 100:11771–11776. doi:10.1073/pnas.2034853100
Olías R, Zakia E, Jun L, Pazalvarez D, Marin-Manzano MC, Mari Carmen M, Pardo JM, Andrés B (2009) The plasma membrane Na+/H+ antiporter SOS1 is essential for salt tolerance in tomato and affects the partitioning of Na+ between plant organs. Plant Cell Environ 32:904–916. doi:10.1111/j.1365-3040.2009.01971.x
Pardo JM (2010) Biotechnology of water and salinity stress tolerance. Curr Opin Biotechnol 21:185–196. doi:10.1016/j.copbio.2010.02.005
Pardo JM, Cubero B, Leidi EO, Quintero FJ (2006) Alkali cation exchangers: roles in cellular homeostasis and stress tolerance. J Exp Bot 57:1181–1199. doi:10.1093/jxb/erj114
Qiu Q, Guo Y, Dietrich MA, Schumaker KS, Zhu JK (2002) Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc Natl Acad Sci USA 99:8436–8441. doi:10.1073/pnas.122224699
Quan R, Lin H, Mendoza I, Zhang Y, Cao W, Yang Y, Shang M, Chen S, Pardo JM, Guo Y (2007) SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell 19:1415–1431. doi:10.1105/tpc.106.042291
Quintero FJ, Ohta M, Shi H, Zhu JK, Pardo JM (2002) Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc Natl Acad Sci USA 99:9061–9066. doi:10.1073/pnas.132092099
Quintero FJ, Martinez-Atienza J, Villalta I, Jiang X, Kim WY, Ali Z, Fujii H, Mendoza I, Yun DJ, Zhu JK, Pardo JM (2011) Activation of the plasma membrane Na+/H+ antiporter salt-overly-sensitive 1 (SOS1) by phosphorylation of an auto-inhibitory C-terminal domain. Proc Natl Acad Sci 108:2611–2616. doi:10.1073/pnas.1018921108
Serrano R (1996) Salt tolerance in plants and microorganisms: toxicity targets and defense response. Int Rev Cytol: A Surv Cell Biol 165:1–52. doi:10.1016/S0074-7696(08)62219-6
Shi H, Ishitani M, Kim C, Zhu JK (2000) The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci USA 97:6896–6901. doi:10.1073/pnas.120170197
Shi H, Quintero FJ, Pardo JM, Zhu JK (2002) The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 14:465–477. doi:10.1105/tpc.010371
Shi H, Lee BH, Wu SJ, Zhu JK (2003) Overexpressing of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nature Biotechnol 21:81–85. doi:10.1038/nbt766
Sunarpi, Horie T, Motoda J et al (2005) Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. Plant J 44:928–938. doi:10.1111/j.1365-313X.2005.02595.x
Tester M, Davenport RJ (2003) Na+ transport and Na+ tolerance in higher plants. Ann Bot 91:503–527. doi:10.1093/aob/mcg058
Volkov V, Wang B, Dominy PJ, Fricke W, Amtmann A (2004) Thellungiella halophila, a salt-tolerant relative of Arabidopsis thaliana, possesses effective mechanisms to discriminate between potassium and sodium. Plant Cell Environ 27:1–14. doi:10.1046/j.0016-8025.2003.01116.x
Wu SJ, Ding L, Zhu JK (1996) SOS1, a genetic locus essential for salt tolerance and potassium acquisition. Plant Cell 8:617–627. doi:10.1105/tpc.8.4.617
Xu H, Jiang X, Zhan K, Cheng X, Chen X, Pardo JM, Cui D (2008) Functional characterization of a wheat plasma membrane Na/H antiporter in yeast. Arch Biochem Biophys 473:8–15. doi:10.1016/j.abb.2008.02.018
Ye J, Zhang W, Guo Y (2013) Arabidopsis SOS3 plays an important role in salt tolerance by mediating calcium-dependent microfilament reorganization. Plant Cell Report 32:139–148. doi:10.1007/s00299-012-1348-3
Yu L, Nie J, Cao C, Jin Y, Yan M, Wang F, Liu J, Xiao Y, Liang Y, Zhang W (2010) Phosphatidic acid mediates salt stress response by regulation of MPK6 in Arabidopsis thaliana. New Phytol 188:762–773. doi:10.1111/j.1469-8137.2010.03422.x
Yue Y, Zhang M, Zhang J, Duan L, Li Z (2011) SOS1 gene overexpression increased salt tolerance in transgenic tobacco by maintaining a higher K+/Na+ ration. J Plant Physiol 169:255–261. doi:10.1016/j.jplph.2011.10.007
Zhu JK (2000) Genetic analysis of plant salt tolerance using Arabidopsis. Plant Physiol 124:941–948. doi:10.1104/pp.124.3.941
Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6:441–445. doi:10.1016/S1369-5266(03)00085-2
Acknowledgments
This study was supported by a grant from the Ministry of Higher Education and Scientific Research of Tunisia and the grants A/8077/07 from the Spanish Agency for International Development Cooperation (AECID), BIO2009-08641 from the Ministry of Science and Innovation (MICINN) and BIO2012-36533 from the Ministry of Economy and Competitiveness (MINECO), with the co-finance of the European Regional Development Fund.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by E. Guiderdoni.
Rights and permissions
About this article
Cite this article
Feki, K., Quintero, F.J., Khoudi, H. et al. A constitutively active form of a durum wheat Na+/H+ antiporter SOS1 confers high salt tolerance to transgenic Arabidopsis . Plant Cell Rep 33, 277–288 (2014). https://doi.org/10.1007/s00299-013-1528-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-013-1528-9