Abstract
Plant fungal pathogens change their cell wall components during the infection process to avoid degradation by host lytic enzymes, and conversion of the cell wall chitin to chitosan is likely to be one infection strategy of pathogens. Thus, introduction of chitosan-degradation activity into plants is expected to improve fungal disease resistance. Chitosanase has been found in bacteria and fungi, but not in higher plants. Here, we demonstrate that chitosanase, Cho1, from Bacillus circulans MH-K1 has antifungal activity against the rice blast fungus Magnaporthe oryzae. Introduction of the cho1 gene conferred chitosanase activity to rice cells. Transgenic rice plants expressing Cho1 designed to be localized in the apoplast showed increased resistance to M. oryzae accompanied by increased generation of hydrogen peroxide in the infected epidermal cells. These results strongly suggest that chitosan exists in the enzyme-accessible surface of M. oryzae during the infection process and that the enhancement of disease resistance is attributable to the antifungal activity of the secreted Cho1 and to increased elicitation of the host defense response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chitosan, a deacetylated form of chitin, is considered to be a component of the fungal cell wall, together with chitin and β-glucan (Latge 2007; Tan et al. 1996). Chitin oligomers (N-acetylchitooligosaccharides) and β-glucan oligomers elicit plant defense responses (Shibuya and Minami 2001) and are defined as microbe-associated molecular patterns (MAMPs). In rice, N-acetylchitooligosaccharides larger than pentaose act as potent elicitors leading to disease resistance, whereas deacetylated chitosan oligomers (chitooligosaccharides) do not (Kishimoto et al. 2010; Kuchitsu et al. 1997; Nishizawa et al. 1999; Tanabe et al. 2006; Yamada et al. 1993). Plants recognize MAMPs through cell surface receptors and induce basal resistance that makes many plants immune to potential pathogens (Boller and He 2009). However, pathogens have evolved a variety of infection strategies to overcome the host basal resistance.
Rice blast, one of the most serious diseases in rice, is caused by the rice blast fungus (Magnaporthe oryzae). Infection commences with the attachment of conidia to rice leaves, followed by formation of a dome-shaped infection structure called the appressorium. The appressorium consists of a melanin layer, which allows high turgor pressure to break through the rice cell wall using a penetration peg. The penetration peg gives rise to primary infection hyphae, and in a susceptible host, infection hyphae invade into neighboring cells within 48 h.
Recently, dynamic changes in the cell wall components of plant pathogens, including M. oryzae, after invasion into the host cell have been reported. Infection hyphae in the first-invaded rice cell show little staining with fluorescently labeled wheat germ agglutinin, a specific probe to detect chitin (Mochizuki et al. 2011). In germ tubes and appressoria, chitin, chitosan, and β-1,3-glucan are detected, whereas in infection hyphae, α-1,3-glucan and chitosan are the major detectable cell wall polysaccharides instead of chitin and β-1,3-glucan (Fujikawa et al. 2009). A change of cell wall chitin to chitosan during the development of infectious structures was analyzed using histochemical immunostaining in rust fungi (El Gueddari et al. 2002). From these results, one may postulate that fungal pathogens avoid attack from the host chitinase through the conversion of chitin to chitosan because chitosan is a poor substrate for chitinases (Ride and Barber 1990) and chitosanase genes have not been isolated from plants. These facts imply that introducing chitosanase activity into plants might improve resistance to fungal pathogens.
Chitosanase (EC 3.2.1.132), an enzyme that specifically hydrolyzes chitosan, has been isolated from several bacteria and fungi. Some chitosanases have antifungal activity in vitro: a chitosanase from Bacillus cereus D-11 inhibits the hyphal growth of Rhizoctonia solani (Gao et al. 2008); Amycolatopsis sp. CsO-2 produces a 27-kDa chitosanase CtoA that inhibits hyphal growth of Rhizopus oryzae (Saito et al. 2009); chitosanase from Streptomyces sp. N174 inhibits hyphal growth of Rhizopus nigricans, Fusarium oxysporum, Verticillium albo-atrum, and Pythium ultimum; and transgenic tobacco plants expressing the N174 chitosanase and a chitosanase from Paenibacillus sp. 61427 show chitosan-degradation activity (El Quakfaoui et al. 1995; Hendrix and Stewart 2003). However, disease resistance in plants expressing chitosanase has not been tested.
Chitosanase from Bacillus circulans MH-K1 is a 29-kDa secretory protein composed of 259 amino acids (Ando et al. 1992) that hydrolyzes both GlcN–GlcN and GlcN–GlcNAc linkages, but not GlcNAc–GlcNAc linkages (Mitsutomi et al. 1996; Yabuki et al. 1988). In the present study, we demonstrated that a chitosanase, designated as Cho1, shows antifungal activity against M. oryzae in vitro, and that transgenic rice plants expressing Cho1 in the apoplast exhibit increased resistance to M. oryzae.
Materials and methods
Antifungal assay for M. oryzae
A conidial suspension of 20 μl (1 × 105 conidia ml−1) from M. oryzae strain Ina86-137 (race 007.0; MAFF Genebank stock number MAFF101511) was spotted onto plastic coverslips (Fisher Scientific, Pittsburgh, PA), and 5 μl of phosphate-buffered saline (PBS; pH 7.4), 2 μg BSA, or 2 μg recombinant Cho1 in PBS was added, suspended by pipetting, and incubated at 25°C in the dark at high humidity. After a 24-h incubation, growth of M. oryzae was observed under an optical microscope. Growth of M. oryzae per conidium was classified into three groups: no germination, germ tube elongation, and appressoria formation. Growth of about 160 conidia was counted and statistical analysis performed using JMP8 software (SAS Institute, Cary, NC). The recombinant Cho1 was prepared using Brevibacillus choshinensis as host and purified as described previously (Saito et al. 1999).
Vector construction and rice transformation
A DNA fragment for Cho1 without the signal sequence region was amplified from the cho1 clone (Ando et al. 1992) with PCR using the following primer set: 5′-GAGCTCCGCTTCTCCTGACGACAATTTC-3′ (underline indicates SacI site) and 5′-GGTACCCTACTTCATTTCCCAGTCCGTG-3′ (underline indicates KpnI site), and ligated into SacI and KpnI sites in pBI333-EN4-RC2ss/ChiC to express Cho1 with the signal sequence of a rice chitinase CHT2, which localizes the adjacent polypeptide to the apoplast (Itoh et al. 2003). To express Cho1 in the cytoplasm, a DNA fragment for the mature Cho1 was amplified with PCR and cloned into pBI333-EN4, which was linearized by XhoI digestion, using the In-Fusion Advantage PCR Cloning Kit (Clontech, Mountain View, CA). Primers used for the PCR were 5′-AACTAGTCTCGAGGCCACCATGGCTTCTCC-3′ and 5′-GCTTGTCGACCTCGAGCTACTTCATTTCCC-3′. Rice transformation was performed as described previously (Toki et al. 2006) using rice (Oryza sativa L. japonica cv. Nipponbare BL no. 2 possessing Pii gene) seeds kindly provided by Dr. H. Satoh of the National Institute of Crop Science in Tsukuba, Japan.
RT-PCR
Expression of the introduced cho1 was confirmed in each transgenic cell line and regenerated plant line using RT-PCR. Total RNA was extracted from rice callus and leaves using the RNeasy Plant Mini Kit (Qiagen, Valencia, CA), and cDNA was synthesized using ReverTra Ace (TOYOBO, Osaka, Japan). PCR was carried out with the following primer sets: 5′-ACTAGTGCCACCATGGCTTCTCCTGACGACAA-3′ and 5′-GGTACCCTACTTCATTTCCCAGTCCGTG-3′ for the cho1 gene, and 5′-CCAGTAAGTCCTCAGCCATGGA-3′ and 5′-GGACACAATGATTAGGGATCAC-3′ for the rice ubiquitin gene.
Protein extraction and Western blot analysis
Rice callus was homogenized in PBS (pH 7.4) containing 10% glycerol and 1 mM ascorbic acid. After centrifugation at 100,000×g for 1 h at 4°C, the supernatant was dissolved in PBS, and the soluble proteins (10 μg per lane) were separated with SDS-PAGE. For Western blot analysis, the proteins were transferred onto a polyvinylidene difluoride membrane (Immobilon-P; Millipore, Billerica, MA) and detected using anti-Cho1 antiserum or anti-GAPDH antibody. Extraction of extracellular and intracellular proteins was performed according to a method described previously (Itoh et al. 2003). Briefly, culms and leaf sheath of rice plants were cut into about 4-cm-long pieces and centrifuged with citrate–phosphate buffer (pH 6.0) to elute extracellular proteins into the buffer. Cytoplasmic proteins were extracted from the tissues after the extracellular proteins were removed.
Chitosanase assay
Soluble proteins were prepared from rice cultured cells that had been washed in the culture medium. A total of 100 μg of soluble protein was diluted in PBS and incubated at 40°C overnight with 750 μg of chitohexaose (Seikagaku Kogyo, Tokyo, Japan). A 2.5-μl aliquot of the reaction mixture was spotted onto the Silica gel 60 TLC plate (Merck, Whitehouse Station, NJ) and developed with n-propanol:aqueous ammonia (2:1) as solvent. Degradation products of chitohexaose were visualized by spraying with 20% sulfuric acid in ethanol, followed by heating at 120°C for 7 min.
Disease resistance tests in the leaf blade
Rice seeds from the T1 generation were germinated on fourfold diluted Murashige and Skoog medium (pH 6.8) containing 1% (w/v) agar and hygromycin B (25 μg ml−1) at 28°C under continuous light. After 7 days, hygromycin-resistant seedlings were transferred to hydroponic culture at 28°C under a 14/10 h photoperiod. M. oryzae strain Ina86-137, which is compatible with rice cv. Nipponbare BL no. 2, was used. Excised rice leaf blades from the sixth leaf were spotted with 10 μl of inoculum (aqueous suspension at 2.5 × 105 conidia ml−1), incubated at 25°C overnight in the dark, and then incubated for 5 days at room temperature on moist filter paper. Mean area of lesions was measured using Image J software (http://rsb.info.nih.gov/ij/).
Disease resistance tests and detection of hydrogen peroxide in the leaf sheath
Leaf sheaths from the sixth leaf were excised, filled with conidia suspension (1 × 105 conidia ml−1) using a syringe, and incubated for 24 or 48 h at 25°C in the dark. Evaluation of hyphal growth in epidermal cells was performed as described previously (Tanabe et al. 2009) using four independent T1 lines. Hydrogen peroxide (H2O2) accumulation was detected using 3,3′-diaminobenzidine-tetrahydrochloride (DAB; Sigma, St. Louis, MO) staining using two independent T1 lines. The inoculated leaf sheaths were soaked in 1 mg ml−1 of DAB solution for 24 h at 25°C in the dark, then boiled in 95% (v/v) ethanol for 10 min and stored in 90% ethanol until microscopic observation.
Results
Antifungal activity of Cho1 to M. oryzae in vitro
To evaluate the antifungal activity of Cho1 to M. oryzae, the conidial suspension was incubated with recombinant Cho1 on plastic coverslips. As shown in Fig. 1, conidia incubated in PBS or PBS including BSA normally germinated and formed appressoria, and septa in conidia clearly remained. In contrast, conidia incubated with Cho1 failed to form appressoria even though conidial germination occurred, and septa in conidia were not clearly observed. These results indicate that Cho1 has antifungal activity against M. oryzae.
Antifungal activity of Cho1 to M. oryzae in vitro. a Conidial growth of M. oryzae after incubation with or without Cho1. Arrows indicate appressoria. Enlarged views of conidia are shown at the lower left. b The levels of conidial growth are presented as percentages of the total number of conidia observed by microscopy. About 160 conidia per microscopic field were counted. Error bars represent standard deviations (n = 4 fields). Asterisks above the bars indicate significant differences compared to –Cho1 at P < 0.05 (Dunnett’s test)
Chitosanase activity of rice cells expressing Cho1
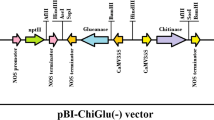
We produced two types of transgenic rice plant expressing Cho1 using a modified CaMV 35 S promoter: Cho1 without a signal sequence (–SS), whose deduced molecular weight is 29 kDa, and Cho1 with the signal sequence of a rice chitinase CHT2 (+SS), whose deduced molecular weight is 32.9 kDa (Fig. 2).
Structure of T-DNA regions in the binary vectors. RB and LB indicates the right-border and left-border sequences of the T-DNA regions, respectively. 35S cauliflower mosaic virus (CaMV) 35S promoter, EN4 enhanced CaMV 35S promoter, NOS3′ terminator of nopaline synthase gene, CaMV3′, terminator of CaMV 35S, HPT hygromycin phosphotransferase gene, RC2/ss rice chitinase CHT2 signal sequence. Junction between RC2/ss and Cho1 is shown in the lower part. ChiC indicates a carry-over sequence during the vector construction. Dashed line indicates the putative cleavage site in the signal sequence of rice CHT2
Chitosanase activity was analyzed using cultured cells in which transcriptional expression of cho1 was confirmed by RT-PCR (data not shown). Accumulation of Cho1 was confirmed by Western blot analysis both in –SS and +SS cultured cells (Fig. 3a). Cho1 showed slower mobility in +SS than in –SS, likely due to the additional signal sequence that was not cleaved inside the cell. Total soluble proteins from the cultured cells were assayed for chitosanase activity using TLC. Although Cho1 from +SS cells is considered to be an unprocessed form, extracts from both cell lines presented chitosanase activity, i.e., chitohexaose was degraded to lower-molecular-weight products (Fig. 3b). Proteins from vector control rice cells demonstrated no activity.
Chitosanase activity of rice cells expressing Cho1. a Expression analysis of the Cho1 protein. Upper panel shows the Western blot analysis using anti-Cho1 antiserum, and the lower panel shows a CBB-stained gel after SDS-PAGE. M size marker, PC recombinant Cho1 protein as control, soluble protein extracted from the –SS line (–SS), +SS line (+SS), and vector control (VC) line. b Detection of chitosanase activity after TLC. M size marker, a mixture of d-glucosamine monomer (GlcN) to hexamer (GlcN6), PC reaction mixture with recombinant Cho1 protein, reaction mixture with protein extract of the –SS line (–SS), +SS line (+SS) and vector control line (VC)
Localization of Cho1 in transgenic rice plants
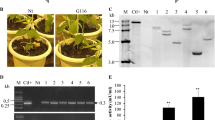
To analyze the cellular localization of Cho1, we prepared soluble proteins from apoplastic and cytoplasmic fractions of –SS and +SS plants in which expression of cho1 was confirmed by RT-PCR (data not shown) and Western blot analysis (Fig. 4). In the +SS plant, Cho1 was mostly detected in the apoplastic fraction, while in the –SS plant, Cho1 was observed in both the apoplastic and cytoplasmic fractions. The level of Cho1 accumulation was higher in the +SS line than in the –SS line.
Localization of Cho1 in rice plants. Western blot analysis of the apoplastic and cytoplasmic fractions of rice plants using anti-Cho1 antiserum and anti-GAPDH antibody. M size marker, PC recombinant Cho1 protein as control, VC soluble protein extracted from the vector control line, –SS from the –SS line, +SS from the +SS line
Disease resistance against M. oryzae in transgenic rice plants
Expression of cho1 in the regenerated leaf blade was confirmed by RT-PCR (data not shown), and their T1 lines were examined for blast resistance (Fig. 5). The sixth leaf blades of six sibling plants from each of four independent transgenic lines were inoculated with a conidial suspension of a compatible isolate of M. oryzae, and the areas of lesions at 6 days postinoculation (dpi) were measured. Note that only the +SS plants exhibited significant resistance to blast.
Disease resistance to M. oryzae in rice leaf blades expressing Cho1. a Photographs of symptoms developed at 6 dpi. Inoculated leaf blades of each representative line are shown. Scale bar indicates 1 cm. b Area of necrotic lesions formed at 6 dpi. Six sibling plants from each of four independent lines in the T1 generation were tested. Error bars represent standard errors (n = 6). Asterisks above the bars indicate significant differences compared to the vector control at P < 0.05 (Dunnett’s test)
We further examined disease resistance of the transgenic rice plants by scoring hyphal growth in the epidermal cells of leaf sheaths. Figure 6a shows different levels of invasion and spread of infection hyphae observed 48 h postinoculation (hpi), and we then categorized each appressorium into one of three infection levels. We tested four independent transgenic lines from –SS and +SS plants; Fig. 6b shows a representative result. In +SS plants, the percentage of appressoria that did not form primary infection hyphae was slightly higher than in the vector control and –SS plants. Although the percentage of ‘one-cell invasion’ did not differ among these lines, the percentage of ‘multicell invasion’ in +SS plants was significantly lower than in the vector control and –SS plants.
Disease resistance to M. oryzae in rice leaf sheaths expressing Cho1. Levels of invasion of M. oryzae in the rice leaf sheath were classified into three groups (a). At 48 hpi, levels of infection were scored for each appressorium and are presented as percentages of the total number of appressoria scored (b). Three sibling plants per line in the T1 generation were tested. Error bars represent standard errors (n = 3). Asterisks above the bars indicate significant differences compared with the vector control at P < 0.05 (Dunnett’s test)
Cellular response to M. oryzae in leaf sheaths of transgenic rice plants
Release of reactive oxygen species (ROS), including H2O2, is a major host defense response to pathogen infection (Torres et al. 2006). We examined the accumulation of H2O2 in epidermal cells of inoculated leaf sheaths using DAB staining at 24 hpi. Figure 7a shows representative DAB-staining patterns in epidermal cells. In +SS plants, the percentage of appressoria that did not cause H2O2 accumulation was significantly lower than in the vector control and –SS plants, and the percentage of appressoria that led to staining of one and more cells was significantly higher than in the other plants (Fig. 7b).
Cellular response to M. oryzae in rice leaf sheaths expressing Cho1. Levels of H2O2 generation were categorized based on the DAB staining patterns (a). At 24 hpi, the DAB-staining patterns were scored for each appressorium and are presented as percentages of the total number of appressoria (b). Four sibling plants per line in the T1 generation were tested. Error bars represent standard deviations (n = 4). Asterisks above the bars indicate significant differences compared with the vector control at P < 0.05 (Dunnett’s test)
Discussion
In this study, we demonstrated that the chitosanase Cho1 from B. circulans MH-K1 exhibits antifungal activity against M. oryzae. Introduction of the cho1 gene conferred chitosanase activity to rice cells, and transgenic rice plants accumulating Cho1 in the apoplast showed increased resistance to M. oryzae. This is the first report describing disease resistance in transgenic plants expressing chitosanase.
Cho1 inhibited appressorial formation of M. oryzae (Fig. 1), which strongly suggests that chitosan exists in the enzyme-accessible surface of M. oryzae during development of the infection structures because Cho1 specifically hydrolyzes chitosan and does not have chitinase activity, i.e., Cho1 does not split GlcNAc–GlcNAc linkages (Yabuki et al. 1988). Chitosan is a polymer of d-glucosamine and is synthesized from chitin by chitin deacetylase. Several putative chitin deacetylase genes of M. oryzae have been reported (Kamakura et al. 2002; Mochizuki et al. 2011) although their enzyme activity has not yet been tested. Chitin deacetylase activity of the broad bean rust fungus Uromyces viciae-fabae increases when the fungus starts infection (Deising and Siegrist 1995), and the conversion of chitin to chitosan occurs in several rust fungi and in the causal agent of maize anthracnose, Colletotrichum graminicola, during infection (El Gueddari et al. 2002). These results suggest that conversion of chitin to chitosan is a strategy for successful infection by several fungal pathogens. Our results imply that M. oryzae also converts the cell wall chitin to chitosan during the infection process to avoid lysis by the host chitinase.
In the suspension-cultured cell lines of +SS, only Cho1 with lower mobility was detected (Fig. 3), probably because the apoplastic Cho1 protein was released into the liquid medium. Rice cells introduced with an empty vector showed no chitosanase activity, which agrees with the fact that no rice genes are annotated as chitosanase (http://rapdb.dna.affrc.go.jp/). In contrast, cytoplasmic proteins from both –SS and +SS cell lines demonstrated chitosanase activity, which indicates that Cho1 is expressed as an active form in rice cells and that Cho1 fusing with the CHT2 signal sequence also carries chitosanase activity.
In the –SS plant, Cho1 was detected both in the apoplast and cytoplasm, while in the +SS plant it was exclusively in the apoplast and at higher levels. +SS plants, but not –SS plants, showed significantly increased levels of resistance against M. oryzae both in leaf blades (Fig. 5) and leaf sheaths (Fig. 6). These results indicate that production of a larger amount of Cho1 in the apoplast is necessary to improve resistance to rice blast.
We consider that two factors are responsible for the enhanced disease resistance: first, the antifungal activity of Cho1, whereby Cho1 inhibited appressoria formation of M. oryzae in vitro. Chitosan has been detected in primary infection hyphae (Fujikawa et al. 2009) in the apoplastic space surrounded by the host plasma membrane (Kankanala et al. 2007). Microscopic observations revealed that fungal penetration through the rice cell walls was inhibited in +SS plants (Fig. 6b), which implies that large amounts of the secreted Cho1 in +SS plants suppress the formation of primary infection hyphae and penetration of hyphae further into the next rice cells. Second, incremental MAMPs generation, whereby significant decreases in multicell invasion observed in +SS plants were correlated with increased generation of H2O2 in epidermal cells of the inoculated leaf sheaths (Fig. 7), also leads to enhanced disease resistance. Generation of ROS is one cellular defense response to MAMPs (Torres et al. 2006). Although the chitosan polymer has been reported to induce generation of ROS (Iriti and Faoro 2009), chitoheptaose does not cause this response in rice cells (Kishimoto and Nishizawa, unpublished data). Natural chitosan is not considered fully deacetylated (Saito et al. 2009), and thus N-acetylchitooligosaccharides could be generated from chitosan through Cho1. In fact, partially acetylated chitooligosaccharides, as well as N-acetylchitooligosaccharides, induce ROS generation (dos Santos et al. 2008). Therefore, in +SS plants, apoplastic Cho1 may hydrolyze chitosan in the cell wall of M. oryzae and result in the increased production of both N-acetylchitooligosaccharides and partially acetylated chitooligosaccharides, which induces higher levels of ROS generation. In addition, degradation of cell wall chitosan could allow easier access of endogenous rice lytic enzymes like chitinase and β-1,3-glucanase to the fungal cell wall.
Chitosan is widely distributed in the cell wall of fungi, particularly zygomycetes (Tan et al. 1996). Some bacterial chitosanases show antifungal activity to several plant fungal pathogens in vitro (El Quakfaoui et al. 1995; Gao et al. 2008; Hendrix and Stewart 2003; Saito et al. 2009). Therefore, the use of chitosanase might provide a novel strategy for crop protection against fungal diseases.
Abbreviations
- BSA:
-
Bovine serum albumin
- GlcN:
-
d-Glucosamine
- GlcNAc:
-
N-Acetyl-d-glucosamine
- PCR:
-
Polymerase chain reaction
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- SDS-PAGE:
-
SDS-polyacrylamide gel electrophoresis
- CBB:
-
Coomassie brilliant blue
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- TLC:
-
Thin-layer chromatography
References
Ando A, Noguchi K, Yanagi M, Shinoyama H, Kagawa Y, Hirata H, Yabuki M, Fujii T (1992) Primary structure of chitosanase produced by Bacillus circulans MH-K1. J Gen Appl Microbiol 38:135–144
Boller T, He SY (2009) Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science 324:742–744
Deising H, Siegrist J (1995) Chitin deacetylase activity of the rust Uromyces viciae-fabae is controlled by fungal morphogenesis. FEMS Microbiol Lett 127:207–211
dos Santos AL, El Gueddari NE, Trombotto S, Moerschbacher BM (2008) Partially acetylated chitosan oligo- and polymers induce an oxidative burst in suspension cultured cells of the gymnosperm Araucaria angustifolia. Biomacromolecules 9:3411–3415
El Gueddari NE, Rauchhaus U, Moerschbacher BM, Deising HB (2002) Developmentally regulated conversion of surface-exposed chitin to chitosan in cell walls of plant pathogenic fungi. New Phytol 156:103–112
El Quakfaoui S, Potvin S, Brzezinski R, Assekin A (1995) A Streptomyces chitosanase is active in transgenic tobacco. Plant Cell Rep 15:222–226
Fujikawa T, Kuga Y, Yano S, Yoshimi A, Tachiki T, Abe K, Nishimura M (2009) Dynamics of cell wall components of Magnaporthe grisea during infectious structure development. Mol Microbiol 73:553–570
Gao X, Ju W, Jung W, Park R (2008) Purification and characterization of chitosanase from Bacillus cereus D-11. Carbphydr Polym 72:513–520
Hendrix B, Stewart J (2003) Chitosanase may enhance anti-fungal defense responses in transgenic tobacco. Discovery 4:27–33
Iriti M, Faoro F (2009) Chitosan as a MAMP, searching for a PRR. Plant Signal Behav 4:66–68
Itoh Y, Takahashi K, Takizawa H, Nikaidou N, Tanaka H, Nishihashi H, Watanabe T, Nishizawa Y (2003) Family 19 chitinase of Streptomyces griseus HUT6037 increases plant resistance to the fungal disease. Biosci Biotechnol Biochem 67:847–855
Kamakura T, Yamaguchi S, Saitoh K, Teraoka T, Yamaguchi I (2002) A novel gene, CBP1, encoding a putative extracellular chitin-binding protein, may play an important role in the hydrophobic surface sensing of Magnaporthe grisea during appressorium differentiation. Mol Plant Microbe Interact 15:437–444
Kankanala P, Czymmek K, Valent B (2007) Roles for rice membrane dynamics and plasmodesmata during biotrophic invasion by the blast fungus. Plant Cell 19:706–724
Kishimoto K, Kouzai Y, Kaku H, Shibuya N, Minami E, Nishizawa Y (2010) Perception of the chitin oligosaccharides contributes to disease resistance to blast fungus Magnaporthe oryzae in rice. Plant J 64:343–354
Kuchitsu K, Yazaki Y, Sakano K, Shibuya N (1997) Transient cytoplasmic pH change and ion fluxes through the plasma memberane in suspension-cultured rice cells triggered by N-acetylchitooligosaccharide elicitor. Plant Cell Physiol 38:1012
Latge JP (2007) The cell wall: a carbohydrate armour for the fungal cell. Mol Microbiol 66:279–290
Mitsutomi M, Kigoh H, Tomita H, Watanabe T (1996) Action patterns of microbial chitinases and chitosanases on partially N-acetylated chitosan. Chitin Enzymol 2:273–284
Mochizuki S, Saitoh K, Minami E, Nishizawa Y (2011) Localization of probe-accessible chitin and characterization of genes encoding chitin-binding domains during rice—Magnaporthe oryzae interactions. J Gen Plant Pathol 77:163–173
Nishizawa Y, Kawakami A, Hibi T, He DY, Shibuya N, Minami E (1999) Regulation of the chitinase gene expression in suspension-cultured rice cells by N-acetylchitooligosaccharides: differences in the signal transduction pathways leading to the activation of elicitor-responsive genes. Plant Mol Biol 39:907–914
Ride J, Barber M (1990) Purification and characterization of multiple forms of endochitinase from wheat leaves. Plant Sci 71:185–197
Saito J, Kita A, Higuchi Y, Nagata Y, Ando A, Miki K (1999) Crystal structure of chitosanase from Bacillus circulans MH-K1 at 1.6-Å resolution and its substrate recognition mechanism. J Biol Chem 274:30818–30825
Saito A, Ooya T, Miyatsuchi D, Fuchigami H, Terakado K, Nakayama SY, Watanabe T, Nagata Y, Ando A (2009) Molecular characterization and antifungal activity of a family 46 chitosanase from Amycolatopsis sp. CsO-2. FEMS Microbiol Lett 293:79–84
Shibuya N, Minami E (2001) Oligosaccharide signaling for defense responses in plant. Physiol Mol Plant Pathol 59:223–233
Tan SC, Tan TK, Wong SM, Khor E (1996) The chitosan yield of zygomycetes at their optimum harvesting time. Carbohydr Poly 30:239–242
Tanabe S, Okada M, Jikumaru Y, Yamane H, Kaku H, Shibuya N, Minami E (2006) Induction of resistance against rice blast fungus in rice plants treated with a potent elicitor, N-acetylchitooligosaccharide. Biosci Biotechnol Biochem 70:1599–1605
Tanabe S, Nishizawa Y, Minami E (2009) Effects of catalase on the accumulation of H2O2 in rice cells inoculated with rice blast fungus, Magnaporthe oryzae. Physiol Plant 137:148–154
Toki S, Hara N, Ono K, Onodera H, Tagiri A, Oka S, Tanaka H (2006) Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice. Plant J 47:969–976
Torres MA, Jones JD, Dangl JL (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141:373–378
Yabuki M, Uchiyama A, Suzuki K, Ando A, Fujii T (1988) Purification and properties of chitosanase from Bacillus circulans MH-K1. J Gen Appl Microbiol 34:255–270
Yamada A, Shibuya N, Kodama O, Akatsuka T (1993) Induction of phytoalexin formation in suspension-cultured rice cells by N-acetylchitooligosaccharides. Biosci Biotechnol Biochem 57:405–409
Acknowledgments
We thank S. Omiya, A. Kikuchi, A. Kimura, M. Tomita, and R. Tokishige of Chiba University for providing information on chitosanase, recombinant Cho1 protein, and technical advice for detecting chitosanase activity. We also thank M. Nishimura and S. Tanabe of the National Institute of Agrobiological Sciences for fruitful discussions on the alteration of cell wall composition of M. oryzae and for providing technical advice regarding M. oryzae inoculation, respectively; and E. Nakajima, H. Kurano, and K. Iwasaki for producing rice transformants. This work was supported by the Program for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry in Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Toriyama.
Rights and permissions
About this article
Cite this article
Kouzai, Y., Mochizuki, S., Saito, A. et al. Expression of a bacterial chitosanase in rice plants improves disease resistance to the rice blast fungus Magnaporthe oryzae . Plant Cell Rep 31, 629–636 (2012). https://doi.org/10.1007/s00299-011-1179-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-011-1179-7