Abstract
BAC FISH (fluorescence in situ hybridization using bacterial artificial chromosome probes) is a useful cytogenetic technique for physical mapping, chromosome marker screening, and comparative genomics. As a large genomic fragment with repetitive sequences is inserted in each BAC clone, random BAC FISH without adding competitive DNA can unveil complex chromosome organization of the repetitive elements in plants. Here we performed the comparative analysis of the random BAC FISH in monocot plants including species having small chromosomes (rice and asparagus) and those having large chromosomes (hexaploid wheat, onion, and spider lily) in order to understand a whole view of the repetitive element organization in Poales and Asparagales monocots. More unique and less dense dispersed signals of BAC FISH were observed in species with smaller chromosomes in both the Poales and Asparagales species. In the case of large-chromosome species, 75–85% of the BAC clones were detected as dispersed repetitive FISH signals along entire chromosomes. The BAC FISH of Lycoris did not even show localized repetitive patterns (e.g., centromeric localization) of signals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant chromosomes vary in size from small to large, being reflected by their genome size and chromosome number. For example, mitotic chromosomes are as small as ~1.5 μm in length in Arabidopsis thaliana (2n = 10) (Lysak et al. 1998), whose genome size (1C) was estimated as 125 Mb from sequencing (Arabidopsis Genome Initiative 2000) or 157 Mb from DNA amount (Bennett et al. 2003), while Lycoris aurea (2n = 15, 1C = 24,000 Mb) have over 20 μm large chromosomes in mitotic metaphase (Kurita 1986, 1987; Zonneveld et al. 2005). Such remarkable difference of genome size of diploid plants is generally explained by different copy number of repetitive elements, including retroelements, in the non-coding regions. Comparative sequence studies in various plant species suggested that amplification of the retroelements drives expansion of genome size (SanMiguel and Bennetzen 1998; Shirasu et al. 2000; Piegu et al. 2006; Hawkins et al. 2006; Neumann et al. 2006). It is noteworthy that fluorescence in situ hybridization (FISH) is frequently used to visualize chromosomal distribution of the repetitive sequences (Miller et al. 1998; Presting et al. 1998; Zhang et al. 2004) as a powerful tool for comparative genomics at the microscopic level.
In the case of monocot plants, most of the recent genomic studies have been done in Poales, including cereal crops (such as rice, maize, barley and wheat), and genomic information of other monocot species, including Asparagales, has so far been limited. In order to elucidate genomic organization of Asparagales, which is different from that of cereal monocots, we have constructed a bacterial artificial chromosome (BAC) library of onion (Allium cepa), and used it for molecular cytogenetic analysis (Suzuki et al. 2001) and sequencing studies (Do et al. 2004; Jakse et al. 2008). In particular, FISH studies using randomly selected BAC clones (here we call it random BAC FISH) without competitive DNA have been performed to compare genomes of onion (Asparagales) and hexaploid wheat (Triticum aestivum, Poales): onion FISH with 91 BAC clones (Suzuki et al. 2001) and wheat FISH with 202 BAC clones (Suzuki and Mukai 2004). As a result, frequent occurrence of dispersed repetitive sequences was revealed in both the monocot species having large chromosomes, in addition to the finding of BAC clones showing (peri) centromeric and (sub) telomeric localization. Similarly, Zhang et al. (2004) reported more detailed FISH experiments of selected 56 BAC clones with subclone sequencing in wheat. Overall results of wheat BAC FISH in Suzuki and Mukai (2004) and Zhang et al. (2004) are comparable.
In the present study, we further constructed the BAC libraries of rice (Oryza sativa, Poales) and asparagus (Asparagus officinalis, Asparagales) for representative small-chromosome species, and that of spider lily (Lycoris radiata) for representative Asparagales with large chromosomes, and performed the random BAC FISH to compare their genomes. Difference of genomic organization between small- and large-chromosome species was revealed by the BAC FISH analysis.
Materials and methods
Chromosomal preparation
Root tips of A. cepa, A. officinalis, L. radiata, and T. aestivum were pretreated at 0°C for 18–20 h with water. For O. sativa (japonica rice), root tips were pretreated with 2 mM 8-hydroxyquinoline for 4 h at room temperature. The pretreated root tips were fixed in ethanol/glacial acetic acid (3:1) for several days, and then squashed in 45% acetic acid on glass slides as described by Mukai et al. (1990).
Construction of BAC libraries
Megabase DNA embedded in agarose plugs was prepared from young leaf tissue using the rapid method described by Suzuki et al. (1997). The megabase DNA in agarose plugs was partially digested with HindIII (Takara) at 37°C for optimum amount of time, and was subjected to pulsed field gel electrophoresis (PFGE) using a CHEF mapper (Bio-Rad) with a 1% low melting point agarose gel (SeaPlaque GTG, FMC) in 0.5× TBE buffer with the conditions of 6 V/cm, constant linearly ramped pulse time of 90 s for 4 h at 14°C, followed by 6 V/cm, constant linearly ramped pulse time of 6 s for 12 h at 14°C. The selected fractions containing the target sizes of DNA fragments were released from the gel with β-Agarase I (New England Biolabs), and ligated to HindIII-digested pBeloBAC11 vector with T4 ligase (Promega). After ligation, DNA was electroporated into Escherichia coli strain ElectroMAX DH10B (Invitrogen) using a Gene Pulser II (Bio-Rad) under the conditions of 1.25 or 1.5 kV, 25 μF, and 100 Ω. The electroporated cells were spread on an LB agar plate containing 12.5 mg/l chloramphenicol (Cm), X-gal and IPTG. Recombinants (white colonies) were clearly distinguished from non-recombinants (blue colonies) after 20–24 h of incubation at 37°C.
Probe labeling
BAC clones (randomly selected white colonies) were picked up, inoculated into 3 ml of LB with 12.5 mg/l Cm, and shaken at 37°C overnight. BAC DNA purified by the standard alkali method was dissolved in 20 μl of TE, and a 5 μl aliquot was used to determine the concentration and insert size by PFGE analysis of NotI digested DNA. Another 5–10 μl aliquot was labeled with digoxigenin-11-dUTP using a Dig-Nick Translation Mix kit (Roche Diagnostics). For the rDNA probe, DNA purified by the QIAGEN Plasmid Midi kit (QIAGEN) was labeled with biotin-16-dUTP with Biotin-Nick Translation Mix kit (Roche Diagnostics).
FISH analysis
Chromosomal DNA was denatured in 70% formamide-2× SSC for 2 min at 69°C and dehydrated in an ethanol series at −20°C. The hybridization mixture (100 μl) consisted of 50% formamide, 10% dextran sulfate, 50 μg of salmon sperm DNA, 2× SSC, and the labeled BAC DNA or rDNA. The mixture was denatured for 10 min at 100°C and immediately quenched in ice for at least 10 min. A 10 μl aliquot of the mixture was applied to each slide. Hybridization took place overnight in a moist chamber at 37°C. After hybridization, the slides were washed in 2× SSC at room temperature for 5 min, 50% formamide-2× SSC at 37°C for 15 min, 2× SSC at room temperature for 15 min, 1× SSC at room temperature for 15 min, and 4× SSC at room temperature for 5 min. For the simultaneous detection of digoxigenin and biotin, slides were incubated in 2 μg/ml rhodamine-conjugated anti-digoxigenin (Roche Diagnostics) and 2 μg/ml fluorescein isothiocyanate (FITC)-conjugated avidin (Roche Diagnostics) in the detection buffer containing 4× SSC-1% BSA for 1 h at 37°C. After incubation, the slides were washed in 4× SSC for 10 min, 0.1% Triton X-100 in 4× SSC for 10 min, 4× SSC for 10 min, and 2× SSC for 5 min, all at room temperature. The slides were mounted in a fluorescence antifade solution (1.25% DABCO, 90% glycerol). DAPI was used as chromosome DNA counterstaining in the antifade solution at 200 ng/ml. Each fluorescent signal on the slide was captured with an Axioskop fluorescence microscope (Zeiss) coupled to a cooled CCD camera (Hamamatsu Photonics, model 4880). Images were pseudo-colored and merged using Photoshop 5.0 software (Adobe).
Results and discussion
Random BAC FISH in rice, asparagus, and spider lily
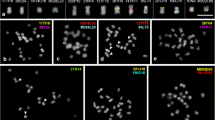
In this study, we constructed partial BAC libraries of rice (O. sativa), asparagus (A. officinalis), and spider lily (L. radiata) with average insert size of 104.0 kb, 60.4 kb, and 63.6 kb, respectively (representative results of PFGE analysis determining the insert size are indicated in Online Resource 1). By using these BAC clones, we conducted random BAC FISH analysis in rice, asparagus, and spider lily to compare the results with those in wheat (T. aestivum) and onion (A. cepa) (Fig. 1) (Suzuki et al. 2001; Suzuki and Mukai 2004). Although successful detection of the single locus by BAC FISH has usually been reported by masking repetitive DNA by adding C 0 t1 DNA as competitor (Jiang et al. 1995), we did not add the competitive DNA in these BAC FISH experiments in order to reveal chromosomal distribution of repetitive DNA included in the BAC insert.
BAC FISH results of randomly selected clones in rice are summarized in Table 1. Although we used only 18 BAC clones as FISH probes, one-third of the clones showed distinct signal(s) detecting one or several loci (Fig. 1a), which we classified as “unique” type signals (Table 1). Easy detection of the unique signals without competitive DNA might be due to less repetitive sequences in the BAC clones, which is characteristic of the small genome plant species. Results of “repetitive” type FISH signals were classified in “localized repetitive” and “dispersed repetitive.” All the localized repetitive type signals were detected in (peri) centromeric regions of the rice chromosomes (Fig. 1b). The (peri) centromeric-repetitive BAC clones were much more frequently observed than BAC clones containing dispersed repetitive sequences, whose BAC FISH signals were detected as “mottled” or “entire chromosomal” localization (Table 1).
In the case of asparagus, whose chromosomes are small but larger than the rice chromosomes (Fig. 1), random BAC FISH of 40 clones resulted in detection of six unique, eight localized repetitive, and 23 dispersed repetitive types of signals (Table 2). In the 15% (6 out of 40) of BAC clones showing the unique FISH signals, two BAC clones were hybridized with the distinct one locus (Fig. 1c), indicating the small proportion of repetitive sequences in the small asparagus genome, but more repetitive than in the rice genome. Confirming such characteristics of the asparagus genome, over half of the BAC clones showed the dispersed repetitive FISH signals (Table 2; Fig. 1e). In the clones showing localized repetitive signals, one clone showed (sub) telomeric FISH signals, whereas all the others showed (peri) centromeric signals (Table 2; Fig. 1d).
Differing from the small-genome monocot species (rice and asparagus), spider lily with the large genome and chromosomes showed highly repetitive signal distribution in random BAC FISH using 211 clones (Table 3; Fig. 1k, l). Only dispersed repetitive type BAC FISH signals were obtained, which were further classified in entire chromosomal (Fig. 1k) and mottled localization (Fig. 1l). These results strongly indicated that highly complex repetitive sequences are dispersed in entire chromosomes, and constitute gene-flanking sequences in the large genome of Lycoris.
Comparison of BAC FISH results in monocot plants
We compared the random BAC FISH results in rice, asparagus, and spider lily (Tables 1, 2, 3; Fig. 1a–e, k, l) with our previous reports in wheat and onion (Suzuki et al. 2001; Suzuki and Mukai 2004). Chromosome number, genome size, and length of mitotic metaphase chromosomes of the five plant species used in this study are summarized in Table 4. Chromosome sizes of rice and asparagus are relatively small (less than 6 μm; Table 4; Fig. 1a–e). The large-genome species (wheat, onion, and spider lily) have extremely large mitotic chromosomes, up to 20 μm (Table 4; Fig. 1f–l).
Figure 2 shows a summary of our random BAC FISH results of the five monocot species. It is obvious that species with larger chromosomes contain more complex repetitive DNA than those with smaller chromosomes in both Poales (rice and wheat) and Asparagales (asparagus, onion and spider lily). Proportion of BAC clones with unique or localized repetitive FISH signals is less in the larger chromosome species and more in the smaller chromosome species. Especially in spider lily, which has the largest genome, there were no BAC clones showing unique or localized repetitive FISH signals out of 211 BAC clones examined (Table 3; Fig. 2). This difference might be mainly due to the amount of the dispersed repetitive DNA; an increased number of BAC clones with dispersed repetitive FISH signals might lead to a reduction of the proportion of the unique or localized category in the larger chromosome species. These dispersed repetitive sequences, which have been accumulated in the large chromosome species, might consist of complex, mixed and nested structures of different kinds of retroelements (SanMiguel et al. 1996; Ramakrishna et al. 2002). Thus, the present comparative study of random BAC FISH analysis successfully reveals differential distribution of repetitive DNA elements vis-à-vis large and small chromosomes in monocots. This random BAC FISH is a unique approach to discern the differences in large-scale chromosome organization with respect to distribution of repetitive DNA elements. Such molecular cytogenetic analysis can be effectively applied to species with extremely large genome size, in which the entire genome sequence has not yet been available.
Regarding the localized repetitive BAC FISH signals, most of them were detected in (peri) centromeric chromosomal regions (Fig. 1b, d, f, h). Patterns of this (peri) centromeric signal localization vary among species; slightly broad signals in rice (Fig. 1b), several spotted signals in asparagus (Fig. 1d), compacted spot signals in wheat (Fig. 1f), and apparently peri-centromeric broad signals in onion (Fig. 1h). The (peri) centromeric signals in wheat (Fig. 1f) might correspond to the cereba sequence, which is a Ty3/gypsy retrotransposon-like sequence family localized near the centromeric region of cereals (Presting et al. 1998; Fukui et al. 2001; Hudakova et al. 2001; Houben and Schubert 2003). Existence of the onion BAC showing (sub) telomeric signals (Fig. 1i) indicates repetitive organization of the characteristic terminal heterochromatin (Suzuki et al. 2001), which suggested the substitution of telomere sequences in Allium (Pich et al. 1996; Pich and Schubert 1998; Do et al. 2001). The asparagus BAC showing (sub) telomeric signals (Table 3) might be related to the onion terminal heterochromatin sequences, which is expected to be characterized further in the future.
Around 10% of BAC probes did not show any signals (classified in “no signal”) in these monocot species. These BAC inserts might contain DNA derived from organelles. Alternatively, single unique sequences could not be detected on chromosomes because of low intensity of the FISH signals, or because of difficulty with the hybridization. In particular, it is possible that a relatively higher proportion (16%) of no-signal BAC clones in spider lily is due to difficulty with the probe hybridization to the target DNA embedded in its highly condensed chromosomal structure with steric complexity.
Thus, the BAC FISH detection of the single locus without the competitive DNA is quite difficult in the large-chromosome species of both Poales and Asparagales. Although we used randomly selected clones without sequence information in the present BAC FISH studies, a similar tendency is observed in the selected BAC clones with gene sequences; the asparagus BAC clones containing AODEF genes (Ito et al. 2005) were easily detected as distinct FISH signals showing the single locus on the chromosome with rDNA in the absence of the competitive DNA (Fig. 3a), and on the other hand, the sequenced onion BAC clones, 1G-12-89 with a MYB–related DNA binding protein-like gene (Jakse et al. 2008) and 4F4-77 with an alliinase-like (ALL1) gene (Do et al. 2004) showed dispersed FISH signals (Fig. 3b, c). Single-locus FISH detection of the 4F4-77 clone has not been successful by BAC FISH adding the competitive DNA or using selected probes with less repetitive fragments. These BAC clones actually contain many degenerated retroelements and transposon sequences (Do et al. 2004; Jakse et al. 2008), which are major components of the large onion genome, and specific in closely related species (Jakse et al. 2008). Similarly, in our selected BAC FISH experiments in wheat, it is not necessarily the case that competitive DNA such as C 0 t1 can successfully repress the dispersed repetitive signals to detect the single locus (data not shown). Again, it suggests the complex nature of the dispersed repetitive sequences in the large-chromosome species.
BAC FISH of the selected BAC clones including gene(s). a Two-color FISH detection of the 48-kb AODEF BAC (red) and rDNA (green) in Asparagus officinalis (A.o.). Close-up pictures of the chromosome with both FISH signals and the corresponding DAPI signal (white) are also shown. The bar represents 10 μm. b, c FISH detection of the 108 kb 1G-12-89 BAC with a MYB-related DNA binding protein-like gene (b, red) and the 86 kb 4F4-77 BAC with an alliinase-like (ALL1) gene (c, red) in Allium cepa (A.c.). The bars represent 10 μm
In conclusion, our BAC FISH analysis in monocot plants reveals that the complex dispersed repetitive elements have been accumulated in the large chromosomes in both Poales and Asparagales, whose genomes are, however, quite different in their GC contents (Do et al. 2004; Kuhl et al. 2004, 2005). Such genome-size expansion by accumulation of repeats and proliferation of transposable elements might have occurred commonly in Asparagales as well as in Poales, although it could have occurred independently in both species.
Abbreviations
- BAC:
-
Bacterial artificial chromosome
- FISH:
-
Fluorescence in situ hybridization
- FITC:
-
Fluorescein isothiocyanate
- PFGE:
-
Pulsed field gel electrophoresis
References
Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408:796–815
Arumuganathan K, Earle ED (1991) Nuclear DNA content of some important plant species. Plant Mol Biol Rep 9:208–218
Bennett MD, Leitch IJ, Price HJ, Johnston JS (2003) Comparisons with Caenorhabditis (approximately 100 Mb) and Drosophila (approximately 175 Mb) using flow cytometry show genome size in Arabidopsis to be approximately 157 Mb and thus approximately 25% larger than the Arabidopsis genome initiative estimate of approximately 125 Mb. Ann Bot 91:547–557
Do GS, Seo BB, Yamamoto M, Suzuki G, Mukai Y (2001) Identification and chromosomal location of tandemly repeated DNA sequences in Allium cepa. Genes Genet Syst 76:53–60
Do GS, Suzuki G, Mukai Y (2004) Genomic organization of a novel root alliinase gene, ALL1, in onion. Gene 325:17–24
Fukui K-N, Suzuki G, Lagudah ES, Rahman S, Appels R, Yamamoto M, Mukai Y (2001) Physical arrangement of retrotransposon-related repeats in centromeric regions of wheat. Plant Cell Physiol 42:189–196
Hawkins JS, Kim H, Nason JD, Wing RA, Wendel JF (2006) Differential lineage-specific amplification of transposable elements is responsible for genome size variation in Gossypium. Genome Res 16:1252–1261
Houben A, Schubert I (2003) DNA and proteins of plant centromeres. Curr Opin Plant Biol 6:554–560
Hudakova S, Michalek W, Presting GG, ten Hoopen R, dos Santos K, Jasencakova Z, Schubert I (2001) Sequence organization of barley centromeres. Nucleic Acids Res 29:5029–5035
International Rice Genome Sequencing Project (2005) The map-based sequence of the rice genome. Nature 436:793–800
Ito T, Suzuki G, Ochiai T, Nakada M, Kameya T, Kanno A (2005) Genomic organization of the AODEF gene in Asparagus officinalis L. Genes Genet Syst 80:95–103
Jakse J, Meyer JD, Suzuki G, McCallum J, Cheung F, Town CD, Havey MJ (2008) Pilot sequencing of onion genomic DNA reveals fragments of transposable elements, low gene densities, and significant gene enrichment after methyl filtration. Mol Genet Genomics 280:287–292
Jiang J, Gill BS, Wang G-L, Ronald PC, Ward DC (1995) Metaphase and interphase fluorescence in situ hybridization mapping of the rice genome with bacterial artificial chromosomes. Proc Natl Acad Sci USA 92:4487–4491
Kuhl JC, Cheung F, Yuan Q, Martin W, Zewdie Y, McCallum J, Catanach A, Rutherford P, Sink KC, Jenderek M, Prince JP, Town CD, Havey MJ (2004) A unique set of 11, 008 onion expressed sequence tags reveals expressed sequence and genomic differences between the monocot orders Asparagales and Poales. Plant Cell 16:114–125
Kuhl JC, Havey MJ, Martin WJ, Cheung F, Yuan Q, Landherr L, Hu Y, Leebens-Mack J, Town CD, Sink KC (2005) Comparative genomic analyses in Asparagus. Genome 48:1052–1060
Kurita S (1986) Variation and evolution in the karyotype of Lycoris, Amaryllidaceae I. General karyomorphological characteristics of the genus. Cytologia 51:803–815
Kurita S (1987) Variation and evolution on the karyotype of Lycoris, Amaryllidaceae II. Karyotype analysis of ten taxa among which seven are native in China. Cytologia 52:19–40
Lysak M, Fransz P, Schubert I (1998) Cytogenetic analysis of Arabidopsis. In: Salinas J, Sanchez-Serrano JJ (eds) Arabidopsis protocols, 2nd edn. Humana Press, Totowa, pp 173–186
Miller JT, Dong F, Jackson SA, Song J, Jiang J (1998) Retrotransposon-related DNA sequences in the centromeres of grass chromosomes. Genetics 150:1615–1623
Mukai Y, Endo TR, Gill BS (1990) Physical mapping of the 5S rDNA multigene family in common wheat. J Hered 81:290–295
Neumann P, Koblízková A, Navrátilová A, Macas J (2006) Significant expansion of Vicia pannonica genome size mediated by amplification of a single type of giant retroelement. Genetics 173:1047–1056
Pich U, Schubert I (1998) Terminal heterochromatin and alternative telomeric sequences in Allium cepa. Chromosom Res 6:315–321
Pich U, Fuchs J, Schubert I (1996) How do Alliaceae stabilize their chromosome ends in the absence of TTTAGGG sequences? Chromosom Res 4:207–213
Piegu B, Guyot R, Picault N, Roulin A, Saniyal A, Kim H, Collura K, Brar DS, Jackson S, Wing RA, Panaud O (2006) Doubling genome size without polyploidization: dynamics of retrotransposition-driven genomic expansions in Oryza australiensis, a wild relative of rice. Genome Res 16:1262–1269
Presting GG, Malysheva L, Fuchs J, Schubert I (1998) A Ty3/gypsy retrotransposon-like sequence localizes to the centromeric regions of cereal chromosomes. Plant J 16:721–728
Ramakrishna W, Dubcovsky J, Park YJ, Busso C, Emberton J, SanMiguel P, Bennetzen JL (2002) Different types and rates of genome evolution detected by comparative sequence analysis of orthologous segments from four cereal genomes. Genetics 162:1389–1400
SanMiguel P, Bennetzen JL (1998) Evidence that a recent increase in maize genome size was caused by the massive amplification of intergene retrotransposons. Ann Bot 82:37–44
SanMiguel P, Tikhonov A, Jin YK, Motchoulskaia N, Zakharov D, Melake-Berhan A, Springer PS, Edwards KJ, Lee M, Avramova Z, Bennetzen JL (1996) Nested retrotransposons in the intergenic regions of the maize genome. Science 274:765–768
Shirasu K, Schulman AH, Lahaye T, Schulze-Lefert P (2000) A contiguous 66-kb barley DNA sequence provides evidence for reversible genome expansion. Genome Res 10:908–915
Suzuki G, Mukai Y (2004) Plant BAC libraries as tools for molecular cytogenetics. In: Williams CR (ed) Focus on genome research. Nova Science, New York, pp 195–210
Suzuki G, Watanabe M, Toriyama K, Isogai A, Hinata K (1997) Direct cloning of the Brassica S locus by using a P1-derived artificial chromosome (PAC) vector. Gene 199:133–137
Suzuki G, Ura A, Saito N, Do G, So BB, Yamamoto M, Mukai Y (2001) BAC FISH analysis in Allium cepa. Genes Genet Syst 76:251–255
Zhang P, Li W, Fellers J, Friebe B, Gill BS (2004) BAC-FISH in wheat identifies chromosome landmarks consisting of different types of transposable elements. Chromosoma 112:288–299
Zhou S, Bechner MC, Place M, Churas CP, Pape L, Leong SA, Runnheim R, Forrest DK, Goldstein S, Livny M, Schwartz DC (2007) Validation of rice genome sequence by optical mapping. BMC Genomics 8:278
Zonneveld BJ, Leitch IJ, Bennett MD (2005) First nuclear DNA amounts in more than 300 angiosperms. Ann Bot 96:229–244
Acknowledgments
This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT) and the Japan Society for the Promotion of Science (JSPS), KAKENHI (18075003, 20780240, 23113001 and 23113006 to G.S., 20380014 to A.K., 22580004 and 19380194 to Y.M.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Toriyama.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
Representative results of PFGE analysis of NotI-digested BAC clones in rice, asparagus, and spider lily (Lycoris). BAC-vector (pBeloBAC11) fragments are indicated by asterisks. Other fragments represent the BAC inserts.
Rights and permissions
About this article
Cite this article
Suzuki, G., Ogaki, Y., Hokimoto, N. et al. Random BAC FISH of monocot plants reveals differential distribution of repetitive DNA elements in small and large chromosome species. Plant Cell Rep 31, 621–628 (2012). https://doi.org/10.1007/s00299-011-1178-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-011-1178-8