Abstract
Due to its reproducibility and sensitivity, real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR) has become the method of choice for quantifying gene expression. However, the accuracy of RT-qPCR is prone to bias if proper precautions are not taken, e.g. starting with intact, non-degraded RNA, considering the PCR efficiency and using the right reference gene(s) for normalization. It has been reported that some of the well-known reference genes are differentially regulated under certain experimental conditions suggesting that there is no gene that could be used as a universal reference. This paper aims at selecting the most suitable reference gene(s) out of six putative genes to be used as normalizer(s) for quantification of gene expression in the grapevine-downy mildew interaction as well as upon induced resistance with chemical elicitors. Moreover, the paper aims at determining the optimal number of reference genes to be used in normalization, since it has been emphasized in the literature that using multiple reference genes increases accuracy. Two different software tools, geNorm and Normfinder, were used to identify the most stable reference genes in grapevine under the aforementioned conditions. The importance of the choice of adequate reference genes is highlighted by studying chitinase expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants are continuously facing stresses or constraints. On one side, they are exposed to biotic stresses, e.g. viral, bacterial, fungal infections as well as competition with other plants. On the other side, plants face many abiotic stresses, e.g. temperature, water availability and salinity. The main and economically relevant consequence of these stresses is yield loss. Indeed, abiotic stresses represent the primary cause of crop loss worldwide (Boyer 1982), while biotic stresses reduce the yield by around 10–20% (Kreps et al. 2002).
Studies on the defense responses of plants to these stresses are numerous and many are based on gene expression (Collinge and Boller 2001; Bezier et al. 2002; Dean et al. 2002). It is important to know the expression patterns of genes differentially expressed under stress (biotic and abiotic) conditions to better understand the complex interactions between genes. Moreover, in combination with functional tests, they allow the identification of genes involved in biological processes (Vandesompele et al. 2002).
Real-time RT-PCR (RT-qPCR) is considered to be an accurate technique for measuring the gene expression due to its reproducibility and sensitivity (Bustin 2000). However, the use of RT-qPCR in gene expression analysis might be biased if normalization is not adequate. Housekeeping genes are needed for pivotal functions during the organism’s life cycle and therefore are expected to have a stable or slightly fluctuating expression even under stress conditions. This is the reason why they are also called reference genes and are used as endogenous controls being subjected to the same conditions (Huggett et al. 2005) (e.g. treatments—RNA extraction—cDNA synthesis and qPCR) as the genes of interest. Unfortunately, there is no ideal, universal reference gene for all experiments as some well-known reference genes may be differentially expressed under specific experimental conditions (Radonic et al. 2004). Therefore reference genes must be validated for each experimental condition (Schmittgen and Zakrajsek 2000). Ideally, for accurate normalization, geometrical averaging of multiple internal control genes is used (Vandesompele et al. 2002). The method relies on the use of several reference genes to remove any variation in the expression of these genes.
Downy mildew, caused by the oomycete Plasmopara viticola, is one of the most destructive grapevine diseases in Europe and in the eastern half of the United States. In 2006, 54% of the global sales of fungicides for downy mildews were for grapevine (Gisi and Sierotzki 2008). Induced resistance is considered to be an alternative to fungicides for the control of this disease. Defense responses can be induced in plants by the application of elicitors prior to actual pathogen infection. As an example, Aziz et al. (2003) have reported regarding the elicitation of defense responses and increased protection against the necrotrophic fungus B. cinerea and the biotrophic P. viticola on grapevine cell suspensions and detached leaves using laminarin. Elicitors induce the defense reaction of the plant by activating genes involved in the defence mechanisms such as, e.g. pathogenesis-related proteins (Aziz et al. 2006). Chitinase has been shown to have direct activity against fungal pathogens (Robert et al. 2002) induced under host and non-host pathogen conditions. After inoculation with Pseudoperonospora cubensis, a non-host grapevine oomycete pathogen, the cell wall of which is not composed of chitin, chitinases are largely accumulated (Gomès and Coutos-Thévenot 2009). Moreover, Uncinula necator, the causal agent of grapevine powdery mildew, induces expression of chitinases in leaves and berries in various grapevine cultivars, including susceptible ones (Gomès and Coutos-Thévenot 2009).
For grapevine, validated reference genes have been reported only in grape berries (Reid et al. 2006). The present paper aims at (a) searching for and selecting the most stable reference gene(s) to be used in gene expression studies in grapevine leaves especially under the grapevine-downy mildew pathosystem and at (b) determining the optimal number of reference genes to be used. Several experimental conditions are compared: control, downy mildew inoculated and elicited plants. The expression of a gene of interest known to be induced by the pathogen, chitinase, is quantified using various combinations of reference genes suggested by geNorm (Vandesompele et al. 2002) as well as by Normfinder (Andersen et al. 2004) in order to demonstrate the impact of the type and number of reference genes used. Results indicate the most stable and therefore suitable set of reference genes in elicited and downy mildew inoculated grapevine leaves.
Materials and methods
Plant cultivation
Two-eye cuttings of Vitis vinifera cv. Riesling (a white grape variety commonly planted in the Rhein region in Germany) were collected from the mature shoots after the first frost, i.e. after being stratified. They were disinfected by soaking them in 0.5% Chinoplant® solution (active ingredient: 8-hydroxychinoline) for 12 h. Thereafter, they were stored at 4°C and 95% humidity until use. Before cultivation, cuttings were soaked in lukewarm water for half an hour in order to gain sufficient moisture. Cuttings were then reduced (by cutting their edge) at both ends by about an inch to remove the dried edges. The lower eye was removed and the cuttings were put in boxes filled with a mixture of 50% perlite and 50% standard soil mixture. Cuttings were cultivated for 10–12 weeks at an average temperature of 24:22°C day:night and irrigated twice a week. Plantlets were fertilized during irrigation (fertigation) once a week (after the emergence of the first leave) with 1 g/l Flory 3 Mega (18 g N, 12 g P, 18 g K, 2 g Mg). Thereafter plantlets were potted in MCI-17 pots filled with standard soil ED 73. Plantlets were fertilized once a week. Young vine plants are best suited for inoculation trials when they have six to eight leaves unfolded.
Inoculation with Plasmopara viticola
Sporangia of the oomycete Plasmopara viticola were collected from previously inoculated plants (about 1 week incubation period) in demineralised (de-ionized) water and used for inoculation at a concentration of 5 × 105 sporangia/ml. Sporangia suspension was sprayed on the abaxial (lower) leaf surface. The control plants were neither inoculated nor elicitor-treated. After spraying, potted vines were immediately covered with a dark plastic wrap that was previously moistened with tap water for 24 h to create an ideal microclimate (liquid water conditions and a very high relative humidity) for the infection process. Inoculation was carried out in three biological replicates.
Elicitor treatments
Two different elicitors were used to induce the plant′s defence response, namely Algin Biovital® (TILCO Biochemie GmbH, Germany) and Frutogard® (TILCO Biochemie GmbH, Germany). The elicitors Frutogard® and Algin are mainly used in organic farming and consist of the following substances: algae extract, plant extracts and phosphate derivatives. The former contains Brown algae extract (Ascophyllum nodosum, Laminaria spp.), plant amino acids, phosphonate (phosphonic acid) and phosphate, while the latter contains algae extract (Ascophyllum nodosum, Laminaria spp.), sugar beet extract and phosphate solely. They were applied separately, at a concentration of 1%, and evenly on both, abaxial (lower) and adaxial (upper) surface of the leaves, using an airbrush gun. The control plants were neither elicitor-treated nor inoculated. Treatments were carried out in three biological replicates.
Greenhouse growth conditions and sampling
During the sampling period, potted vines were grown in a greenhouse cabin with a day–night rhythm of 16 h:8 h and at a temperature of 22°C. Young leaves (the first apical three leaves) were collected at intervals of 24 h for 5 days and immediately flash-frozen in liquid nitrogen. Leaf samples were then stored in the freezer at −80°C until RNA isolation.
Total RNA extraction
RNA was extracted from 100 mg of frozen leaves according to Wielgoss and Kortekamp (2006), and modified according to Chang et al. (1993). RNA was purified and treated with DNase I (on column digestion) (Qiagen, RNeasy cleanup kit, Hilden, Germany) to remove DNA contamination. RNA was washed (according to the manufacturer guidelines) using two washing buffers to get rid of DNase I and eluted in RNase-free water.
RNA purity and concentration were measured at 260/280 nm using a spectrophotometer (NanoDrop-1000, Thermo Scientific, Villebon sur Yvette, France) after RNA cleaning while RNA integrity was measured on the Agilent 2100 Bioanalyzer with the RNA 6000 Nano LabChip kit (Agilent, Diegem, Belgium). RIN (RNA Integrity Number) was calculated using an algorithm adapted for plant RNA profiles. All RIN values were between 7.0 and 7.8.
Two-step real-time RT-PCR
cDNA was synthesized from 1 μg of total RNA using MultiScribe® Reverse Transcriptase (Applied Biosystems, USA) and random hexamers (Invitrogen, Carlsberg, USA). cDNA synthesis was performed using the following parameters (incubation: 25°C for 10 min, RT: 48°C for 30 min, enzyme inactivation: 95°C for 5 min). cDNAs were stored at −20°C for short periods.
PCR reactions were performed on an ABI PRISM® 7500 fast Sequence Detection System (Applied Biosystems, USA) using SYBR®. Reactions were made in 25 μl containing 100 nM of primers (forward and reverse), 5 μl cDNA (10 ng cDNA equivalent RNA), and 12.5 μl 2× SYBR MESA GREEN MasterMix Plus, Low ROX (Eurogentec, Liège, Belgium). A dissociation curve was done at the end of each run to check the specificity of the primers.
To ensure reliability of the results, PCR efficiency was determined (Radonic et al. 2004). To this end, 5 μl were taken from each cDNA sample and pooled. Five serial tenfold dilutions of pooled cDNAs were made starting from 10 to 0.001 ng/μl using DNase free water. These dilutions were used with the corresponding primer pairs and real-time RT-Mix. The raw C t values were plotted against log-transformed concentrations of the serial dilutions to obtain the slope from the following equation (E = 10(−1/slope) − l). All PCRs displayed efficiencies between 90 and 97% (Table 1).
Aliquots from the same cDNA sample were used with all primer sets in each experiment. Reactions, performed on three biological replicates, were run in duplicates using the manufacturer’s recommended cycling parameters of [holding stage: 95°C for 10 min, cycling stage: 40 cycles of 95°C for 15 s (denaturation), and 60°C for 1 min (annealing and extension)]. No-template controls were included for each primer pair.
Six housekeeping genes were selected: Elongation factor 1 (EF1) (Trouvelot et al. 2008), Cyclophilin, Ubiquitin-conjugating enzyme (UBQ), SAND family protein (SAND) (Reid et al. 2006), Actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Heibertshausen, personal communication). Chitinase (Trouvelot et al. 2008), a PR-3, was selected as gene of interest (Table 1).
Statistical analysis
geNorm was used to determine the stability of expression as well as the optimal number of reference genes to be used for normalization. Normfinder allowed to assess the stability of expression as well as the combination of the two best reference genes. A normalization factor based on the best housekeeping genes was used to calculate the chitinase gene expression. For statistical analysis of these data, a two-tailed t test with unequal variance was performed on log-transformed datasets (PASW 18 software).
Results and discussion
Variations in the transcription of housekeeping genes
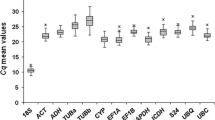
Gene expression of the six housekeeping genes and the gene of interest, chitinase, was monitored by qPCR. To study the variations in the transcription of the housekeeping genes, the C t values of all treatments combined (control, inoculated, Algin and Frutogard) were used as input. The boxplot in Fig. 1 shows the variations in the expression values (represented by the C t) for all data for each of the six housekeeping genes and the gene of interest individually. Chitinase had the most variable C t range. This is not surprising since it is an inducible PR gene with an anticipated role in the plant defence against pathogens (Robert et al. 2002). Levene’s test (PASW18 software) indicated no statistically significant difference in the variance between all housekeeping genes investigated; a statistically significant difference in the variance was only observed between chitinase C t values and the housekeeping genes.
Expression stability of the housekeeping genes
To decide which are the best reference genes for normalization, geNorm and Normfinder were used. geNorm calculates the gene expression stability measure (M) for each gene based on the average pairwise (V) expression ratio between this gene and each of the other genes being compared. Moreover, it performs a stepwise exclusion of the least stable gene and recalculates M until only the two most stably expressed genes are left. Normfinder ranks the candidate genes according to their expression stability. The input data for both software tools were the relative expression values based on the C t values obtained from the samples across the time course (1–5 days). These values were obtained from the equation (\( {\text{RQ}} = \, E \, ^{{(\min C_{\text{t}} --{\text{sample}}C_{\text{t}} )}} \)). This formula transforms C t values into relative quantities with the highest expression level set to 1. The method involves the choice of a calibrator sample having the lowest C t value (minC t) obtained for each gene. The gene expression stability as well as stability value was calculated separately for the datasets ‘control’, ‘inoculation’, ‘elicitation’ and the combined data from ‘control and elicitation’ and ‘control and inoculation’, to evaluate the stability and the ranking of the housekeeping genes in each experimental setup.
Expression stability using geNorm
As expected, the order of the housekeeping genes was not the same when comparing the different datasets within the same software tool and/or between the two software tools (Fig. 2a–f). According to the geNorm software, SAND and UBQ were the most stable genes in all treatments (Fig. 2a–f), while EF1 was the least stable gene in all treatments, except in the Algin treatment, where GAPDH was the least stable gene (Fig. 2c). The order of the housekeeping genes was the same for the datasets ‘control’ and ‘control and elicitation’ (Algin and Frutogard) (Fig. 2a, f): SAND and UBQ were the most stable genes followed by GAPDH, actin, cyclophilin and EF1. Both ‘inoculation’ and ‘control and inoculation’ also had the same order of the housekeeping genes (Fig. 2b, e): SAND and UBQ were the most stable genes followed by actin, GAPDH, Cyclophilin and EF1. Inoculation had little effect on the order of the housekeeping genes (Fig. 2a, b) where Actin and GAPDH switched the positions between 3rd and 4th. In the elicitor treatments, both for Algin and for Frutogard, SAND and UBQ had the first rank but the order of the rest of the housekeeping genes differed (Fig. 2c, d). Indeed, for the elicitor treatment Algin, EF1 had the second position after SAND and UBQ (Fig. 2c); for the elicitor treatment Frutogard, EF1 was the least stable (Fig. 2d) while the rest of the housekeeping genes were differently ordered. To conclude, in the present experimental system, SAND and UBQ were the most stable reference genes according to geNorm.
Average expression stability (M) and stability value in different data sets obtained from the softwares geNorm and Normfinder, respectively. a No treatment ‘control’, b inoculation, c elicitor treatment ‘Algin’, d elicitor treatment ‘Frutogard’, e control and inoculation, f control and elicitation (Algin treatment and Frutogard treatment)
Expression stability using Normfinder
According to Normfinder, GAPDH and Actin were the most stable genes in ‘control and inoculation’ as well as in ‘control and elicitation’ (Fig. 2e, f), while in the separate treatments (control, inoculation, Algin and Frutogard), the two most stable genes were different in each case (Fig. 2a–d). It is worth mentioning that according to Normfinder, EF1 was the least stable gene in four conditions out of six (Fig. 2a, d–f), while in the Algin treatment, GAPDH had the last ranking as in geNorm (Fig. 2c). The order of the housekeeping genes was more varied. GAPDH was the most stable housekeeping gene in three treatments out of six: ‘control’ ‘inoculation’ and ‘control and inoculation’ (Fig. 2a, b, e). For the treatments ‘Algin’, ‘Frutogard’ and ‘control and elicitation’ Actin, SAND and UBQ were the most stable genes (Fig. 2c, d, f).
The selection of the optimal reference gene(s) has been investigated in several plants under stress. In a study of potato, Nicot et al. (2005) reported EF1 to be the most stable housekeeping gene under both abiotic and biotic stress (late blight infection). Recently, Mascia et al. (2010) showed in different tomato tissues that GAPDH and ubiquitin were the most stable reference genes. In a sunflower leaf senescence transcript analysis, α-TUB1 was the most stable gene as determined by geNorm (Fernandez et al. 2011). Moreover, it was reported that GAPDH used together with actin or SAND was the most stable reference gene(s) for grape berry development studies (Reid et al. 2006). While the involvement of GAPDH in metabolic functions is well known, it has been recently reported that it is involved in several non-metabolic processes such as transcription activation and initiation of apoptosis (Hu et al. 2009). Our study showed that under Algin treatment, GAPDH was the least stable gene, according to both softwares, indicating that Algin treatment strongly affects the GAPDH expression. On one hand, it was suggested that GAPDH might have a role in defence; indeed, it was shown that cytosolic GAPDH, one of three forms of GAPDH in plants, transcripts accumulated in potato plants infected by P. infestans (Laxalt et al. 1996). However, the possibility that this accumulation is simply due to an increase in metabolic turnover of the protein, and not a specific stress response, should not be overlooked (Amey et al. 2008). The above-mentioned information from the literature together with our results suggests that identification of the reference genes depends not only on the treatments or the type of stress but also on the plant species. It also supports the notion that there is no ideal housekeeping gene that could be used as a universal reference gene under all conditions.
Determination of the optimal number of reference genes for normalization by geNorm
It was suggested that normalization using multiple reference genes gives more accurate results (Vandesompele et al. 2002). geNorm calculates the optimal number of genes to be used as reference genes. M value (average expression stability) is important to determine which gene has a stable expression, while V value (pairwise variation) is used to determine the optimal number of reference genes to be used. A cutoff value of 0.15 was proposed for the pairwise variation below which the inclusion of an additional reference gene is not required (Vandesompele et al. 2002).
V values were determined for the experimental datasets ‘control’, ‘inoculation’, ‘elicitation’, ‘control and elicitation’ and ‘control and inoculation’ individually. Interestingly, the optimal number of reference genes to be used for normalization differed from one dataset to another (Fig. 3; Table 2). In the control, the V value for using two genes was below the cutoff value 0.15 (0.144); thus the use of a third housekeeping gene was not required. For the dataset ‘inoculation’, using three housekeeping genes was sufficient for normalization (0.148). As for the datasets ‘elicitors’ (Algin and Frutogard), the V value did not reach the cutoff value; thus six housekeeping genes would be needed for accurate normalization. In the case of the dataset ‘control and inoculation’, using three housekeeping genes was sufficient for normalization (0.137). In the dataset ‘control and elicitation’, the V value of using five housekeeping genes was 0.143 and therefore a sixth housekeeping gene was not needed.
The elicitor treatments seem to have an important effect on the stability of the housekeeping genes indicating high metabolic impacts. These elicitors are phosphate derivatives. Algin contains phosphonate (phosphonic acid) and phosphate, while Frutogard contains phosphate. Phosphate derivatives are usually used as fertilizers but it was reported that they improve the resistance status of plants when they are used as a foliar spray. KH2PO4 and K2HPO4 have shown efficacy against powdery mildews on tomato and cucumber (Ehret et al. 2002; Orober et al. 2002). It has not been proven that phosphates are toxic to pathogens or have a direct effect on pathogens, which might indicate that the observed resistance is due to the activation of plant defence. Other phosphate derivatives, the phosphites have already been used in plant protection against downy mildews and Phytophthora species for 30 years (Cohen and Coffey 1986). Although the mode of action of phosphites is not yet fully understood, there is evidence that phosphites have direct antimicrobial activity on oomycetes (Smillie et al. 1989). Besides their direct activity, phosphites act indirectly by reinforcing defence reactions in plants. Phosphite treatments induce oxidative burst, hypersensitive response, PR proteins and accumulation of phenolic compounds (Bécot et al. 2000; Daniel and Guest 2006; Andreu et al. 2006). Phosphonates are also known to possess powerful antifungal activity against some Phytophthora species (Ouimette and Coffey 1989). Moreover, the fungicide Fosetyl-Al, marketed as Aliette® (active ingredient O-ethyl phosphonate), is known to have both a direct effect on the pathogen and an indirect effect via stimulation of defense responses in resistant and susceptible tobacco cultivars infected with Phytophthora nicotianae var. nicotianae (Nemestothy and Guest 1990).
Chitinase expression
Chitinases (EC 3.2.1.14) are ubiquitous enzymes in bacteria, fungi and plants. They hydrolyze the β-1,4-linkage between N-acetylglucosamine residues of chitin, a structural polysaccharide of the cell wall of many fungi. Chitinase induction upon pathogen infection has been reported in many plants (Bowles 1990; Collinge et al. 1993; Graham and Sticklen 1994). Although Plasmopara viticola is an oomycete and contains glucan in its cell wall and not chitin, chitinase is thought to be co-regulated with other PR proteins. Indeed, the expression of PR genes in general and of PR-1 in particular is used as a molecular marker for a successful induction of systemic acquired resistance (SAR) (Durrant and Dong 2004).
To validate the results obtained by geNorm and Normfinder, chitinase expression was calculated using the best reference genes (the best two) according to the average expression stability (M) and stability value suggested by geNorm and Normfinder, respectively. Chitinase expression was also calculated using the optimal number of reference genes according to the pairwise variation (V) analysis obtained from geNorm (Fig. 3). Thus, chitinase expression was normalized using four different sets of reference genes: ‘four best reference genes suggested by both softwares’ (SAND, UBQ, Actin, GAPDH), ‘two best reference genes suggested by geNorm’ (SAND, UBQ), ‘two best reference genes suggested by Normfinder’ (Actin, GAPDH), ‘three best genes as the optimal number of reference genes suggested by geNorm’ (SAND, UBQ, Actin) in the dataset inoculation and ‘five best genes as the optimal number of reference genes suggested by geNorm’ (SAND, UBQ, Actin, GAPDH, Cyclophilin) in the dataset elicitation.
After inoculation, chitinase expression significantly increased to reach a maximum on the first and the second day and decreased thereafter, while in the control plants (not infected) changes in chitinase expression were small (Fig. 4a). When comparing the differences in chitinase expression with the four different normalizations, using four different sets of reference genes in each case, chitinase expression level did not differ significantly (Fig. 4a).
Chitinase expression after inoculation, elicitation and in the controls. The normalized gene expression using the optimal number, the two best and the four best reference genes is shown. Asterisks indicate significant differences between control and inoculated/elicited plants at the following P values: *0.05 > P > 0.01; **0.01 > P > 0.005; ***0.005 > P > 0.001. HKG housekeeping gene, GN geNorm, NF Normfinder
After elicitation with Algin (Fig. 4b), chitinase transcript abundance was low as compared to inoculation. Chitinase expression was only slightly affected by Algin treatment. There was no significant difference when comparing chitinase expression with the different normalizations (Fig. 4b). Similar results were obtained for the elicitation with Frutogard (Fig. 4b).
Induction of chitinase genes in grapevine depends on the infecting pathogen (Robert et al. 2002). In V. vinifera cultivars susceptible to P. viticola such as Riesling, but inoculated with Pseudoperonospora cubensis (downy mildew of cucumber), a non-host pathogen in grapevine, chitinases (PR-3) are more induced than in the compatible interaction (Gomès and Coutos-Thévenot 2009). U. necator, the causal agent of grapevine powdery mildew, induces expression of chitinases in leaves and berries in various grapevine cultivars, including susceptible ones. Jacobs et al. (1999) showed that the hydrolytic activity of chitinase was directly related to the severity of infection at the pathogen location. In V. vinifera, most of the data regarding chitinases are related to their activity in leaves (Robert et al. 2002). There are 13 chitinase isoforms detected in grapevine tissues, 6 of which were found in untreated leaves and 4 new isoforms appeared in wounded leaves or leaves treated with salicylic acid after wounding (Derckel et al. 1996). Among these four wound-induced isoforms, three were also induced in leaves inoculated with B. cinerea (Derckel et al. 1999). Busam et al. (1997) have reported high transcript accumulations of class I and class III chitinase genes in leaves infected with P. viticola. Their accumulation was also observed in leaves infected with U. necator (Jacobs et al. 1999). Chitinase activity was also reported in healthy grapevine tissues, e.g. in mature healthy berries and in healthy leaves. Interestingly, in this report, the activity in mature healthy berries was ten times higher than in the healthy leaves (Derckel et al. 1996). Furthermore, expression of a rice chitinase in grapevine plants enhanced the resistance to fungal diseases, namely powdery mildew and anthracnose (Yamamato et al. 2000).
Our results indicate that using four reference genes, suggested by both softwares, the two most stable reference genes, or the optimal number of reference genes did not significantly affect the final results. Therefore, in the present experimental system, it could be acceptable to normalize expression results with the two most stable housekeeping genes, especially if labor cost and time are to be taken into account. It can, however, be assumed that the use of the optimal number of reference genes will result in more accurate results.
Conclusion
Our results support the notion that there is no universal reference gene for all experimental conditions. Therefore, housekeeping genes have to be tested under each experimental condition to ensure the stability of the selected genes. According to the results from both software, four reference genes were considered the best (SAND, UBQ, Actin, GAPDH). More specifically, geNorm suggested SAND and UBQ under all selected conditions, while Normfinder suggested different reference genes for different treatment. geNorm results also showed that the optimal number of reference genes to be used varied from one condition to another. However, normalization of the gene of interest, chitinase, with different sets of reference genes did not lead to significantly different results, though they are expected to be more accurate. Thus, the number of genes used for geometric averaging is a trade-off between practical consideration (time and labour cost) and accuracy (Vandesompele et al. 2002). It is also worth mentioning that EF1 and Cyclophilin were considered the least stable housekeeping gene under the selected conditions according to both programs used.
References
Amey RC, Schleicher T, Slinn J, Lewis M, Macdonald H, Neill SJ, Spencer-Phillips PTN (2008) Proteomic analysis of a compatible interaction between Pisum sativum (pea) and the downy mildew pathogen Peronospora viciae. Eur J Plant Pathol 122:41–55
Andersen CL, Jensen JL, Orntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data. A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64:5245–5250
Andreu AB, Daleo G, Caldiz DO, Guevara MG, Wolski EA (2006) Enhancement of natural disease resistance in potatoes by chemicals. Pest Manag Sci 62:162–170
Aziz A, Poinssot B, Daire X, Adrian M, Bezier A, Lambert B, Joubert JM, Pugin A (2003) Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol Plant Microbe Interact 16:1118–1128
Aziz A, Trotel-Aziz P, Couderchet M, Vernet G (2006) Chitosan stimulates defence reactions in grapevine leaves and inhibits development of Botrytis cinerea. Eur J Plant Pathol 114:405–413
Bécot S, Pajot E, Le Core D, Monot C, Silué D (2000) Phytogard® (K2HPO3) induces localized resistance in cauliflower to downy mildew of crucifers. Crop Prot 19:417–425
Bezier A, Lambert B, Baillieul F (2002) Study of defense-related gene expression in grapevine leaves and berries infected with Botrytis cinerea. Eur J Plant Pathol 108:111–120
Bowles DJ (1990) Defence-related proteins in higher plants. Ann Rev Biochem 59:873–907
Boyer JS (1982) Plant productivity and environment. Science 218:443–448
Busam G, Kassemeyer HH, Matern U (1997) Differential expression of chitinases in Vitis vinifera L responding to systemic acquired resistance activators or fungal challenge. Plant Physiol 115:1029–1038
Bustin SA (2000) Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 2:169–193
Chang S, Puryear J, Cairney J (1993) A simple method for isolating RNA from pine trees. Plant Mol Biol Rep 11:113–116
Cohen Y, Coffey MD (1986) Systemic fungicides and the control of oomycetes. Annu Rev Phytopathol 24:311–338
Collinge M, Boller T (2001) Differential induction of two potato genes, Stprx2 and StNAC, in response to infection by Phytophthora infestans and to wounding. Plant Mol Biol 46:521–526
Collinge DB, Kragh KM, Mikkelsen JD, Nielsen KK, Rasmussen U, Vad K (1993) Plant chitinases. Plant J 3:31–40
Daniel R, Guest D (2006) Defence responses induced by potassium phosphonate in Phytophthora palmivora-challenged Arabidopsis thaliana. Physiol Mol Plant Pathol 67:194–201
Dean JD, Goodwin PH, Hsiang T (2002) Comparison of relative RT-PCR and northern blot analyses to measure expression of β-1, 3-glucanase in Nicotiana benthamiana infected with Colletotrichum destructivum. Plant Mol Biol Rep 20:347–356
Derckel JP, Legendre L, Audran JC, Haye B, Lambert B (1996) Chitinases of the grapevine (Vitis vinifera L.): five isoforms induced in leaves by salicylic acid are constitutively expressed in other tissues. Plant Sci 119:31–37
Derckel JP, Baillieul F, Manteau S, Audran JC, Haye B, Lambert B, Legendre L (1999) Differential induction of grapevine defenses by two strains of Botrytis cinerea. Phytopathol 89:197–203
Durrant WE, Dong X (2004) Systemic acquired resistance. Ann Rev Phytopathol 42:185–209
Ehret DL, Menzies JG, Bogdanoff C, Ukthede RS, Frey B (2002) Foliar applications of fertilizer salts inhibit powdery mildew on tomato. Can J Plant Pathol 24:437–444
Fernandez P, Rienzo JA, Moschen S, Dosio GA, Aguirrezábal L, Hopp HE, Paniego N, Heinz RA (2011) Comparison of predictive methods and biological validation for qPCR reference genes in sunflower leaf senescence transcript analysis. Plant Cell Rep 30:63–74
Gisi U, Sierotzki H (2008) Fungicide modes of action and resistance in downy mildews. Eur J Plant Pathol 122:157–167
Gomès E, Coutos-Thévenot P (2009) Molecular aspects of grapevine pathogenic fungi interactions. In: Roubelakis-Angelakis KA (ed) Grapevine molecular physiology & biotechnology, 2nd edn. Springer, Berlin, pp 407–428
Graham LS, Sticklen MB (1994) Plant chitinases. Can J Bot 72:1057–1083
Hu Y, Wu G, Cao Y, Wu Y, Xiao L, Li X, Lu C (2009) Breeding response of transcript profiling in developing seeds of Brassica napus. BMC Mol Biol 10:49
Huggett J, Dheda K, Bustin S, Zumla A (2005) Real-time RT-PCR normalisation; strategies and considerations. Genes Immun 6:279–284
Jacobs AK, Dry IB, Robinson SP (1999) Induction of different pathogenesis-related cDNAs in grapevine infected with powdery mildew and treated with ethephon. Plant Pathol 48:325–336
Kreps JA, Wu Y, Chang HS, Zhu T, Wang X, Harper JF (2002) Transcriptome changes for Arabidopsis in response to salt, osmotic and cold stress. Plant Physiol 130:2129–2141
Laxalt AM, Cassia RO, Sanllorenti PM, Madrid EA, Andreu AB, Daleo GR et al (1996) Accumulation of cytosolic glyceraldehyde-3-phosphate dehydrogenase RNA under biological stress conditions and elicitor treatments in potato. Plant Mol Biol 30:961–972
Mascia T, Santovito E, Gallitelli D, Cillo F (2010) Evaluation of reference genes for quantitative reverse-transcription polymerase chain reaction normalization in infected tomato plants. Mol Plant Pathol 11:805–816
Nemestothy GN, Guest DI (1990) Phytoalexin accumulation, phenylalanine ammonia lyase activity and ethylene biosynthesis in Fosetyl-Al treated resistant and susceptible tobacco cultivars infected with Phytophthora nicotianae var nicotianae. Physiol Mol Plant Pathol 37:207–219
Nicot N, Hausman JF, Hoffmann L, Evers D (2005) Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J Exp Bot 56:2907–2914
Orober M, Siegrist J, Buchenauer H (2002) Mechanism of phosphate induced disease resistance in cucumber. Eur J Plant Pathol 108:345–353
Ouimette DG, Coffey MD (1989) Comparative antifungal activity of four phosphonate compounds against isolates of nine Phytophthora species. Phytopathol 79:761–767
Radonic A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A (2004) Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun 313:856–862
Reid KE, Olsson N, Schlosser J, Peng F, Lund ST (2006) An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol 6:27
Robert N, Roche K, Lebeau Y, Breda C, Boulay M, Esnault R, Buffard D (2002) Expression of grapevine chitinase genes in berries, leaves infected by fungal, bacterial pathogens. Plant Sci 162:389–400
Smillie R, Grant BR, Guest D (1989) The mode of action of phosphite: evidence for both direct and indirect modes of action on three Phytophthora spp in plants. Phytopathol 79:921–926
Schmittgen TD, Zakrajsek BA (2000) Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J Biochem Biophys Meth 46:69–81
Trouvelot S, Varnier AL, Allègre M, Mercier L, Baillieul F, Arnould C, Gianinazzi-Pearson V, Klarzynski O, Joubert JM, Pugin A, Daire X (2008) A β-1, 3 Glucan sulfate induces resistance in grapevine against Plasmopara viticola through priming of defense responses, including HR-like cell death. Mol Plant Microbe Interact 21:232–243
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3(7):0034.1–0034.11
Wielgoss A, Kortekamp A (2006) Comparison of PR1 expression in grapevine cultures after inoculation with a host- and a non-host pathogen. Vitis 45:9–13
Yamamato T, Iketani H, Ieki H, Nishizawa Y, Nostuka K, Hibi T, Hayashi T, Matusta N (2000) Transgenic grapevine plants expressing a rice chitinase with enhanced resistance to fungal pathogens. Plant Cell Rep 19:639–646
Acknowledgments
This work was funded by the National Research Fund (FNR), Ministry of Higher Education and Research, Luxembourg (BFR08-079). The authors gratefully acknowledge H. Findeis, M. Sandmann and W. Schoenbach for the technical assistance in the greenhouse trials at the Geisenheim research center, Germany. They also acknowledge S. Contal and L. Solinhac for the technical assistance in the molecular biology section and Dr T. Bohn for the help with the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Harwood.
Rights and permissions
About this article
Cite this article
Selim, M., Legay, S., Berkelmann-Löhnertz, B. et al. Identification of suitable reference genes for real-time RT-PCR normalization in the grapevine-downy mildew pathosystem. Plant Cell Rep 31, 205–216 (2012). https://doi.org/10.1007/s00299-011-1156-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-011-1156-1