Abstract
Nitric oxide (NO) is a key signal molecule involved in many physiological processes in plants. To study the mechanisms of exogenous NO contribution to alleviate the aluminum (Al) toxicity, roots of rice (Oryza sativa) seedlings pre-treated with sodium nitroprusside (SNP, a NO donor) were used to investigate the effect of Al in this study. Results indicated that NO alleviated the lipid peroxidation induced by Al and promoted the root elongation, whereas butylated hydroxyanisole (BHA), an efficient lipophilic antioxidant, alleviated the lipid peroxidation only. Rice seedling roots pre-treated with SNP followed by Al treatment had lower contents of pectin and hemicellulose, lower Al accumulation in root tips and cell walls, higher degree of methylation of pectin and lower wall Al-binding capacity than the roots with Al treatment only. Therefore, the decreased Al accumulation in the cell walls of rice roots is likely to be the reason for the NO-induced increase of Al tolerance in rice, and it seems that exogenous NO enhanced Al tolerance in rice roots by decreasing the contents of pectin and hemicellulose, increasing the degree of methylation of pectin, and decreasing Al accumulation in root cell walls.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aluminum (Al) toxicity is a major limiting factor in affecting crop productivity on acidic soils, which accounts for about 40% of the World’s arable land (Uexküll and Mutert 1995). The primary toxic symptom of Al ion is the inhibition of root growth (Yamamoto et al. 2003). Al ions can cause significant inhibition of root elongation at micromolar concentrations (Horst et al. 2010; Kochian 1995). Numerous studies indicated that root apex is the primary site perception (Horst et al. 2010; Ma 2007; Tian et al. 2007; Yang et al. 2007) and expression of Al toxicity and resistance (Wang et al. 2010a). Plants have diverse mechanisms to tolerate Al toxicity in acid soils (Horst et al. 2010; Ma 2007; Yang et al. 2007). There are two distinct mechanisms of resisting Al toxicity in plants. One class of mechanisms is excluding Al from the root apex, while the other class allows the plant to tolerate Al accumulation in the root and shoot symplasm (Kikui et al. 2005). Although progress has been made during recent years, the mechanisms of Al-induced inhibition of root elongation and Al resistance are still not well understood.
Numerous studies indicate that Al interacts with root cells at multiple sites including cell wall, plasma membrane and symplasm, resulting in disrupted structures and/or functions of the cell wall and plasma membrane, signal transduction pathway, and Ca homeostasis (Ma et al. 2004; Ma 2007). Cell wall is the first site in contact with Al and about 85–90% of the total Al accumulated by roots is tightly bound to cell walls (Chang et al. 1999; Rengel and Reid 1997; Yang et al. 2007). Cell elongation is also regulated by turgor pressure and physical properties of cell walls (Ma et al. 2004). Recent studies showed that Al caused the changes of polysaccharides in cell walls and increased wall-bound ferulic and diferulic acids, which may lead to a decrease in cell wall extensibility and an increase in cell wall rigidity (Eticha et al. 2005; Hossain et al. 2006; Ma 2007; Matsumoto 2000; Tabuchi and Matsumoto 2001). Al3+ binds to the negatively charged carboxylic groups of pectin matrix in cell walls (Eticha et al. 2005; Yang et al. 2007). Previous study has shown that pectin content is positively correlated with the Al-induced loss of cell viability in maize suspension cells, suggesting that the binding of Al to the pectin matrix is an important step in the manifestation of Al toxicity (Ma 2007; Schmohl and Horst 2000). Hence, the amount of Al binding to cell walls is controlled by the pectin content and its methylation degree (Schmohl and Horst 2000; Yang et al. 2007). In rice, the pectin content of the root apex in the Al-resistant cultivars was lower than that in Al-sensitive cultivars (Yang et al. 2007). Moreover, Al-sensitive cultivars showed a higher pectin methylesterase (PME) activity and a higher portion of demethylated pectin than that in Al-resistant cultivars (Eticha et al. 2005; Yang et al. 2007), indicating a higher portion of free pectic acid from demethylated pectin residues in cell walls resulted in the higher Al contents in the root tips and cell walls. Strong binding of Al to the pectin matrix may prevent cell wall extension by decreasing the effectiveness of cell wall-loosening enzymes (Wehr et al. 2004). Rice (O. sativa) is a worldwide staple crop, an important monocotyledon model plant and the most Al-resistant species among small grain cereal crops (Foy 1988). However, the mechanism of rice resistance to Al toxicity is little known. In rice, correlations between cell wall polysaccharide content and Al exclusion might be the basis for a novel Al resistance mechanism (Yang et al. 2007).
As an important signaling molecule in plants, NO is involved in responses to abiotic and biotic stresses such as drought, salt, heavy metal, heat stress, disease resistance and apoptosis (Delledonne et al. 1998; Durner and Klessig 1999; García-Mata and Lamattina 2002; Wang et al. 2009; Zhao et al. 2007). In plants, NO also modulates numerous physiological processes (Crawford and Guo 2005; Lamattina et al. 2003; Neill et al. 2003; Wang et al. 2010b), including promoting root growth and mediating IAA-induced adventitious roots in cucumber (Cucumis sativus) (Pagnussat et al. 2003), modulating the expression of cell cycle regulatory genes and the lateral root formation in tomato (Correa-Aragunde et al. 2004, 2006), as well as affecting the composition of cell walls in tomato and rice roots (Correa-Aragunde et al. 2008; Xiong et al. 2009).
It has been reported that NO can reduce Al toxicity by alleviating Al-induced oxidative stress and promoting root elongation in Cassia tora L. and Hibiscus moscheutos L. (Tian et al. 2007; Wang and Yang 2005). However, Yamamoto et al. (2001) indicated that the Al-induced oxidative stress eliminating by BHA could not promote the root elongation, suggesting that there might be other mechanisms of NO in alleviating Al stress. Considering the role of cell wall in Al toxicity and root elongation, NO may be involved in the regulation of cell wall composition in alleviating heavy Al toxicity to rice roots. In this study, we provide the useful information in understanding the roles and mechanisms of exogenous NO in alleviating Al toxicity in rice.
Materials and methods
Plant material and chemical treatments
Rice (O. sativa sp.) seeds were surface sterilized in 1% (v/v) sodium hypochlorite solution for 18 min, washed thrice with de-ionized water and then soaked in de-ionized water overnight at 30°C in the dark. Germinated seeds were cultured in the solution (0.5 mM CaCl2, pH 4.5) in a culture room at 25 ± 2°C with 16-h day/8-h dark photoperiod under a photon flux density of 120 μmol photons m−2 s−1.
After 4 days culture, the seedlings were subjected to various treatments. AlCl3 was included in the culture solution for Al treatment. SNP (NO donor) and/or PTIO (NO scavenger) were added into the culture solution for 24-h pre-treatment, then the rice roots were rinsed with 0.5 mM CaCl2 (pH 4.5) thrice and exposed to the AlCl3 solution. Root elongation was measured with a ruler after different treatments.
Ion leakage measurement
Relative ion leakage was determined according to Sairam and Srivastava (2002) with some modifications. The rice roots (10 tips for each sample) were placed in beaker with 10 ml of de-ionized water at 25°C for 2 h. After the incubation, the conductivity in the bathing solution was determined (C 1). Then, the samples were boiled for 15 min, and the conductivity was read again in the bathing solution after cooling (C 2). Relative ion leakage was calculated from the equation: relative ion leakage % = C 1/C 2 × 100.
Determination of Al content
The content of Al bound to cell walls of rice roots was estimated by homogenizing the frozen root apices with the ice-cold distilled water. The homogenate was centrifuged at 13,000g for 10 min and the pellet was washed three times with 80% ethanol, methanol and chloroform mixture (1:1, v/v), and 100% acetone, respectively. After drying, the pellet was resuspended in 2 N HCl for 24 h with occasional shaking. For the total Al content in the root apices, the root apices were directly suspended in 2 N HCl for 24 h. Al content was determined through graphite furnace atomic absorption spectrophotometry according to the method of Wang and Yang (2005).
Cell wall extraction and measurement of polysaccharide content
The rice root cell wall was extracted according to Zhong and Lauchl (1993). Root cell walls were estimated by homogenizing the frozen root apices with 80% ethanol. The homogenate was kept in a centrifuge tube undisturbed for 20 min in an ice-water bath. Then, the homogenate was centrifuged at 10,000g for 10 min and the pellet was washed thrice each with a methanol and chloroform mixture (1:1, v/v), and 100% acetone, respectively. The supernatant of each wash was discarded and the final pellet was freeze dried overnight.
The cell wall was fractionated into three fractions: pectin, hemicellulose 1 (HC1), and hemicellulose 2 (HC2) according to Yang et al. (2007). The pectin fraction was extracted twice by 0.5% ammonium oxalate buffer containing 0.1% NaBH4 (pH 4.0) in a boiling water bath for 1 h. Pellets were subsequently subjected to triple extractions with 4% KOH containing 0.1% NaBH4 for 24 h, followed by the extraction with 24% KOH containing 0.1% NaBH4. The pooled supernatants from 4 and 24% KOH extraction thus yielded the HC1 and HC2 fractions. Uronic acid content in each cell wall fraction was assayed according to the method of Blumenkrantz and Asboe-Hansen (1973). Galacturonic acid was used as a calibration standard, thus the root pectin content was expressed as galacturonic acid equivalents (GaE).
Al association and dissociation kinetics
The Al association and dissociation kinetics were analyzed as described earlier by Zheng et al. (2004). The association solution consisted of 10 μM AlCl3 otherwise stated in 0.5 mM CaCl2 at pH 4.5. The solution was sipped by a peristaltic pump set at a speed of 2 ml 10 min−1 after running through a 2-ml column packed with the cell wall sample. Then the flowthrough was collected by a fraction collector at 10 min intervals. The fractions were collected until the Al concentration was the same as that in the association solution. The unbound Al was washed with 0.5 mM CaCl2 at pH 4.5 at a speed of 6 ml min−1 for 1 h. Then the bound Al was dissociated by 2.5 mM CaCl2 at pH 4.5 at the same speed as association, and the fraction was collected until the Al concentration in the dissociation solution was below the detection limit. Finally, the accumulated bound or unbound Al was calculated and plotted. All the kinetic studies were carried out twice independently, and one set of association and dissociation curve was presented in the result.
Al was measured spectrophotometrically with pyrocatechol violet (PCV) according to Kerven et al. (1989) with some modifications. As only simple dilute solution was used in the present study, MES buffer at pH 6.2 was applied instead of hexamine buffer. The reaction solution consisted of 2 ml sample solution + 0.8 ml de-ionized water + 0.2 ml 0.0375% PCV. After mixing, 1.0 ml of 50 mM MES was added and mixed. The absorbance at 590 nm was recorded 15 min later. In preliminary experiments, it was shown that the pH of the reaction solution could be maintained at 6.2 throughout the measurement. The absorbance of the standard was stable and repeatable during the whole experimental period.
Pectin methylesterase activity assay
PME in rice roots was extracted according to the method described by Richard et al. (1994). Cell wall was resuspended in 1 M NaCl solution (pH 6.0) for 1 h, and then centrifuged at 12,000g for 15 min. The supernatant was collected. The extract was added to the PME activity assay buffer (0.5% citrus pectin, 0.2 M NaCl, and 0.002% methyl red, pH 6.8) for 1 h at 37°C. Pectin de-esterification decreases the pH, thus changes the color from yellow to red. The color change was recorded at 525 nm with a spectrophotometer. A calibration curve was obtained by adding 0.01 M HCl to the PME activity assay buffer and the respective OD values were measured at 525 nm. The PME activity was obtained according to the calibration curve.
The plasma membrane integrity determination
The plasma membrane integrity of rice roots was evaluated according to the method described by Yamamoto et al. (2001). The roots were stained with 0.025% (w/v) Evans Blue and then washed by de-ionized water. The stained region (10 mm) was excised and placed together. The trapped Evans Blue was released by homogenizing the root sections in 1 ml of 1% (w/v) SDS. The homogenate was centrifuged at 10,000g for 10 min. The supernatant was determined at 600 nm.
Histochemical analyses
The localization of Al was detected with hematoxylin according to the method of Sasaki et al. (1997). In this staining procedure, Al acts as a mordant and causes the binding of oxidized hematoxylin to constituents of cells with the formation of colored complexes (Havas 1986). The localization of the loss of plasma membrane integrity was detected with Evans Blue according to the method of Yamamoto et al. (2001). The root was stained with 0.025% (w/v) Evans Blue solution for 15 min and then washed thrice with deionized water.
Determination of lipid peroxidation
Lipid peroxidation was measured in terms of thiobarbituric acid-reactive substances (TBARS) content following the method of Heath and Packer (1968) with some modifications. Rice root tips (0.3 g) were homogenized in 10% trichloroacetic acid and then the homogenate was centrifuged at 4,000g for 30 min. A 2 ml aliquot of the supernatant was mixed with 2 ml of 10% trichloroacetic acid containing 0.5% thiobarbituric acid. The mixture was heated at 100°C for 30 min, and then centrifuged at 10,000g for 10 min. The supernatant was measured at 532 nm, with a reading at 600 nm subtracted from it to reduce non-specific turbidity. The amount of TBARS was calculated using an extinction coefficient of 155 mM−1 cm−1.
Determination of methyl esterification degree of pectin
Methyl esterification degree (MED) of pectin was determined through FT-IR spectroscopy analysis as described by Manrique and Lajolo (2002). MED of pectin was calculated according to the absorbance spectra of the samples, using a relationship involving absorbance intensities for the 1,630 and 1,745 cm−1 band.
Results
Effect of NO on Al-induced inhibition of root elongation
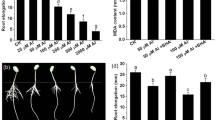
The growth of rice roots treated with 0–200 μM Al3+ in 0.5 mM CaCl2 solution (pH 4.5) for 24 h was obviously inhibited in a dose-dependent manner. Approximately, 50% inhibition of root elongation was observed at 75 μM Al3+ treatment (Fig. 1a). Therefore, 75 μM Al3+ was used in subsequent experiments. In order to determine the effects of NO on the Al-induced inhibition of rice root growth, 0–150 μM SNP pre-treatments in rice seedlings was performed. As shown in Fig. 1b, the pre-treatment with 25–100 μM SNP clearly removed the Al-induced inhibition of rice root elongation. The root length increased about twofold in rice seedlings pre-treated with 25 μM SNP followed by 75 μM Al3+ treatment compared with that of the control (75 μM Al3+ treatment alone). The root growth almost recovered to the normal level by 25–50 μM SNP pre-treatment (followed by 75 μM Al treatment). However, in the presence of higher SNP concentrations (75–100 μM), the recovery of the Al-induced inhibition decreased and no recovery was observed at 150 μM SNP (Fig. 1b). The root elongation of SNP pre-treated rice seedlings under Al treatment for 0–24 h increased along with the treatment time (Fig. 1c). The effect of 25 μM SNP on recovering the Al-induced root growth inhibition was reversed by application of 100 μM 2-phenyl-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide (PTIO), a NO-specific scavenger (Fig. 1d). These results suggested that NO could relief the Al toxicity on rice roots.
Effect of Al and NO on root elongation of rice seedlings. a Changes of root elongation under 50–200 μM Al treatments for 24 h. AlCl3 was added to the culture solution (0.5 mM CaCl2, pH 4.5) for treatment. b Effect of SNP on root elongation. Roots were pre-treated with 0, 25, 50, 100, 200 μM SNP for 24 h and then exposed to treatment solutions (0.5 mM CaCl2, pH 4.5) containing 0, 75 μM Al for 24 h. c Time course of root growth under 75 μM Al and 25 μM SNP treatment. Seedlings were pre-treated with 25 μM SNP for 24 h and then treated with 75 μM Al. d Effect of NO on the Al-induced inhibition of root growth of rice seedlings. The seedlings were pretreated with 25 μM SNP and 100 μM PTIO for 24 h and then transferred into the culture solution containing 75 μM Al for 24 h. Data are mean values ± SE of three independent experiments. Within each set of experiments, bars with different letters were significantly different at P < 0.05
Effect of NO on Al accumulation in root apices and cell wall of rice seedlings
Al accumulation in root apices is correlated with the Al-induced inhibition of root elongation. In the root apex, Al mainly accumulates in the cell walls (Horst et al. 2010; Schmohl and Horst 2000). As shown in Fig. 2, Al mainly accumulated in the cell wall fraction (about 87.7%). The pre-treatment with 25 μM SNP obviously reduced the Al accumulation in rice roots (60.4%) and cell walls (59.0%) compared with that of 75 μM Al treatment alone. The effect of NO on the decrease of Al accumulation in rice root tips and cell walls was reversed by PTIO application. The inhibition of Al accumulation in root apices by SNP was further supported by histochemical staining (Fig. 4a). Compared to the control roots (75 μM Al alone), the root apex of seedlings pre-treated with SNP was weakly stained by hematoxylin, an indicator of Al presence. Higher level of Al accumulation appeared in the root apical region.
Changes of Al content in root tips (a) and cell walls (b) of rice seedlings. The seedlings were treated with 25 μM SNP, 100 μM PTIO and 75 μM Al as in Fig. 1 and then root tips (10 mm from the apex) were used for the measurement of Al content. Cell wall was extracted from the cut-off root tips. Data are mean values ± SE of three independent experiments. Within each set of experiments, bars with different letters were significantly different at P < 0.05
Effect of NO on Al-induced lipid peroxidation and plasma membrane integrity
To study the role of NO in mediating Al-induced lipid peroxidation, the content of TBARS, an indicator of lipid peroxidation, was measured in the rice root apices. Butylated hydroxyanisole (BHA) was used as a positive control of efficient lipophilic antioxidant to evaluate the relationship between lipid peroxidation and root elongation. The content of TBARS in rice roots pre-treated with 25 μM SNP (followed by 75 μM Al treatment) was only 50% of the control (75 μM Al alone), and the root elongation in rice seedlings pre-treated with 25 μM SNP (followed by 75 μM Al treatment) was recovered to 85% of the seedlings without SNP and Al treatment. BHA application also reduced the content of TBARS induced by Al to the normal level (without Al treatment). BHA, however, had little effect on alleviating the inhibition of root elongation caused by Al (Fig. 3). The integrity of plasma membrane in rice root apices was evaluated by a spectrophotometric assay of Evans Blue uptake and the membrane permeability (MP). The more intense staining indicated a higher level of the loss of plasma membrane integrity at the root apical region (Yamamoto et al. 2001). A 2.3-fold increase in MP and 1.8-fold increase in Evans Blue uptake in rice root apices under 75 μM Al treatment were observed compared with control. These increases were reduced by SNP pre-treatment before the rice seedlings were exposed to 75 μM Al solution (Fig. 5). The inhibition of Evans Blue uptake in rice root apices by SNP pre-treatment was supported by histochemical staining (Fig. 4b). Compared with the control roots (Al treatment only), the SNP pre-treated root tips were stained less by Evans Blue.
Effect of NO and BHA on the content of TBARS (a) and the elongation of roots (b). The seedlings were treated with 25 μM SNP, 100 μM PTIO and 75 μM Al as in Fig. 1. The seedlings without pre-treatment were treated with 75 μM Al and/or 100 μM BHA. Data are mean values ± SE of three independent experiments. Within each set of experiments, bars with different letters were significantly different at P < 0.05
Effect of NO on Al accumulation in the root apices (a) and Al-induced loss of plasma membrane integrity (b). The seedlings were treated with 25 μM SNP, 100 μM PTIO and 75 μM Al as in Fig. 1. After treatment, the roots were rinsed with 0.5 mM CaCl2 (pH 4.5) solution and then the roots were stained with hematoxylin (a) or Evans Blue (b). The scale bars in the graph indicate 1 mm
Effect of NO on pectin and hemicellulose contents in the cell wall of rice roots under Al treatment
It has been reported that Al mainly accumulates in the cell wall and the accumulation exerts a detrimental effect on root growth and function (Blamey 1993). The cell wall composition of the rice root apex was measured. As shown in Fig. 6, polysaccharides including pectin, hemicellulose 1 and hemicellulose 2 in cell wall of rice roots increased obviously under Al treatment compared with control. The increase in polysaccharides induced by Al treatment was reduced by SNP pre-treatment. The application of PTIO eliminated the effect of SNP on relieving the increased polysaccharides caused by Al (Fig. 6).
Effect of NO on pectin methylesterase activity and the degree of pectin methylation
The degree of pectin methylation is an important factor affecting the properties of the cell wall (Yang et al. 2007). Lower degree of pectin methylation in the cell wall leads to more Al accumulation (Cosgrove 2005; Yang et al. 2007) PME can reduce the degree of pectin methylation. In order to determine the degree of pectin methylation, the PME activity in rice root apex (0–10 mm) was analyzed. The result showed that the PME activity in rice root under Al treatment increased obviously as compared with the control (without Al treatment) (Fig. 7a). With SNP pre-treatment, the PME activity in the rice root apex under Al treatment was maintained at the control level, and the effect of SNP could be counteracted by PTIO application (Fig. 7a). Concomitantly, the degree of pectin methylation decreased significantly in Al-treated seedlings, nearly decreased to 25% of the control (without Al treatment). The decrease of pectin methylation caused by Al treatment was greatly restored by SNP pre-treatment (Fig. 7b). In addition, the effect of SNP on restoring pectin methylation could be eliminated by PTIO application. In order to investigate the effect of NO on Al binding to the cell wall of rice roots, a time-course kinetic study of Al association and dissociation in cell wall of rice root was conducted. The cell walls of rice root in the SNP pre-treatment bind less Al and the bound Al was less tightly retained than that of the control (Fig. 8).
Discussion
Al toxicity is harmful to plant growth and interferes with a lot of physiological and biochemical processes (Kochian 1995). Al toxicity is the main cause of inhibition of root growth of barley, rye and rice in acidic soil (Hartwell and Pember 1918; Huang et al. 2009; Yang et al. 2007). Many previous reports show that NO is not only involved in modulating plant growth and development, but also involved in plant responses to biotic and abiotic stresses (Lamattina et al. 2003). In this study, we found that exogenous NO alleviated the Al-induced inhibition of root elongation in rice (Fig. 1b, c) and reduced Al accumulation in root apexes and cell wall (Fig. 2). Our results indicate that NO-promoted root elongation is correlated with the decrease in Al accumulation in root apexes and cell wall. Furthermore, the NO scavenger PTIO could reverse the effect of SNP on the root growth (Fig. 1d). These results suggest that alteration of endogenous NO level in root apexes of rice seedling may be closely associated with plant tolerance to Al toxicity.
Previous reports show that Al-induced oxidative stress and changes in cell wall properties have been suggested as the two major factors involving in Al toxicity (Matsumoto 2000; Yamamoto et al. 2003; Zheng and Yang 2005). Furthermore, Al-induced oxidative stress has been observed in many plant species and in various systems including soybean root tips (Horst et al. 1992), detached rice leaves (Kuo and Kao 2003) and red kidney bean roots (Wang et al. 2010a), and barley roots (Simonovicova et al. 2004). Also, the level of reactive oxygen species (ROS) will increase under Al toxicity, such as H2O2 and O2 −, the excess formation of reactive oxygen species is reported to be one of the primary responses to Al exposure (Kuo and Kao 2003; Yamamoto et al. 2003). There are also many reports showing that the expression of genes for antioxidative enzymes such as superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD), ascorbate peroxidase (APX) is activated under Al stress (Ezaki et al. 2000; Wang et al. 2010a). The activated antioxidative systems under Al stress are beneficial for plant to remove excess ROS and inhibit lipid peroxidation (Mittler 2002; Wang et al. 2004). Excess ROS often caused lipid peroxidation that only observed after prolonged treatment in Al (24 h or more) (Cakmak and Horst 1991). Concerning lipid peroxidation induced by Al toxicity, there are different conclusions in different plant species. For example, Al treatment induced lipid peroxidation in red kidney bean roots (Wang et al. 2010a), in triticale roots (Liu et al. 2008), detached rice leaves (Kuo and Kao 2003). Al treatment failed to induce lipid peroxidation in maize and they concluded that the Al-induced oxidation was not the primary cause for the inhibition of root growth (Boscoloa et al. 2003). In this study, we found that exogenous NO alleviated the Al-induced lipid peroxidation and inhibition of rice root elongation in rice seedling (Fig. 3a, b). In addition, BHA could also suppress the Al-induced TBARS production (Fig. 3a), but it could not alleviate the inhibition of rice root elongation induced by Al (Fig. 3b). Our results indicated that SNP can alleviate the Al-induced inhibition of root elongation and the lipid peroxidation in rice. The further study showed that lipid peroxidation and root elongation inhibition under Al stress had no direct correlation, because BHA only had effect on Al-induced TBARS production but could not promote root elongation. This result is consistent with Yamamoto et al. (2001). Al rapidly affects the properties of plasma membrane when it binds to the sites in the plasma membrane (Ishikawa and Wagatsuma 1998). The interaction of Al with membrane lipids and proteins induces modifications of membrane fluidity, permeability, and other structural properties (Wagatsuma et al. 2005). Our results confirmed the results of these reports. Histochemical observation and quantification of the loss in plasma membrane integrity by Evans Blue staining suggest that the membrane damage induced by Al can be alleviated by exogenous NO application (Figs. 4b, 5b). Similar phenomenon was found in the plasma membrane permeability of rice roots under Al stress (Fig. 5a). The function of NO as an antioxidant molecule preventing plant from abiotic stresses was proved in many reports (Lamattina et al. 2003). Furthermore, NO also has some other function. Recent reports show that nitric oxide affects cell wall metabolism in tobacco BY-2 cells (Pacoda et al. 2004) and exogenous nitric oxide enhances cadmium tolerance of rice by affecting pectin and hemicellulose contents in root cell wall (Xiong et al. 2009). Considering the function of NO on affecting the cell wall, we focus our attention on the changes of cell wall under the Al stress.
Effect of NO on the integrity of the plasma membrane. Seedlings were pre-treated with 25 μM SNP for 24 h and then treated with 75 μM Al for 24 h. Relative ion leakage (a) and Evans Blue uptake (b) were measured. Data are mean values ± SE of three independent experiments. Within each set of experiments, bars with different letters were significantly different at P < 0.05
Not only Al rapidly affects the properties of the plasma membrane, but also its accumulation in the root apoplast modifies cell wall composition and properties (Horst et al. 2010). Previous study has shown that the negativity of cell wall mainly depends on the pectin content and its degree of methylation (Yang et al. 2007). Cellulose synthesis was inhibited in favor of callose synthesis in barley (Teraoka et al. 2002). It has been demonstrated that Al stress increases cell wall pectin content in a number of plant species such as squash (Van et al. 1994), maize (Eticha et al. 2005), rice (Yang et al. 2007) and common bean (Rangel et al. 2009b). In this study, we observed an increase of pectin and hemicelluloses in rice root apex under Al stress. The increase of pectin and hemicelluloses under Al stress was reduced by SNP (Fig. 6). This phenomenon illustrated that matrix polysaccharides may play an important role in rice roots against Al toxicity. However, in all plant species studied so far, there is no consensus on differences in constitutive pectin contents with regard to Al resistance. For example, Al-resistant and Al-sensitive maize cultivars did not differ in pectin content in the 5 mm root apex (Eticha et al. 2005). It has been found that the pectin content of the rice root apex in the Al-resistant cultivars was lower than that in the Al-sensitive cultivars (Yang et al. 2007). In common bean, the Al-resistant cultivar showed higher pectin content, higher Al sensitivity and Al accumulation than those in Al-sensitive cultivars during the 4 h Al treatment (Rangel et al. 2009b). The Al-resistant cultivar prior to the induction of citrate exudation was related to higher unmethylated pectin content in the 5 mm root tips (Rangel et al. 2009a). Furthermore, the degree of pectin methylation also determined Al binding to the cell wall. The Al-sensitive cultivar had more low-methylated pectin than an Al-resistant cultivar in maize and rice, which resulted in higher Al binding levels in the Al-sensitive cultivar (Eticha et al. 2005; Yang et al. 2007). In order to investigate the degree of methylation of pectin in cell walls, we measured the activity of PME and the degree of pectin methylation in rice roots. The results showed that the PME activity in rice roots treated with Al increased by 60% as compared with the control. The increase of PME activity in rice roots under Al stress was alleviated by pre-treatment with SNP (Fig. 7a). The result suggested that NO could affect PME activity to keep the degree of pectin methylation at a high level to minimize Al toxicity. By directly measuring the degree of methylation of pectin using FT-IR spectroscopy, we found that rice seedlings pre-treated with SNP had higher degree of pectin methylation than that of seedlings with the Al treatment only (Fig. 7b). The lower content of polysaccharides in cell walls with a higher degree of methylation led to less carboxylic groups available for Al binding. The results of Al association and dissociation kinetics showed that the cell walls of rice roots pre-treated with SNP bind less Al and the bound Al was less tightly retained than that in the rice roots without SNP pre-treatment (Fig. 8). These results indicated that rice roots pre-treated with SNP has less Al binding sites and lower Al-binding capacity than those in the rice roots without SNP pre-treatment.
Uronic acid content in cell wall fractions of rice roots. The seedlings were treated with 25 μM SNP, 100 μM PTIO and 75 μM Al as in Fig. 1. Root apices were cut and cell wall polysaccharides were fractionated into pectin (a), HC1 (b), and HC2 (c) for uronic acid content measurement. Data are mean values ± SE of three independent experiments. Within each set of experiments, bars with different letters were significantly different at P < 0.05
Effect of NO on the PME activity in the root apex of rice (a) and the degree of pectin methylation (b). Seedlings were treated with 25 μM SNP, 100 μM PTIO and 75 μM Al as in Fig. 1. Root apices were cut for cell wall and PME extraction. The PME activity was determined spectrophotometrically and the degree of pectin methylation was analyzed by FT-IR spectroscopy. Data are mean values ± SE of three independent experiments. Within each set of experiments, bars with different letters were significantly different at P < 0.05
The kinetics of Al association (a) and dissociation (b) in the cell walls of rice roots. The seedlings were exposed to 0.5 mM CaCl2 solution (pH 4.5) with or without 25 μM SNP pre-treated for 24 h and then cell wall materials were extracted. Cell wall materials (20 mg) were packed in a 2-ml column and kinetics was conducted
The cell wall porosity is largely controlled by the pectin matrix (Baron-Epe et al. 1988). The cross-linking of pectins with Al reduces the cell wall porosity and subsequently decreases the permeability of cell walls for macromolecules such as proteins (Schmohl and Horst 2000). Recent studies showed that Al and other metals reduced the hydraulic conductivity of bacterial cellulose–pectin composites by binding to the composites and changing pectin porosity (McKenna et al. 2010). Pectin plays an important role in cell growth, because pectin can form hydrated gels and push microfibrils apart (Cosgrove 2005). Any change in cell wall structure, hydrophobicity, cell wall chemical composition, and physical properties may result in the subsequent alteration of porosity (Yang et al. 2010). Therefore, the increase of pectin in the cell wall of rice root apices under Al stress (Fig. 6) may change the structure of cell wall, consequently resulting in a rearrangement of wall polymers and affecting the porosity. NO plays a role in changing the structure of cell walls by blocking the increase of pectin (Fig. 6a).
In conclusion, our results give an indication that NO plays an important role in alleviating the Al-induced damage of plasma membrane and lipid peroxidation and improving root elongation in rice. Moreover, we provided evidence that the role of NO in alleviating Al-induced oxidative stress under Al stress was not in direct correlation with root elongation. Recent studies reported that the strong binding of Al to the pectin matrix of the cell wall was a main factor of Al toxicity but not a resistance mechanism. The inhibition of Al to root elongation was not induced by Al accumulation in the symplasts, but by apoplastic Al in common bean (Rangel et al. 2009b). In rice, the Al-binding capacity in the cell wall contributes to differential Al resistance, and the Al-binding capacity in the cell wall is determined by the content and the degree of pectin (Yang et al. 2007). Our results show that NO reduces Al toxicity and improves root elongation by decreasing the contents of pectin and hemicellulose, and by increasing the degree of methylation of pectin, which determines the Al-binding capacity in the cell walls and leads to less Al accumulation in cell walls.
Abbreviations
- BHA:
-
Butylated hydroxyanisole
- GaE:
-
Galacturonic acid equivalents
- HC1:
-
Hemicellulose 1
- HC2:
-
Hemicellulose 2
- MP:
-
Membrane permeability
- NO:
-
Nitric oxide
- PME:
-
Pectin methylesterase
- PTIO:
-
2-Phenyl-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide
- SNP:
-
Sodium nitroprusside
- TBARS:
-
Thiobarbituric acid-reactive substances
References
Baron-Epe O, Gharyal PK, Schindler M (1988) Pectins as mediators of wall porosity in soybean cells. Planta 175:389–395
Blamey FPC (1993) In vitro evidence of aluminium effects on solution movement through root cell walls. J Plant Nutr 16:555–562
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54:484–489
Boscoloa PRS, Menossi M, Jorge RA (2003) Aluminum-induced oxidative stress in maize. Phytochemistry 62:181–189
Cakmak L, Horst WJ (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83:463–468
Chang YC, Yamamoto Y, Matsumoto H (1999) Accumulation of aluminium in the cell wall pectin in cultured tobacco (Nicotiana tabacum L.) cells treated with a combination of aluminium and iron. Plant Cell Environ 22:1009–1017
Correa-Aragunde N, Graziano M, Lamattina L (2004) Nitric oxide plays a central role in determining lateral root development in tomato. Planta 218:900–905
Correa-Aragunde N, Graziano M, Chevalier C, Lamattina L (2006) Nitric oxide modulates the expression of cell cycle regulatory genes during lateral root formation in tomato. J Exp Bot 57:581–588
Correa-Aragunde N, Lombardo C, Lamattina L (2008) Nitric oxide: an active nitrogen molecule that modulates cellulose synthesis in tomato roots. New Phytol 179:386–396
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6:850–861
Crawford NM, Guo FQ (2005) New insights into nitric oxide metabolism and regulatory functions. Trends Plant Sci 10:195–200
Delledonne M, Xia Y, Dixon RA, Lamb C (1998) Nitric oxide functions as a signal in plant disease resistance. Nature 394:585–588
Durner J, Klessig DF (1999) Nitric oxide as a signal in plants. Curr Opin Plant Biol 2:369–374
Eticha D, Stass A, Horst WJ (2005) Cell-wall pectin and its degree of methylation in the maize root-apex: significance for genotypic differences in aluminium resistance. Plant Cell Environ 28:1410–1420
Ezaki B, Gardner RC, Ezaki Y, Matsumoto H (2000) Expression of aluminum-induced genes in transgenic Arabidopsis plants can ameliorate aluminum stress and/or oxidative stress. Plant Physiol 122:657–665
Foy CD (1988) Plant adaptation to acid aluminum-toxic soils. Comm Soil Sci Plant Anal 19:959–987
García-Mata C, Lamattina L (2002) Nitric oxide and abscisic acid cross talk in guard cells. Plant Physiol 128:790–792
Hartwell BL, Pember FR (1918) The presence of aluminium as a reason for the difference in the effect of so-called acid soil on barley and rye. Soil Sci 6:259–281
Havas M (1986) A hematoxylin staining technique to locate sites of aluminum binding in aquatic plants and animals. Water Air Soil Poll 30:735–741
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Horst WJ, Asher CJ, Cakmak I, Szulkiewicz P, Wissemeier AH (1992) Short-term responses of soybean roots to aluminium. J Plant Physiol 140:174–178
Horst WJ, Wang YX, Eticha D (2010) The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Ann Bot 106:185–197
Hossain A, Koyama H, Hara T (2006) Growth and cell wall properties of two wheat cultivars differing in their sensitivity to aluminum stress. J Plant Physiol 163:39–47
Huang CF, Yamaji N, Mitani N, Yano M, Nagamura Y, Ma JF (2009) A bacterial-type ABC transporter is involved in aluminum tolerance in rice. Plant Cell 21:655–667
Ishikawa S, Wagatsuma T (1998) Plasma membrane permeability of root-tip cells following temporary exposure to Al ions is a rapid measure of Al tolerance among plant species. Plant Cell Physiol 39:516–525
Kerven GL, Edward DC, Asher CJ, Hallman PS, Kokot S (1989) Aluminum determination in soil solution. II. Short-term colorimetric procedures for the measurement of inorganic monomeric aluminum in the presence of organic acid ligands. Aust J Soil Res 27:91–102
Kikui S, Sasaki T, Maekawa M, Miyao A, Hirochika H, Matsumoto H, Yamamoto Y (2005) Physiological and genetic analyses of aluminium tolerance in rice, focusing on root growth during germination. J Inorg Biochem 99:1837–1844
Kochian LV (1995) Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol 46:237–260
Kuo MC, Kao CH (2003) Aluminum effects on lipid peroxidation and antioxidative enzyme activities in rice leaves. Biol Plant 46:149–152
Lamattina L, García-Mata C, Graziano M, Pagnussat G (2003) Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol 54:109–136
Liu Q, Yang JL, He LS, Li YY, Zheng SJ (2008) Effect of aluminum on cell wall, plasma membrane, antioxidants and root elongation in triticale. Biol Plant 52:87–92
Ma JF (2007) Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. Int Rev Cytol 264:225–252
Ma JF, Shen R, Nagao S, Tanimoto E (2004) Aluminum targets elongating cells by reducing cell wall extensibility in wheat roots. Plant Cell Physiol 45:583–589
Manrique GD, Lajolo FM (2002) FT-IR spectroscopy as a tool for measuring degree of methyl esterification in pectins isolated from ripening papaya fruit. Postharvest Biol Technol 25:99–107
Matsumoto H (2000) Cell biology of aluminium toxicity and tolerance in higher plants. Int Rev Cytol 200:1–46
McKenna BA, Kopittke PM, Wehr JB, Blamey FP, Menzies NW (2010) Metal ion effects on hydraulic conductivity of bacterial cellulose pectin composites used as plant cell wall analogs. Physiol Plant 138:205–214
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Neill SJ, Desikan R, Hancock JT (2003) Nitric oxide signaling in plants. New Phytol 159:11–35
Pacoda D, Montefusco A, Piro G, Dalessandro G (2004) Reactive oxygen species and nitric oxide affect cell wall metabolism in tobacco BY-2 cells. J Plant Physiol 161:1143–1156
Pagnussat GC, Lanteri ML, Lamattina L (2003) Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol 132:1241–1248
Rangel AF, Rao IM, Braun HP, Horst WJ (2009a) Aluminium resistance in common bean (Phaseolus vulgaris L.) involves induction and maintenance of citrate exudation from root apices. Physiol Plant 138:176–190
Rangel AF, Rao IM, Horst WJ (2009b) Intracellular distribution and binding state of aluminum in root apices of two common bean (Phaseolus vulgaris) genotypes in relation to Al toxicity. Physiol Plant 135:162–173
Rengel Z, Reid RJ (1997) Uptake of Al across the plasma membrane of plant cells. Plant Soil 192:31–35
Richard L, Qin LX, Gadal P, Oldberg R (1994) Molecular cloning and characterization of a putative pectin methylesterase cDNA in Arabidopsis thaliana (L.). FEBS Lett 355:135–139
Sairam RK, Srivastava GC (2002) Changes in antioxidant activity in subcellular fraction of tolerant and susceptible wheat genotypes in response to long term salt stress. Plant Sci 162:897–904
Sasaki M, Yamamoto Y, Ma JF, Matsumoto H (1997) Early events induced by aluminum stress in elongating cells of wheat root. Soil Sci Plant Nutr 43:1009–1014
Schmohl N, Horst WJ (2000) Cell wall pectin content modulates aluminium sensitivity of Zea mays (L.) cell grown in suspension culture. Plant Cell Environ 23:735–742
Simonovicova M, Tamas L, Huttova J, Mistrík I (2004) Effect of aluminium on oxidative stress related enzyme activities. Biol Plant 48:261–266
Tabuchi A, Matsumoto H (2001) Changes in cell-wall properties of wheat (Triticum aestivum) roots during aluminum-induced growth inhibition. Physiol Plant 112:353–358
Teraoka T, Kaneko M, Mori S, Yoshimura E (2002) Aluminum rapidly inhibits cellulose synthesis in roots of barley and wheat seedlings. J Plant Physiol 159:17–23
Tian QY, Sun DH, Zhao MG, Zhang WH (2007) Inhibition of nitric oxide synthase (NOS) underlies aluminum-induced inhibition of root elongation in Hibiscus moscheutos L. New Phytol 174:322–331
Uexküll HR, Mutert E (1995) Global extent, development and economic impact of acid soils. Plant Soil 171:1–15
Van HL, Kuraishi S, Sakurai N (1994) Aluminum-induced rapid root inhibition and changes in cell-wall components of squash seedlings. Plant Physiol 106:971–976
Wagatsuma T, Ishikawa S, Uemura M, Mitsuhashi W, Kawamura T, Khan MSH, Tawaraya K (2005) Plasma membrane lipids are the powerful components for early stage aluminum tolerance in Wticale. Soil Sci Plant Nutr 51:701–704
Wang YS, Yang ZM (2005) Nitric oxide reduces aluminum toxicity by preventing oxidative stress in the roots of Cassia tora L. Plant Cell Physiol 46:1915–1923
Wang SH, Yang ZM, Lu B, Li SQ, Lu YP (2004) Copper-induced stress and antioxidative responses in roots of Brassica juncea L. Bot Bull Acad Sin 45:203–212
Wang HH, Liang XL, Wang XM, Bi YR (2009) Ethylene and nitric oxide are involved in maintaining ion homeostasis in Arabidopsis callus under salt stress. Planta 230:293–307
Wang HH, Huang JJ, Bi YR (2010a) Nitrate reductase-dependent nitric oxide production is involved in aluminum tolerance in red kidney bean roots. Plant Sci 179:281–288
Wang XM, Li JS, Liu J, He WL, Bi YR (2010b) Nitric oxide increases mitochondrial respiration in the cGMP-dependent manner in the callus from Arabidopsis. Nitric Oxide 23:242–250
Wehr JB, Menzies NW, Blamey FPC (2004) Inhibition of cell-wall autolysis and pectin degradation by cations. Plant Physiol Biochem 42:485–492
Xiong J, An L, Lu H, Zhu C (2009) Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta 230:755–765
Yamamoto Y, Kobayashi Y, Matsumoto H (2001) Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol 125:199–208
Yamamoto Y, Kobayashi Y, Devi SR, Rikiishi S, Matsumoto H (2003) Oxidative stress triggered by aluminum in plant roots. Plant Soil 255:239–243
Yang JL, Li YY, Zhang YJ, Zhang SS, Wu YR, Wu P, Zheng SJ (2007) Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol 146:602–611
Yang ZB, Eticha D, Rao IM, Horst WJ (2010) Alteration of cell-wall porosity is involved in osmotic stress-induced enhancement of aluminium resistance in common bean (Phaseolus vulgaris L.). J Exp Bot 61:3245–3258
Zhao MG, Tian QY, Zhang WH (2007) Nitric oxide synthase-dependent nitric oxide production Is associated with salt tolerance in Arabidopsis. Plant Physiol 144:206–217
Zheng SJ, Yang JL (2005) Target sites of aluminum phytoxicity. Biol Plant 49:321–331
Zheng SJ, Lin XY, Yang JL, Liu Q, Tang C (2004) The kinetics of aluminum adsorption and desorption by root cell walls of an aluminum resistant wheat (Triticum aestivum L.) cultivar. Plant Soil 261:85–90
Zhong H, Lauchl A (1993) Changes of cell wall composition and polymer size in primary roots of cotton seedlings under high salinity. J Exp Bot 44:773–778
Acknowledgments
This work was supported by the Major Project of Cultivating New Varieties of Transgenic Organisms (2009ZX08009-029B), the National High Technology Research and Development Program (863 Program) (2007AA021401) and the National Natural Science Foundation of China (No. 90917019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Chong.
Rights and permissions
About this article
Cite this article
Zhang, Z., Wang, H., Wang, X. et al. Nitric oxide enhances aluminum tolerance by affecting cell wall polysaccharides in rice roots. Plant Cell Rep 30, 1701–1711 (2011). https://doi.org/10.1007/s00299-011-1078-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-011-1078-y