Abstract
Aims
Glucose-6-phosphate dehydrogenase (G6PDH) has been reported to be involved in resistance to various environmental stresses. However, the role of G6PDH in aluminum (Al) toxicity remains unclear.
Methods
Physiological and biochemical methods together with histochemical analysis were used to investigate the participation of G6PDH in Al-induced inhibition of root growth.
Results
Exposure to high Al concentration caused a significant increase in the activities of total and cytosolic G6PDH in roots of soybean. Al-induced inhibition of root growth and oxidative stress were alleviated by a G6PDH inhibitor. Reactive oxygen species (ROS) accumulation in Al-treated root apexes could be abolished by a NADPH oxidase inhibitor. Furthermore, treatment with a G6PDH inhibitor reduced NADPH content and NADPH oxidase activity in Al-treated root apexes. Further investigation demonstrates that nitric oxide (NO) mediates Al-induced increase in cytosolic G6PDH activity by modulating the expression of genes encoding cytosolic G6PDH. In addition, nitrate reductase pathway is mainly responsible for Al-induced NO production in root apexes.
Conclusions
These results indicate that NADPH produced by NO-modulated cytosolic G6PDH in root apexes is responsible for ROS accumulation mediated by NADPH oxidase under Al stress, subsequently suffering from oxidative stress and thus causing the inhibition of root elongation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aluminum (Al) toxicity becomes the primary constraint limiting crop growth and yield in acid soils (Kochian 1995). Inhibition of root elongation has been reported to be the most important and distinct symptoms of Al toxicity. In acid soil (pH < 5.0), phytotoxic forms of Al appears and rapidly inhibit root growth and function, leading to poor nutrient and water acquisition and thus reducing crop yield (Ma 2005). In the past few years, some hypotheses have been suggested for Al toxicity, one of which is the induction of oxidative stress that results in lipid peroxidation. For example, previous studies indicated that reactive oxygen species (ROS) generation played a critical role in Al-induced root growth inhibition in some plant species (Wang and Yang 2005; Yamamoto et al. 2003). It has been reported that Al intensified the levels of ROS production and lipid peroxidation in roots of wheat (Sun et al. 2014) and red kidney bean (Wang et al. 2010). Yamamoto et al. (2002) also showed that Al-triggered ROS production in plant cells might be the critical event in Al-induced cell growth inhibition. Recently, we have demonstrated that ROS production is of paramount importance in Al-induced root growth inhibition in soybean seedlings (Wang et al. 2016a). Although extensive efforts have been made during the last decades, the primary mechanisms underlying the Al phytotoxicity are still poorly characterized. For example, the signaling process of how Al activates ROS generation in plants remains unclear.
Plasma membrane-localized NADPH oxidase is considered one of the main sources of ROS production in plants under biotic and abiotic stresses (Hao et al. 2006; Scharte et al. 2009; Torres et al. 2002; Zhang et al. 2007). Importantly, the activity of NADPH oxidase is dependent on rapid regeneration of NADPH in cytosol. Glucose-6-phosphate dehydrogenase (G6PDH, EC 1.1.1.49), widely present in plant tissues, catalyzes the first committed step of the oxidative pentose phosphate pathway that produces NADPH to meet cellular needs for reductive biosynthesis. G6PDH activity has been detected in both cytosol and plastids, and evidence has indicated that at least two cytosolic (Cyt) and four plastidic isoforms (P1 and P2) are present (Wakao and Benning 2005; Wendt et al. 1999). Increasing evidence has demonstrated that G6PDH is involved in response to various stresses, such as salinity, heat, drought and pathogens (Dal Santo et al. 2012; Gong et al. 2013; Scharte et al. 2009; Wang et al. 2016b). Ślaski et al. (1996) also reported that G6PDH activity was increased by Al stress in Al-resistant wheat cultivar but not in Al-sensitive wheat cultivar. However, to this day, the studies on the role of G6PDH in response to Al toxicity are still lacking in plants. In the last few years, the roles of G6PDH isoforms have also been investigated in plants. The plastidic isoforms have been reported to be involved in providing reducing power for nutrient assimilation (Bowsher et al. 2007; Esposito et al. 2005). Asai et al. (2011) reported that NADPH produced by P2-class G6PDH was responsible for hypersensitive response cell death. In addition, Cyt-G6PDH isoforms are crucial in supply of NADPH which is required for plant defense responses to pathogen infection in tobacco (Scharte et al. 2009) and drought stress in soybean (Wang et al. 2016b). Therefore, each G6PDH isoform seems to play a diverse role in responses to stresses in plants. However, the regulatory mechanism of each G6PDH isoform under stress conditions is still poorly understood.
Nitric oxide (NO) has been considered as an important signaling molecule involved in responses of plants to biotic and abiotic stresses (Lamattina et al. 2003; Neill et al. 2003). Apart from NO synthase (NOS), nitrate reductase (NR) is another enzyme that is responsible for NO production in plants (Neill et al. 2003). The roles of NO in Al stress were also reported in plants. However, the results on the role of NO in Al toxicity are contradictory. For example, it has been reported that NO could enhance plant resistance to Al toxicity (Sun et al. 2015; Wang et al. 2010; Wang and Yang 2005). Besides, there was also report indicating that NO exaggerated Al-induced inhibition of root growth in rice bean (Zhou et al. 2012). The discrepancy of NO on Al toxicity may be related to different NO concentration and plant species used.
In this study, the response pattern of G6PDH to Al toxicity was analyzed in soybean roots, and the roles of G6PDH isoforms in Al toxicity were investigated. Furthermore, the role of NO in regulating G6PDH isoforms of soybean roots under Al stress was examined. In addition, the source of endogenous NO production was also explored. We demonstrate that NO-mediated cytosolic G6PDH of soybean roots plays a pivotal role in Al-induced inhibition of root growth through regulating NADPH oxidase-dependent ROS production.
Materials and methods
Plant culture and treatments
Soybean (Glycine max L.) seeds were placed on wet sponge for 2 d to germinate at 26 °C. After germination, the uniform seeds were chosen to be cultured in a container containing 400 ml of culture solution (0.5 mM CaCl2, pH 4.5). The solution for plant culture was renewed every day. Seedlings were grown in a controlled growth environment (26/23 °C for day/night temperature, a light intensity of 300 μmol m−2 s−1, and 16/8 h light/dark photoperiod). After 3 d of culture, seedlings were exposed to various reagents in the presence of culture solution for 1 d. Different concentrations of AlCl3 (Al stress), sodium nitroprusside (SNP, NO donor), 100 μM 2-phenyl-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxyde (PTIO, NO scavenger), 0.5 m M glucosamine 6-phosphate (GN6P, G6PDH inhibitor), 20 μM diphenylene iodonium (DPI, NADPH oxidase inhibitor), 100 μM N ω-nitro-L-Arg (L-NNA, NO synthase inhibitor), 20 μM NaN3 (nitrate reductase inhibitor) were added to the basal solution (0.5 mM CaCl2) for various treatments. In order to keep the hydroponic system consistent, the pH of the solution was monitored and adjusted to 4.5 with 1 M HCl or KOH every 3 h. After collection and washing, the root apexes (1 cm) were used for parameters assay.
Assay of G6PDH activity
G6PDH extraction and assay were performed as described by Hauschild and von Schaewen (2003). Briefly, root apexes (0.2 g) were ground in liquid nitrogen and extracted in 2 ml extraction buffer containing Hepes-Tris (50 mM, pH 7.8), MgCl2 (3 mM), EDTA (1 mM), and phenylmethylsulfonyl fluoride (1 mM). After centrifugation (12,000 g, 4 °C) for 20 min, the supernatant was used to determine enzyme activity. To determine the total G6PDH activity, a 100 μl aliquot of the extract was added to the assay buffer containing Hepes-Tris (50 mM, pH 7.8), MgCl2 (3.3 mM), glucose-6-phosphate (0.5 mM), and NADPNa2 (0.5 mM). G6PDH activity was assayed at 25 °C by monitoring the reduction of NADP+ to NADPH at 340 nm. For the assay of cytosolic G6PDH activity, the sample was incubated with DTTred (final concentration 62.5 mM) for 5 min at room temperature prior to measuring enzyme activity (Wenderoth et al. 1997). Plastidic G6PDH activity was calculated as the difference between total and cytosolic G6PDH activities.
Determination of lipid peroxidation
Lipid peroxidation was estimated by monitoring the malondialdehyde (MDA) content as described by Liu et al. (2007). Root apexes (0.2 g) was ground and extracted with 10% trichloroacetic acid (2 ml). After centrifugation at 5000 g for 15 min, the supernatant (1.5 ml) was added to 1.5 ml of reaction solution containing 0.5% thiobarbituric acid and 10% trichloroacetic acid. After reaction at 100 °C for 30 min, the absorbance values of product were read at 440, 532 and 600 nm, respectively.
Determination of ROS production
Total ROS accumulation in root apexes was labelled by using 2, 7-dichlorofluorescein diacetate (H2DCF-DA), a ROS-specific fluorescent probe, as described by Maffei et al. (2006). Briefly, root apexes were incubated with 10 mM Hepes-NaOH buffer (pH 7.5) containing 10 μM H2DCF-DA for 30 min in dark. The root apexes were then washed with fresh buffer prior to detection using an epifluorescence microscope (Nikon, Tokyo, Japan). For histochemical visualization of H2O2, root apexes were incubated with 50 mM Tris-HCl (pH 3.8) containing 1 mg/ml diaminobenzidine (DAB) for 3 h. The in situ formation of O2 ·- level in root apexes was assayed by nitroblue tetrazolium (NBT) staining. The root apexes were immersed in 50 mM Tris-HCl (pH 6.5) containing 0.1% NBT for 15 min. Staining root apexes were then washed with fresh buffer prior to observation under a stereomicroscope (Nikon, Tokyo, Japan).
Assay of PM NADPH oxidase activity
Plasma membrane (PM) isolation was performed as described by Yang et al. (2003). The enzyme activity in isolated PM was assayed by monitoring the reduction of nitroblue tetrazolium (NBT) by O2 ·- (Van Gestelen et al. 1997). The reaction mixture consisted of Tris-HCl buffer (50 mM, pH 7.4), sucrose (250 mM), NBT (0.1 mM), and membrane protein (15–20 μg). The reaction was initiated by the addition of NADPH (0.1 mM). After incubation for 10 min at room temperature, the reaction solution was used for assay of NBT formazan formation by monitoring the absorbance at 530 nm. Rates of O2 ·- production were calculated using an extinction coefficient (12.8 mM−1 cm−1). NBT reduction by O2 ·- was determined from the difference of NBT reduction rates in the presence and absence of 50 U SOD.
Determination of NADPH content
Root apexes (0.5 g) were ground and extracted with 3 ml of 0.1 M NaOH solution. The extracts were boiled for 5 min, and then were cooled on ice. After centrifugation (12,000 g, 10 min), the supernatants were used to assay the NADPH content by the enzyme cycling method described by Matsumura and Miyachi (1980).
Determination of NO production
NO in root apexes was labelled by diaminofluorescein-FM diacetate (DAF-FM DA), a specific NO fluorescent probe, according to the method of Zhao et al. (2007). Root apexes were incubated with HEPES-NaOH buffer (20 mM, pH 7.4) containing 20 μM DAF-FM DA for 30 min. Staining root apexes were then washed with fresh buffer prior to observation under an epifluorescence microscope (Nikon, Tokyo, Japan).
Determination of NR and NOS activities
NR activity was assayed according to the method of Mackintosh et al. (1995). Sample (root apexes) was ground and extracted with the extract solution containing HEPES-KOH (50 mM, pH 7.5), glycerol (5%), MgCl2 (10 mM), PMSF (1 mM), DTT (1 mM), and flavin adenine dinucleotide (FAD, 10 μM). The homogenate was then centrifuged at 15000 g for 15 min at 4 °C. To determine the enzyme activity, a 250 μl aliquot of the extract (supernatant) was added to the 250 μl of reaction solution containing HEPES-KOH (50 mM, pH 7.5), DTT (1 mM), MgCl2 (10 mM), KNO3 (2 mM), and NADH (200 μM). After incubation (30 °C, 10 min ), the reaction was then stopped by addition of 50 μl of Zn-acetate (0.5 M). After addition of 1 ml of sulfanilamide (1%) and 1 ml of N-(1-naphthyl) ethylenediamine (0.02%), the absorbance value of nitrite produced was read at 540 nm.
NOS activity assay was performed as described by Lin et al. (2012). Root apexes sample was homogenized in extract buffer containing HEPES-KOH (100 mM, pH 7.5), EDTA (1 mM), DTT (5 mM), glycerol (10%), PMSF (0.5 mM), Triton X-100 (0.1%), polyvinylpyrrolidone (1%) and FAD (20 μM). After centrifugation (15,000 g, 15 min, 4 °C), the crude extract (supernatant) was used to measure enzyme activity. After adding 100 μl of supernatant, the reaction was conducted in 1 ml of reaction solution containing phosphate buffer (100 mM, pH 7.0), L-Arginine (1 mM), CaCl2 (0.3 mM), MgCl2 (2 mM), BH4 (4 μM), flavin mononucleotide (FMN, 1 μM), FAD (1 μM), DTT (0.2 mM), and 0.2 mM NADPH. The enzyme activity was detected by reading the changes of absorbance values at 340 nm.
RNA isolation and semi-quantitative RT-PCR
Total RNA was isolated from 100 mg root apexes using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. First-strand cDNA was synthesized from DNA-free total RNA (2 μg) with 2 μM of Oligo-dT primer and PrimeScript RTase (Takara). Specific primers of genes for PCR were as follows: Gm-G6PDH1 (LOC100793462), forward ATGAAAGATGGGAAGGTGTT and reverse ATGCCGG TAAATAGACTTCA, amplifying a 719 bp fragment; Gm-G6PDH2 (LOC100793462), forward TTCTTGAATCAGTGCTCCCT and reverse AAGGGAAGAAGCCTCACA AG, amplifying a 720 bp fragment; Gm-G6PDH3 (LOC100806107), forward GAAAGATGGGAGGGTGTT and reverse GCATTCGTAGGCAGATAAT, amplifying a 684 bp fragment; Gm-β-tubulin, forward TTGATAATGAGGCGCTCT AC and reverse CAGGCATCTTTCACATTCAG, amplifying a 771 bp fragment. To standardize the results, β-tubulin was used as the internal standard. Cycling conditions were as follows: each cycle was composed of a 40 s denaturation at 94 °C, 40 s of annealing at 55, 55, 58 and 55 °C for Gm-G6PDH1, Gm-G6PDH2, Gm-G6PDH3, and β-tubulin, respectively, a 45 s extension at 72 °C followed by 5 min of prolonged extension at 72 °C. Transcript levels of the genes indicated were measured by RT-PCR with 30–35 cycles for β-tubulin, Gm-G6PDH1, Gm-G6PDH2 and Gm-G6PDH3. The PCR reaction products were electrophoresed in 1% agarose gels stained with ethidium bromide. For quantification, filters were scanned and band intensities were determined with image-analysis software (Image Lab 4.0).
Statistical analysis
All data in this study were statistically analyzed by one-way analysis of variance (ANOVA) procedures using the SPSS 13.0 software, and LSD test (P < 0.05) was performed to determine the differences among various treatments.
Results
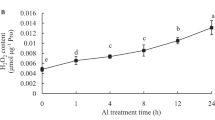
Effect of Al toxicity on G6PDH activity
To investigate the response of G6PDH to Al toxicity in root apexes, the activities of total G6PDH, cytosolic G6PDH (Cyt-G6PDH) and plastidic G6PDH (P-G6PDH) were determined under different Al concentrations (0–5000 μM). As shown in Fig. 1, total G6PDH activity began to increase significantly when the Al concentration was higher than 200 μM, suggesting that high Al concentration activates total G6PDH activity in root apexes. To further investigate the contributions of Cyt-G6PDH and P-G6PDH to enhanced G6PDH activity, the activities of Cyt-G6PDH and P-G6PDH were also measured in Al-treated soybean roots. Cyt-G6PDH activity displayed a similar pattern as that of total G6PDH activity under Al stress, whereas Al stress had no significant effect on P-G6PDH activity (Fig. 1), suggesting that Cyt-G6PDH contributes to the enhanced total G6PDH activity in soybean roots exposed to high Al concentration.
Effect of Al on the activities of total G6PDH, cytosolic G6PDH (Cyt-G6PDH) and plastidic G6PDH (P-G6PDH). Seedlings were exposed to different Al concentrations for 24 h. Data represent means ± SD of at least three biological replicates. Bars with different letters indicate significant differences at P < 0.05. CK, control
G6PDH is involved in Al-induced inhibition of root growth
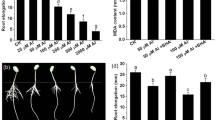
To elucidate the role of G6PDH in Al toxicity, glucosamine 6-phosphate (GN6P), a competitive inhibitor of G6PDH (Scharte et al. 2009; Wang et al. 2016b), was used in this study. Since root elongation inhibition is the most distinct symptom of Al toxicity, we investigated the effect of GN6P on root elongation under Al stress. As shown in Fig. 2a, root growth was inhibited by 70.8 and 80.8% in the presence of 0.5 and 2 mM Al compared with control seedlings (without Al treatment), respectively. Application of GN6P alone had almost no effect on root growth (Fig. 2a). However, GN6P alleviated inhibition of root growth in the presence of Al stress (Fig. 2a). For example, in the presence of 0.5 mM Al, the root elongation increased by 35% in GN6P-treated seedlings. The alleviation effect of GN6P on root growth under Al stress was also morphologically observed (Fig. 2b).
Effect of G6PDH inhibitor on the root elongation under Al stress (a, b). Seedlings were exposed to Al in the presence or absence of 0.5 mM GN6P for 24 h. Data represent means ± SD of at least three biological replicates. Bars with different letters indicate significant differences at P < 0.05. CK, control
The alleviation of Al toxicity by GN6P may result from chelation of Al by GN6P. To exclude this possibility, we examined the effect of pretreatment of roots with GN6P on root elongation and compared this with the effect of treatment with GN6P and Al simultaneously on root elongation. Results showed that a similar effect of GN6P on the Al-induced inhibition of root elongation was observed regardless of whether GN6P and Al were present together or separately (Fig. S1). These findings exclude the possibility that the elimination of Al toxicity by GN6P is the result of chelating effect of GN6P with Al in the incubation solution. These results suggest that G6PDH plays a critical role in Al-induced inhibition of root growth.
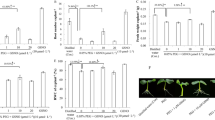
G6PDH mediates Al-induced oxidative stress
Recently, we have revealed that lipid peroxidation contributes to Al-induced inhibition of root growth in soybean seedlings (Wang et al. 2016a). To explore the role of G6PDH in mediating oxidative stress caused by Al, malondialdehyde (MDA), an indicator of lipid peroxidation, was determined in Al-treated root apexes. As shown in Fig. 3, Al increased MDA content in root apexes, and GN6P application significantly reduced MDA content under Al stress. For example, MDA content in root apexes increased by 42.1% in the presence of 0.5 mM Al, and GN6P reduced Al-induced increase in MDA content by 64.2%. However, application of GN6P alone had no significant effect on MDA content in root apexes (Fig. 3). Since lipid peroxidation could be caused by ROS accumulation, H2O2 and O2 ·- levels were detected in root apexes by histochemical approach. Fluorescent visualization by H2DCF-DA staining showed that Al triggered total ROS accumulation (Fig. 4a). Furthermore, the fluorescence signal induced by Al was inhibited by GN6P treatment in root apexes (Fig. 4a). Histochemical visualization by DAB and NBT staining showed that Al increased H2O2 and O2 ·- production in root apexes, whereas GN6P treatment reduced H2O2 and O2 ·- accumulation under Al stress (Fig. 4b and c).
Effect of G6PDH inhibitor on the lipid peroxidation under Al stress. Treatments for seedlings were performed as described in Fig. 2. Data represent means ± SD of at least three biological replicates. Bars with different letters indicate significant differences at P < 0.05. CK, control
Effect of G6PDH inhibitor on the accumulation of total ROS (a), H2O2 (b) and O2 ·- (c) under Al stress. Treatments for seedlings were performed as described in Fig. 2. At least three biological replicates were performed. Representative images showing endogenous ROS production are given. CK, control
Al-induced ROS production origins from NADPH oxidase
It has been reported that NADPH oxidase localized at plasma membrane is an important source of ROS production under stress conditions (Torres et al. 2002). To elucidate whether NADPH oxidase was responsible for Al-induced ROS accumulation in root apexes, diphenylene iodonium (DPI), an inhibitor of NADPH oxidase, was used in this study. As shown in Fig. 5a, the enhanced fluorescence signals by Al were almost abolished by DPI treatment. Furthermore, the Al-induced increase in H2O2 and O2 ·- levels were also inhibited in root apexes (Fig. 5b and c). To further confirm that Al-induced ROS production resulted from NADPH oxidase, the NADPH oxidase activity was tested in root apexes. As shown in Fig. 6a, NADPH oxidase activity increased by 83.7 and 140.7% in the presence of 0.5 and 2 mM Al, respectively. Furthermore, Al-induced increase in NADPH oxidase activity was completely inhibited by DPI (Fig. 6a). To further confirm the role of ROS mediated by NADPH oxidase, we tested the effect of DPI on lipid peroxidation under Al stress. The results showed that reduction of ROS production by DPI in Al-treated root apexes alleviates Al-induced lipid peroxidation as indicated by the decrease of MDA content (Fig. S2).
Effect of NADPH oxidase inhibitor on the contents of total ROS (a), H2O2 (b) and O2 ·- (c) under Al stress. Seedlings were exposed to Al in the presence or absence of 20 μM DPI for 24 h. At least three biological replicates were performed. Representative images showing endogenous ROS production are given. CK, control
Effect of inhibitors of G6PDH and NADPH oxidase on the NADPH oxidase activity (a) and NADPH content (b) under Al stress. Treatments for seedlings were performed as described in Fig. 2. Data represent means ± SD of at least three biological replicates. Bars with different letters indicate significant differences at P < 0.05. CK, control
Inhibition of G6PDH activity interferes with NADPH oxidase activity and NADPH production under Al stress
G6PDH is known to regulate NADPH production via the oxidative pentose phosphate pathway. Therefore, we examined whether G6PDH-dependent NADPH production was critical for the activation of NADPH oxidase under Al stress. As shown in Fig. 6, Al treatment significantly increased NADPH oxidase activity and NADPH content in root apexes of soybean. Furthermore, application of GN6P to soybean roots prevented the increase of NADPH oxidase activity and NADPH content under Al stress (Fig. 6a and b). These observations indicate that G6PDH-dependent NADPH production contributes to Al-induced increase in NADPH oxidase activity.
NO is involved in Al-induced increase in G6PDH activity
To test the role of NO in regulating G6PDH activity under Al stress, NO donor (SNP) and NO scavenger (PTIO) were used to manipulate NO level. As shown in Fig. 7a, generally, SNP treatment significantly increased the activities of total G6PDH and Cyt-G6PDH in a concentration-dependent manner under Al stress, while SNP had no significant effect on P-G6PDH activity. Furthermore, reduction of endogenous NO content by PTIO inhibited the activities of total G6PDH and Cyt-G6PDH in Al-treated roots of soybean (Fig. 7b). To further confirm the role of endogenous NO in regulating G6PDH activity under Al stress, NO content in root apexes was detected by an NO-specific fluorescent probe (DAF-FM DA). As shown in Fig. 7c, slight fluorescence signal was observed in control roots (without Al), whereas Al treatment significantly intensified the fluorescence signal. Also, SNP treatment further enhanced fluorescence signal, while PTIO abolished Al-induced fluorescence signal (Fig. 7c). These results indicate that NO is required for the induction of G6PDH activity under Al stress.
Effect of NO donor and scavenger on the activities of total G6PDH, Cyt-G6PDH and P-G6PDH (a, b) and NO content (c) under Al stress. Seedlings were exposed to SNP at indicated concentrations and 100 μM PTIO in the presence of 0.5 or 2 mM Al for 24 h. Data represent means ± SD of at least three biological replicates. Bars with different letters indicate significant differences at P < 0.05. CK, control
Al-induced NO production results from NR pathway
To elucidate the source of endogenous NO production in Al-treated roots, inhibitors of NR (NaN3) and NOS (L-NNA) were used in this study. As shown in Fig. 8a, NaN3 almost abolished Al-induced NO accumulation in root apexes, while L-NNA had no significant effect on NO production in Al-treated root apexes, suggesting that NR-dependent pathway is responsible for Al-induced NO production in root apexes. To further confirm this result, the effects of Al stress on the activities of NR and NOS in root apexes were investigated. Al treatment significantly increased NR activity by 86.3 and 147.1% in the presence of 0.5 and 2 mM Al, respectively, while NOS activity was significantly inhibited in Al-treated root apexes (Fig. 8b and c).
Effect of NR and NOS inhibitors on NO production (a) and the changes of activities of NR (b) and NOS (c) in the presence of 0.5 or 2 mM Al for 24 h. Seedlings were exposed to 20 μM NaN3 and/or 100 μM L-NNA at the indicated Al concentrations. Data represent means ± SD of at least three biological replicates. Bars with different letters indicate significant differences at P < 0.05. CK, control
NO positively regulates Cyt-G6PDH genes expression
To explore whether the increase of G6PDH activity in Al-treated roots was modulated at transcriptional level, the expression of genes encoding G6PDH was tested. In soybean, G6PDH1, G6PDH2 and G6PDH3 encode Cyt-G6PDH proteins. As shown in Fig. 9, the expression of G6PDH1, G6PDH2 and G6PDH3 were all detected in roots of soybean. Also, Al enhanced the expression levels of G6PDH1 and G6PDH2, while G6PDH3 expression had almost no change in response to Al stress. To further clarify how the expression of each Cyt-G6PDH was modulated in Al-treated roots of soybean, the effect of NO on the expression of Cyt-G6PDH was investigated. Under Al stress, the expression of G6PDH1 and G6PDH2 in soybean roots was further enhanced by SNP treatment, but suppressed by PTIO treatment (Fig. 9). However, G6PDH3 showed no sensitivity in response to SNP or PTIO under Al stress (Fig. 9).
Effect of NO donor and scavenger on the expression of Cyt-G6PDH genes under Al stress. Seedlings were treated as described in Fig. 7. Lane 1, CK; lane 2, 0.5 mM Al; lane 3, 0.5 mM Al + SNP; lane 4, 0.5 mM Al + PTIO; lane 5, 2 mM Al; lane 6, 2 mM Al + SNP; lane 7, 2 mM Al + PTIO. Under the gel image panels (a), a graph with relative intensity of the signals ± SD is shown (b). Bars with different letters represent significant differences at P < 0.05. CK, control
Discussion
Although it has been demonstrated that G6PDH plays a critical role in adaptation to various environmental stresses (Dal Santo et al. 2012; Liu et al. 2007; Scharte et al. 2009; Wang et al. 2016b), the involvement of G6PDH in response to Al stress is largely unknown in plants until now. In this study, we first obtained evidence that high Al concentration stimulated G6PDH activity resulting from the enhanced Cyt-G6PDH activity in soybean roots (Fig. 1). During the past few years, some efforts have been made to elucidate the roles of G6PDH isoforms in plants. For example, the P-G6PDH isoforms participated in responses to nitrogen starvation and plant immunity by providing NADPH (Asai et al. 2011; Esposito et al. 2005). Regarding Cyt-G6PDH isoforms, previous studies in plants indicated that Cyt-G6PDH plays an important role in plant defense responses to drought stress and salt stress (Dal Santo et al. 2012; Wang et al. 2016b). Besides, Wakao et al. (2008) showed that Cyt-G6PDH is of important for the oil metabolism of developing seeds in Arabidopsis. These observations suggest that G6PDH isoform seems to play a diverse role in response to different stress stimuli and developmental stages.
What might be the biological role of Al-induced increase in G6PDH activity? It has been reported that oxidative stress is one of the responses of plants to Al toxicity (Wang et al. 2010; Wang and Yang 2005; Yamamoto et al. 2001). Our recent work has demonstrated that Al-induced inhibition of root growth is associated with oxidative stress caused by Al toxicity in soybean seedlings (Wang et al. 2016a). In the present work, we revealed that Al-induced root inhibition and oxidative stress could be alleviated by a G6PDH inhibitor in soybean seedlings (Figs. 2-4), suggesting that G6PDH mediates Al-induced oxidative stress leading to root growth inhibition. However, Ślaski et al. (1996) demonstrated that the induction of G6PDH activity in Al-resistant wheat cultivar may be associated with Al resistance, which is inconsistent with our results presented here. This discrepancy could be due to the different plant species used. It is well documented that plasma membrane NADPH oxidase is generally a main source of ROS production, especially under stress conditions (Torres et al. 2002; Wang et al. 2012). Recently, Sun et al. (2014) showed that Al-induced ROS production was observed on the plasma membrane in wheat roots. Our results showed that Al-induced ROS accumulation and NADPH oxidase activity in root apexes were abolished by a NADPH oxidase inhibitor (Figs. 5 and 6a), suggesting that Al-induced ROS production in root apexes results from the activation of NADPH oxidase. Hao et al. (2006) reported that NADPH oxidase-mediated ROS was involved in nickel-induced oxidative stress in wheat roots, which is in line with our results presented here. However, it has also been reported that ROS production from NADPH oxidase is involved in defense response (Scharte et al. 2009; Wang et al. 2012; Zhang et al. 2007). It is possible that the roles of NADPH oxidase under stress conditions could be due to the different plant species and/or environmental stimuli. Since NADPH can be used as a substrate for NADPH oxidase, we tested whether G6PDH-dependent NADPH is responsible for the activation of NADPH oxidase. In this study, we observed that treatment with a G6PDH inhibitor significantly reduced the NADPH content and NADPH oxidase activity in Al-treated roots of soybean (Fig. 6a and b). Therefore, it is possible that the Cyt-G6PDH modulates ROS production in Al-treated roots through activation of NADPH oxidase. We have previously found that lower Al concentrations caused oxidative stress that contributes to root growth inhibition (Wang et al. 2016a). However, in this study, we found only high concentrations of Al induced G6PDH activity, which was also associated with oxidative stress by modulating ROS accumulation mediated by NADPH oxidase. These observations indicate that oxidative stress induced by low concentrations of Al has a diverse regulatory mechanism in root apexes of soybean. Yamamoto et al. (2002) reported that Al toxicity decreases mitochondrial activities and respiration, but increases ROS production in tobacco cells. Since electron transport in mitochondria is also an important source of ROS production, it is possible that ROS production induced by low Al concentration is mainly due to mitochondria in soybean roots.
However, how Al activates G6PDH activity is still unknown in plants. The role of NO in responses to various stresses is well documented in plants, including Al stress (Sun et al. 2015; Wang et al. 2009; Zhao et al. 2009). In this study, the role of NO in regulating the activities of G6PDH isoforms under Al stress was examined in soybean roots using NO donor and NO scavenger. Our results showed that NO is required for Al-induced increase in the activities of total G6PDH and Cyt-G6PDH (Fig. 7a and b). This observation was also supported by the determination of endogenous NO levels in Al-treated root apexes (Fig. 7c). In this study, we demonstrated that G6PDH activity was induced by high Al concentration (≥200 μM) but not by low Al concentration (Fig. 1). However, our previous work showed that NO level was also elevated by low Al concentration (e.g. 50 μM) in soybean roots (Wang et al. 2016a). Since our results demonstrated that endogenous NO content increased more under high Al concentration than low Al concentration (Fig. S3), this discrepancy could be explained by the level of endogenous NO concentration. Some changes induced by NO may require NO production (at a relative high level) to be effective: when NO content is relatively low under low Al concentration, NO is not sufficient in itself to induce G6PDH activity. In contrast, when NO content is at a high level under high Al concentration, the Al-induced NO signal in soybean roots is able to activate downstream targets that induce G6PDH activity. Further investigation will be required to clarify at what level NO exerts its fundamental effect. Our results further demonstrated that Al-induced NO generation in root apexes of soybean was mainly mediated by NR pathway using inhibitors of NR and NOS (Fig. 8a). The analysis of NOS and NR activities in root apexes further supported this result (Fig. 8b and c). Previous studies suggested that NR-mediated NO production is involved in freezing tolerance of Arabidopsis (Zhao et al. 2009), salt tolerance of Phaseolus vulgaris (Liu et al. 2007) and copper tolerance of Hordeum vulgare (Hu et al. 2015). On the other hand, there are also reports to demonstrate that NOS-mediated NO generation participates in zinc tolerance of Solanum nigrum (Xu et al. 2010) and salt tolerance of Arabidopsis (Zhao et al. 2007). The observation that both NR- and NOS-mediated pathways contribute to iron deficiency-induced NO production was also found in Arabidopsis (Chen et al. 2010). It is possible that the discrepancies in the contribution of NR and/or NOS to NO production might be due to different plant species and environmental stresses.
To further clarify the molecular mechanism of Al-induced increase in Cyt-G6PDH activity, we tested the effects of Al stress and NO on the transcriptional regulation of Cyt-G6PDH in root apexes. In this work, we observed that among the three Cyt-G6PDH genes tested, the expression of G6PDH1 and G6PDH2 was induced by Al stress, while G6PDH3 did not respond to Al stress in root apexes (Fig. 9), suggesting that both G6PDH1 and G6PDH2 contribute to enhanced Cyt-G6PDH activity under Al stress. We also further showed that NO modulates the Al-induced expression of Cyt-G6PDH genes (G6PDH1 and G6PDH2) in root apexes (Fig. 9). These observations highlight the critical role of NO in regulating Cyt-G6PDH expression under Al stress. However, it was reported that abscisic acid (ABA) application increased the expression of P-G6PDH but failed to induce Cyt-G6PDH expression in barley roots (Cardi et al. 2011). In contrast, the expression of G6PDH in wheat seedlings did not respond to ABA treatment (Nemoto and Sasakuma 2000). Our recent work demonstrated that ABA modulated Cyt-G6PDH activity in drought-treated soybean roots (Wang et al. 2016b). It is therefore that each G6PDH isoform seems to have a diversified regulatory mechanism dependent on plant species and environmental stimuli.
It seems that the obtained results from low Al concentration and high Al concentration were somewhat surprising. Our previous work demonstrated that NO mediated Al tolerance by regulating ascorbate-glutathion cycle under low Al concentration (Wang et al. 2016a). However, the present work showed that NO mediated Al toxicity by regulating G6PDH-mediated ROS production under high Al concentration. Previous studies reported that low NO donor concentration could alleviate Al toxicity but high NO donor concentration exaggerated Al toxicity (Sun et al. 2015; Wang and Yang 2005). In our experimental system, we demonstrated that NO production in root apexes of soybean was induced more by high Al concentration than by low Al concentration (Fig. S3). So the discrepancy of NO on Al toxicity could be also explained by the level of endogenous NO concentration.
In summary, our work provides a new insight into how G6PDH works in the Al toxicity in soybean roots. We found that Cyt-G6PDH is responsible for NADPH oxidase-dependent ROS production and Al toxicity. On basis of our results presented here and previous studies, we propose a simple model to show the central role of Cyt-G6PDH in mediating Al toxicity in soybean seedlings (Fig. 10). In this model, Al induces NO production by stimulating NR activity. NO mediates Al-induced increase in Cyt-G6PDH activity by modulating the expression of Cyt-G6PDH genes. The NADPH produced by the enhanced Cyt-G6PDH activity could be used as a substrate to activate NADPH oxidase, thus leading to ROS accumulation. These mechanisms cause the inhibition of root elongation by increasing the level of lipid peroxidation.
Abbreviations
- DAB:
-
Diaminobenzidine
- DPI:
-
Diphenylene iodonium
- G6PDH:
-
Glucose-6-phosphate dehydrogenase
- GN6P:
-
Glucosamine 6-phosphate
- L-NNA:
-
N ω-nitro-L-Arg
- MDA:
-
Malondialdehyde
- NBT:
-
Nitroblue tetrazolium
- NOS:
-
Nitric oxide synthase
- NR:
-
Nitrate reductase
- PTIO:
-
2-phenyl-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide
- ROS:
-
Reactive oxygen species
- SNP:
-
Sodium nitroprusside
References
Asai S, Yoshioka M, Nomura H, Tone C, Nakajima K, Nakane E, Doke N, Yoshioka H (2011) A plastidic glucose-6-phosphate dehydrogenase is responsible for hypersensitive response cell death and reactive oxygen species production. J Gen Plant Pathol 77:152–162
Bowsher CG, Lacey AE, Hanke GT, Clarkson DT, Saker LR, Stulen I, Emes JM (2007) The effect of Glc6P uptake and its subsequent oxidation within pea root plastids on nitrite reduction and glutamate synthesis. J Exp Bot 58:1109–1118
Cardi M, Chibani K, Cafasso D, Rouhier N, Jacquot JP, Esposito S (2011) Abscisic acid effects on activity and expression of barley (Hordeum vulgare) plastidial glucose-6-phosphate dehydrogenase. J Exp Bot 62:4013–4023
Chen WW, Yang JL, Qin C, Jin CW, Mo JH, Ye T, Zheng SJ (2010) Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiol 154:810–819
Dal Santo S, Stampfl H, Krasensky J, Kempa S, Gibon Y, Petutschnig E, Rozhon W, Heuck A, Clausen T, Jonak C (2012) Stress-induced GSK3 regulates the redox stress response by phosphorylating glucose-6-phosphate dehydrogenase in Arabidopsis. Plant Cell 24:3380–3392
Esposito S, Guerriero G, Vona V, Di Martino RV, Carfagna S, Rigano C (2005) Glutamate synthase activities and protein changes in relation to nitrogen nutrition in barley: the dependence on different plastidial glucose-6P dehydrogenase isoforms. J Exp Bot 56:55–64
Gong H, Chen G, Li F, Wang X, Hu Y, Bi Y (2013) Involvement of G6PDH in heat stress tolerance in the calli from Przewalskiatangutica and Nicotianatabacum. Biol Plant 56:422–430
Hao F, Wang X, Chen J (2006) Involvement of plasma-membrane NADPH oxidase in nickel-induced oxidative stress in roots of wheat seedlings. Plant Sci 170:151–158
Hauschild R, von Schaewen A (2003) Differential regulation of glucose-6-phosphate dehydrogenase isoenzyme activities in potato. Plant Physiol 133:47–62
Hu Y, You J, Liang X (2015) Nitrate reductase-mediated nitric oxide production is involved in copper tolerance in shoots of hulless barley. Plant Cell Rep 34:367–379
Kochian LV (1995) Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol 46:237–260
Lamattina L, Garcia-Mata C, Graziano M, Pagnussat G (2003) Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol 54:109–136
Lin A, Wang Y, Tang J, Xue P, Li C, Liu L, Hu B, Yang F, Loake GJ, Chu C (2012) Nitric oxide and protein S-nitrosylation are integral to hydrogen peroxide-induced leaf cell death in rice. Plant Physiol 158:451–464
Liu Y, Wu R, Wan Q, Xie G, Bi Y (2007) Glucose-6-phosphate dehydrogenase plays a pivotal role in nitric oxide-involved defense against oxidative stress under salt stress in red kidney bean roots. Plant Cell Physiol 48:511–522
Ma JF (2005) Plant root responses to three abundant soil minerals: silicon, aluminum and iron. Crit Rev Plant Sci 24:267–281
Mackintosh C, Douglas P, Lillo C (1995) Identification of a protein that inhibits the phosphorylated form of nitrate reductase from spinach (Spinacia oleracea) leaves. Plant Physiol 107:451–457
Maffei ME, Mithofer A, Arimura G, Uchtenhagen H, Bossi S, Bertea CM, Cucuzza LS, Novero M, Volpe V, Quadro S, Boland W (2006) Effects of feeding Spodoptera Littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiol 140:1022–1035
Matsumura H, Miyachi S (1980) Cycling assay for nicotinamide adenine dinucleotides. Methods Enzymol 69:465–470
Neill SJ, Desikan R, Hancock JT (2003) Nitric oxide signaling in plants. New Phytol 159:11–35
Nemoto Y, Sasakuma T (2000) Specific expression of glucose-6-phosphate dehydrogenase (G6PDH) gene by salt stress in wheat (Triticum aestivum L.). Plant Sci 158:53–60
Scharte J, Schön H, Tjaden Z, Weis E, von Schaewen A (2009) Isoenzyme replacement of glucose-6-phosphate dehydrogenase in the cytosol improves stress tolerance in plants. Proc Natl Acad Sci U S A 106:8061–8066
Ślaski JJ, Zhang G, Basu U, Stephens JL, Taylor GJ (1996) Aluminum resistance in wheat (Tritucum aestivum) is associated with rapid, Al-induced changes in activities of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in root apices. Physiol Plant 98:477–484
Sun C, Lu L, Liu L, Liu W, Yu Y, Liu X, Hu Y, Jin C, Lin X (2014) Nitrate reductase-mediated early nitric oxide burst alleviates oxidative damage induced by aluminum through enhancement of antioxidant defenses in roots of wheat (Triticum aestivum). New Phytol 201:1240–1250
Sun C, Liu L, Yu Y, Liu W, Lu L, Jin C, Lin X (2015) Nitric oxide alleviates aluminum-induced oxidative damage through regulating the ascorbate-glutathione cycle in roots of wheat. J Integr Plant Biol 57:550–561
Torres MA, Dangl JL, Jones JDG (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci U S A 99:517–522
Van Gestelen P, Asard H, Caubergs RJ (1997) Solubilization and separation of a plant plasma membrane NADPH-O2 − synthase from other NAD(P)H oxidoreductase. Plant Physiol 115:543–550
Wakao S, Benning C (2005) Genome-wide analysis of glucose-6-phosphate dehydrogenases in Arabidopsis. Plant J 41:243–256
Wakao S, Andre C, Benning C (2008) Functional analyses of cytosolic glucose-6-phosphate dehydrogenases and their contribution to seed oil accumulation in Arabidopsis. Plant Physiol 146:277–288
Wang YS, Yang ZM (2005) Nitric oxide reduces aluminum toxicity by preventing oxidative stress in the roots of Cassia tora L. Plant Cell Physiol 46:1915–1923
Wang HH, Liang XL, Wan Q, Wang XM, Bi YR (2009) Ethylene and nitric oxide are involved in maintaining ion homeostasis in Arabidopsis Callus under salt stress. Planta 230:293–307
Wang HH, Huang JJ, Bi YR (2010) Nitrate reductase-dependent nitric oxide production is involved in aluminum tolerance in red kidney bean roots. Plant Sci 179:281–288
Wang H, Huang J, Liang X, Bi Y (2012) Involvement of hydrogen peroxide, calcium, and ethylene in the induction of the alternative pathway in chilling-stressed Arabidopsis Callus. Planta 235:53–67
Wang H, Li Y, Hou JJ, Huang JJ, Liang WH (2016a) Nitrate reductase-mediated nitric oxide production alleviates Al-induced inhibition of root elongation by regulating the ascorbate-glutathione cycle in soybean roots. Plant Soil. doi:10.1007/s11104-016-3045-4
Wang H, Yang L, Li Y, Hou J, Huang J, Liang W (2016b) Involvement of ABA- and H2O2-dependent cytosolic glucose-6-phosphate dehydrogenase in maintaining redox homeostasis in soybean roots under drought stress. Plant Physiol Biochem 107:126–136
Wenderoth I, Scheibe R, von Schaewen A (1997) Identification of the cysteine residues involved in redox modification of plant plastidic glucose-6-phosphate dehydrogenase. J Biol Chem 272:26985–26990
Wendt UK, Hauschild R, Lange C, Pietersma M, Wenderoth I, von Schaewen A (1999) Evidence for functional convergence of redox regulation in G6PDH isoforms of cyanobacteria and higher plants. Plant Mol Biol 40:487–494
Xu J, Yin H, Li Y, Liu X (2010) Nitric oxide is associated with long-term zinc tolerance in Solanum nigrum. Plant Physiol 154:1319–1334
Yamamoto Y, Kobayashi Y, Matsumoto H (2001) Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol 125:199–208
Yamamoto Y, Kobayashi Y, Devi SR, Rikishi S, Matsumoto H (2002) Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiol 128:63–72
Yamamoto Y, Kobayashi Y, Devi SR, Rikiishi S, Matsumoto H (2003) Oxidative stress triggered by aluminum in plant roots. Plant Soil 255:239–243
Yang YL, Zhang F, He WL, Wang XM, Zhang LX (2003) Iron-mediated inhibition of H+-ATPase in plasma membrane vesicles isolated from wheat roots. Cell Mol Life Sci 60:1249–1257
Zhang F, Wang Y, Yang Y, Wu H, Wang D, Liu J (2007) Involvement of hydrogen peroxide and nitric oxide in salt resistance in the calluses from Populus euphratica. Plant Cell Environ 30:775–785
Zhao MG, Tian QY, Zhang WH (2007) Nitric oxide synthase-dependent nitric oxide production is associated with salt tolerance in Arabidopsis. Plant Physiol 144:206–217
Zhao MG, Chen L, Zhang LL, Zhang WH (2009) Nitric reductase-dependent nitric oxide production is involved in cold acclimation and freezing tolerance in Arabidopsis. Plant Physiol 151:755–767
Zhou Y, Xu XY, Chen LQ, Yang JL, Zheng SJ (2012) Nitric oxide exacerbates Al-induced inhibition of root elongation in rice bean by affecting cell wall and plasma membrane properties. Phytochemistry 76:46–51
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 31301252), Science Foundation of the Henan Normal University for Outstanding Young Scholars (No. 14YQ003), and Program for Innovative Research Team (in Science and Technology) in University of Henan Province (No. 15IRSTHN020).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jian Feng Ma.
Electronic supplementary material
Fig. S1
Root elongation in response to GN6P and AlCl3 separately. Seedlings were pretreated with GN6P for 12 h and then exposed to AlCl3 for 24 h. Data represent means ± SD of at least three biological replicates. Bars with different letters indicate significant differences at P < 0.05. CK, control. (JPEG 53 kb)
Fig. S2
Effect of NADPH oxidase inhibitor on the lipid peroxidation under Al stress. Treatments for seedlings were performed as described in Fig. 5. Data represent means ± SD of at least three biological replicates. Bars with different letters indicate significant differences at P < 0.05. CK, control. (JPEG 49 kb)
Fig. S3
Effect of different Al concentrations on NO production in soybean roots. Seedlings were exposed to the indicated Al concentrations for 24 h. Representative images showing endogenous NO production are given. CK, control. (JPEG 34 kb)
Rights and permissions
About this article
Cite this article
Wang, H., Hou, J., Li, Y. et al. Nitric oxide-mediated cytosolic glucose-6-phosphate dehydrogenase is involved in aluminum toxicity of soybean under high aluminum concentration. Plant Soil 416, 39–52 (2017). https://doi.org/10.1007/s11104-017-3197-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3197-x