Abstract
Transient genetic transformation of plant organs is an indispensable way of studying gene function in plants. This study was aimed to develop an optimized system for transient Agrobacterium-mediated transformation of the Arabidopsis leaves. The β-glucuronidase (GUS) reporter gene was employed to evaluate growth and biochemical parameters that influence the levels of transient expression. The effects of plant culture conditions, Agrobacterial genetic backgrounds, densities of Agrobacterial cell suspensions, and of several detergents were analyzed. We found that optimization of plant culture conditions is the most critical factor among the parameters analyzed. Higher levels of transient expression were observed in plants grown under short day conditions (SDs) than in plants grown under long day conditions (LDs). Furthermore, incubation of the plants under SDs at high relative humidity (85–90%) for 24 h after infiltration greatly improved the levels of transient expression. Under the optimized culture conditions, expression of the reporter gene reached the peak 3 days after infiltration and was rapidly decreased after the peak. Among the five Agrobacterial strains examined, LAB4404 produced the highest levels of expression. We also examined the effects of detergents, including Triton X-100, Tween-20, and Silwet L-77. Supplementation of the infiltration media either with 0.01% Triton X-100 or 0.01% Tween-20 improved the levels of expression by approximately 1.6-fold. Our observations indicate that transient transformation of the Arabidopsis leaves in the infiltration media supplemented with 0.01% Triton X-100 and incubation of the infiltrated plants under SDs at high relative humidity are necessary for maximal levels of expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gene expression studies in homologous or heterologous systems constitute a critical component of molecular biological researches in plants. A wide array of methods or techniques has been developed during the past decade to transfer exogenous genes into plants or specific plant organs and to produce transgenic plants through stable or transient transformation. One benefit of the transient genetic transformation is its rapid process compared to the stable genetic transformation, and we can frequently get the results in days.
Most of the transient expression techniques have been developed by employing particle bombardment of epidermal cells and leaves (Sato et al. 1993; Schweizer et al. 1999) or by protoplast transformation and electroporation- and polyethyleneglycol (PEG)-mediated methods (Lindsey and Jones 1987; Sheen 2001). The particle bombardment is an efficient way of gene delivery applicable to virtually all plant species. However, it suffers from several drawbacks: it usually delivers the gene of interest only to a few cells, and the sizes of the genes are limited (Christou 1997; Fisher and Emans 2000). It also requires expensive and specialized equipments. The PEG-mediated protoplast transformation can be readily carried out using ordinary lab supplies and equipments. However, the experimental procedures are quite complicated and time-consuming (Yang et al. 2000).
Transient Agrobacterium-mediated gene expression assays have been successfully used to analyze gene function, gene silencing, and gene-for-gene interactions between host resistance and pathogen avirulence genes (Baulcombe 1999; Frederick et al. 1998; Kapila et al. 1997; Scofield et al. 1996). They have also been employed to characterize production of recombinant antibodies in tobacco and lettuce (Negrouk et al. 2005; Vaquero et al. 1999). Notably, the experimental procedures are relatively simple and do not require expensive supplies and equipments. In particular, this method efficiently works in many plant species, such as tobacco, potato, tomato, lettuce, pea, grapevine, and Arabidopsis (Mestre et al. 2000; Santos-Rosa et al. 2008; Van der Hoorn et al. 2000; Wroblewski et al. 2005).
However, current transient transformation systems used in Arabidopsis still need to be improved, because optimal plant culture conditions and affordable supplements have not yet been well-defined (Wroblewski et al. 2005).
Genetic backgrounds of the Agrobacterial strains considerably influence the efficiency of transient expression. Different Agrobacterial strains are defined by their chromosomal backgrounds and the presence of resident Ti plasmids. Most of the widely used Agrobacterial strains originate from two representative wild isolates: C58 (Hamilton and Fall 1971) and Ach5 (Hoekema et al. 1983). The AGL1, EHA105, and GV3101 strains have the C58 origin. The LBA4404 strain, one of the most commonly used laboratory strains, originates from the Ach5 chromosomal background.
It is known that some Agrobacterial strains are more virulent than others, depending on the target plant species. Such variations are caused by differences in the ability of the bacterial cells to attach to plant cells or by differences in either bacterial-encoded or plant-encoded T-DNA transfer mechanisms (Nam et al. 1997; Yanofsky et al. 1985a, b). However, it is unknown what host compounds affect the recognition process, the transfer of DNA, and the integration of the T-DNA into the host (Ditt et al. 2001). The T-DNA region of the Ti plasmid carries genes encoding tumor-specific low-molecular-weight compounds, opines (Montoya et al. 1977). Opines are metabolized by the bacterial cells as an important source of carbon and energy.
Agrobacterium Ti-plasmids are classified into several types based on the ways of utilizing the opines: octopine (LBA4404 and GV2260), nopaline (C58C1, GV3100, GV3101, GV3850, A136, and EHA 101), agropine (A281 and A543), and succinamopine (EHA105 and AGL1) types (Dessaux et al. 1992). It seems that specific Agrobacterial strains work better than other strains in specific plant species. For example, it has been suggested that the C58C1 strain is most suitable for the transient expression assays in lettuce, tobacco, and Arabidopsis (Wroblewski et al. 2005).
Detergents are amphiphilic compounds having measurable aqueous solubility both as aggregates and as monomers. They belong to a class of compounds, collectively called surfactants, which reduce the surface tension within the external surface layers of water by absorbing at the liquid–gas interface. They also reduce the interfacial tension between oil and water by absorbing at the liquid–liquid interface. Silwet L-77 is a unique surfactant in that it can improve cuticular penetration of spray mix into plant surfaces. It is compatible with most pesticides and fertilizer solutions. Notably, it has been reported that Silwet L-77 improves the efficiency of the transient Agrobacterium-mediated genetic transformation in Arabidopsis (Clough and Bent 1998) and wheat (Cheng et al. 1997; Wu et al. 2003).
Tween 20 is a polysorbate surfactant and used as detergent and emulsifier in a number of biological, pharmaceutical, industrial, and food applications. It is distinguished from other Tween members by the lengths of polyoxyethylene chains and fatty acid ester moieties. Triton X-100 is one of the most commonly used, nonionic detergents in various biochemical applications in order to solubilize proteins.
In this work, we developed an optimized system for transient Agrobacterium-mediated transformation of the Arabidopsis leaves. The parameters explored included plant culture conditions, Agrobacterial genetic backgrounds and cell densities, and effects of detergents. We found that optimization of plant culture conditions is the most important factor among the parameters analyzed. The optimized conditions for the transient expression system will be widely applicable to studies on gene function in Arabidopsis.
Materials and methods
Plants and growth conditions
Arabidopsis thaliana line used was Columbia (Col-0) ecotype. Arabidopsis plants were routinely grown in a controlled culture room set at 22°C either under LDs (16 h light/8 h dark) or SDs (8 h light/16 h dark) with white light illumination (120 μmol photons/m2 s) provided by fluorescent FLR40D/A tubes (Osram, Seoul, Korea). For transient expression assays, plant pots were covered with clear polyethylene film for 24 h after infiltration to maintain the infiltrated plants at high humidity before further growing for 1–6 additional days under identical growth conditions. The relative humidity was retained at approximately 85–90% with covering.
Agrobacterial strains and expression vector
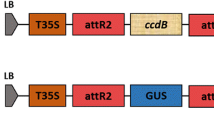
We included five Agrobacterial strains, as listed in Table 1, and a binary vector pCAMBIA1304 containing the GUS reporter gene was used. The expression vector comprises: the right and left borders of the Ti region, the Cauliflower Mosaic Virus (CaMV) 35S promoter, the Nos-3′ terminator, and the mGFP and GUS reporter genes. It also contains a kanamycin resistance gene for bacterial selection and a hygromycin phosphotransferase gene for selection in plants.
Infiltration of the Arabidopsis leaves
The YEP medium (Bacto trypton 10 g/L, yeast extract 10 g/L, NaCl 5 g/L, pH7.5) was used to culture Agrobacterial cells at 28°C. The AGL1 cells harboring the pCAMBIA1304 vector were maintained in YEP media supplemented with rifampicin (25 mg/L), kanamycin (50 mg/L), and ampicillin (100 mg/L). Other Agrobacterial cells harboring the pCAMBIA1304 vector were maintained in YEP media supplemented with rifampicin (25 mg/L) and kanamycin (50 mg/L). The Agrobacterial cells were cultured in 10 mL YEP media overnight at 28°C with vigorous shaking. Small aliquots of the cell cultures were transferred to fresh medium (1:10 ratio, v/v) and grown until the cell density reaches OD600 of 1.0. The cell cultures were centrifuged, and the collected cells were resuspended in the infiltration medium (0.5% D-glucose, 10 mM MES, 10 mM MgCl2, 200 μM acetosyringone) at OD600 = 0.6 prior to infiltration. Acetosyringone is a phenolic compound that can attract Agrobacterial cells to wounded plant tissues via chemotaxis and induce the Vir genes to initiate T-DNA transfer (Hiei et al. 1994).
Infiltration of the Agrobacterial cell suspensions into the Arabidopsis leaves was carried out as described previously (Schob et al. 1997). Arabidopsis plants were grown in soil pots at 22°C for 4 weeks under LDs or for 6 weeks under SDs, and the 4th–5th leaves were used for infiltration. Equal volumes, each containing approximately 0.3 mL, of the Agrobacterial cell suspension were injected to the abaxial side of leaf with a 1 mL syringe lacking needle. Six injections, three injections on each side of the leaf vein, were carried out for individual leaves. After infiltration, the LD-grown or SD-grown plants were incubated at 22°C for 24 h either under LDs or under SDs, respectively, with or without polyethylene film covering. The film cover was then removed, and the plants were further incubated under identical light regime for 1–6 additional days before histochemical GUS staining and fluorometric measurements of GUS activities. Various detergents, such as Triton X-100 (Roche Diagnostics GmbH, Mannheim, Germany), Tween-20 (Sigma-Aldrich, St. Louis, MO, USA), and Silwet L-77 (Lehle Seeds, Round Rock, TX, USA), were added to the infiltration media at a series of varying concentrations.
β-Glucuronidase (GUS) activity assays
Histochemical GUS staining and fluorometric measurements of the GUS activity were conducted as described previously (Jefferson et al. 1987). The infiltrated leaves were cut into pieces and soaked in the GUS staining buffer (100 mM sodium phosphate, 1 mM 5-bromo-4-chloro-3-indolyl-β-d-glucuronide, 0.5 mM potassium ferrocyanide, 0.5 mM potassium ferricyanide, 10 mM Na2EDTA, pH 7.0). They were incubated for 16 h at 37°C. The stained leaf tissues were rinsed with 70% ethanol until pigments, such as chlorophylls, were completely cleared and photographed.
Fluorometric measurements of the GUS activity were carried out using 4-methyl-umbelliferyl-β-d-glucuronide (4-MUG, Sigma-Aldrich) as a substrate. The infiltrated leaf samples were homogenized in the extraction buffer (50 mM sodium phosphate, 10 mM Na2EDTA, 0.1% Triton X-100, 0.1% sarcosyl, 10 mM β-2-mercaptoethanol, pH 7.0). The homogenates were centrifuged for 15 min at 13,000 rpm, and aliquots of the supernatants, containing approximately 25 μg proteins, were incubated for 1 h in the extraction buffer containing 1 mM 4-MUG at 37°C. The reaction was terminated by adding 0.2 M Na2CO3. Fluorescence measurements were carried out using a fluorescence spectrophotometer (Multiple Plate Reader Victor3, Perkin Elmer, Waltham, MA, USA). The standard was calibrated using 4-methylumbelliferone (4MU, Sigma-Aldrich). Protein concentrations were determined using BSA as standard (Bradford 1976).
Results
Determination of optimal Agrobacterial cell density
With an aim of optimizing the conditions for transient Agrobacterium-mediated expression assays in Arabidopsis, we extensively analyzed the effects of plant culture conditions and different Agrobacterial strains as well as those of detergents that are frequently used in plant laboratories. The Agrobacterial strains we examined included AGL1, EHA105, C58C1, GV3101, and LBA4404. Whereas the AGL1 strain is resistant to rifampicin and carbenicillin, other strains have an intrinsic resistance to rifampicin. The pCAMBIA1304 vector containing the GUS reporter gene was for quantitation of the expression levels.
It has been known that the cell density of bacterial suspension cultures is important for high transient expression in Arabidopsis. Whereas cell suspensions with the cell density of less than OD600 = 0.1 often result in low transient expression, those with the cell density with above OD600 = 1.0 result in tissue yellowing or wilting (Wroblewski et al. 2005). To determine the cell density that gives rise to maximal transient expression, we tested several different cell densities at OD600 = 0.3, 0.6, 0.9, or 1.2. While severe wilting and yellowing symptoms were observed when cell suspensions with OD600 = 1.2 were used, lower transient expression was obtained using cell suspensions at OD600 = 0.3 or lower. We obtained the highest transient gene expression with cell suspensions with OD600 = 0.6–0.9 (Table 2). Notably, the LBA4404 and GV3101 strains showed relatively higher expression at OD600 = 0.6. In contrast, the EHA105 strain showed the highest transient expression at OD600 = 0.3. Transient expression using the C58C1 strain was not detectably affected by variations in cell densities, indicating that the optimal cell densities of individual Agrobacterial strains are different from each other.
Effects of daylengths and humidity
Environmental factors, such as daylengths and humidity, significantly influence plant growth and physiology. We therefore examined the effects of different daylengths and relative humidity on the efficiency of transient expression. The LBA4404 cells containing the GUS reporter gene was used at OD600 = 0.6 for infiltration.
The Arabidopsis plants grown either under LDs for 4 weeks or under SDs for 6 weeks were used for infiltration. The infiltrated plants were incubated at 22°C for 24 h either under LDs or under SDs, respectively. The infiltrated plants in soil pots were covered with clear polyethylene vinyl film to maintain a high relative humidity. By doing this, whereas the relative humidity was approximately 50% in the growth chamber, it was maintained at approximately 85–90% with covering. The vinyl film was then removed, and the infiltrated plants were further incubated for 2 more days under identical light regime.
To access the efficiency of transient expression, histochemical GUS staining and fluorometric measurement of GUS activity were carried out. As illustrated in Fig. 1, the level of reporter gene expression was approximately 2 times higher when plants grown under SDs were used for infiltration than those grown under LDs, as determined by fluorometric GUS activity measurements. Notably, the GUS activity was increased significantly when the plants in soil pots were covered with vinyl film both under LDs and SDs. At high relative humidity, the GUS activities were elevated by 4.3-fold and 2.7-fold in the SD-grown and LD-grown plants, respectively (Fig. 1). These observations indicate that use of SD-grown plants for infiltration and maintenance of the infiltrated plants under SDs at high relative humidity for 24 h after infiltration are necessary for higher levels of transient expression in Arabidopsis. We therefore used these conditions for further studies.
Effects of different culture conditions on the transient GUS expression. The 4th–5th leaves of the Arabidopsis plants grown in soil pots either under LDs for 4 weeks or under SDs for 6 weeks were infiltrated. Agrobacterial strain LBA4404 cells containing the GUS reporter gene were infiltrated to the abaxial surfaces of the Arabidopsis leaves. a Histochemical GUS staining. The infiltrated plants were incubated at 22°C for 24 h under LDs with (+) or without (−) polyethylene film covering. The infiltrated plants were further incubated under identical conditions but without polyethylene film covering. Histochemical GUS staining was carried out 3 days after infiltration. b Fluorometric measurements of GUS activities. The measurements were carried out using the leaves shown in Fig. 1a. Three measurements, each consisting of five different leaves, were averaged. Bars indicate standard error of the mean
Effects of incubation times
Expression of the foreign gene continues only for a short time period in the transient system. It is therefore critical to know when the gene expression reaches the peak and thus when the plants should be harvested after infiltration to obtain maximal transient expression.
To determine the optimal incubation time, the infiltrated, LD-grown or SD-grown plants were incubated at 22°C either under LDs or under SDs, respectively, with vinyl film covering for 24 h, the cover was then removed, and the infiltrated plants were further incubated for various time periods upto 7 days under identical light regime. The GUS activities were gradually elevated until 3 days after infiltration. After the peak, they were gradually decreased (Fig. 2), indicating that the highest expression can be obtained in the plants 3 days after incubation.
Levels of transient GUS expression at different time points after incubation. The 4th–5th leaves of the Arabidopsis plants grown in soil under SDs for 6 weeks were infiltrated. Agrobacterial strain LBA4404 cells containing the GUS reporter gene were infiltrated to the abaxial surfaces of the Arabidopsis leaves. The infiltrated plants were incubated at 22°C for 24 h under SDs with polyethylene film covering. The infiltrated plants were further incubated under identical conditions but without polyethylene film covering. The leaves were harvested at the indicated time points after infiltration and subject to histochemical GUS staining and fluorometric measurements of GUS activities. a Histochemical GUS staining. b Fluorometric measurements of GUS activities. Measurements of the GUS activities were carried out as described in Fig. 1b. Bars indicate standard error of the mean
Determination of most effective Agrobacterial strains
Our data indicated that the highest reporter gene expression occurs when the infiltrated SD-grown plants were incubated at 22°C under SDs at high relative humidity for 3 days after incubation. A question was whether the optimized conditions established with the LBA4404 strain are also valid for other Agrobacterial strains.
We examined the levels of transient expression under the optimized conditions but using different Agrobacterial strains. Notably, the levels of transient expression, as determined by histochemical GUS staining and fluorometric GUS activity measurements, were highly variable, depending on the strains used. The LBA4404 strain exhibited the highest expression among the strains examined (Fig. 3). The levels of transient expression in the plants infiltrated with the LBA4404 cells were higher by approximately1.6-fold than those obtained with the nopaline strains (C58C1 and GV3101) and by higher than 3.5-fold those achieved with the succinamopine strains (EHA105 and AGL1). These observations revealed that the LBA strain is a choice of Agrobacterial strains for transient expression in Arabidopsis.
Effects of different Agrobacterial strains on the transient GUS expression. Incubation conditions of the plants were as described in Fig. 2. Five different Agrobacterial strains, including AGL1, EHA105, C58C1, GV3101, and LBA4404, containing the GUS reporter gene were used for infiltration assays. Histochemical GUS staining and fluorometric measurements of GUS activities were carried out 3 days after infiltration. a Histochemical GUS staining. b Fluorometric measurements of GUS activities. Measurements of the GUS activities were carried out as described in Fig. 1b. Bars indicate standard error of the mean
Effects of detergents
Some chemicals, such as acetosyringone, l-cysteine, l-glutamine, and azaserine, have been reported to influence transformation efficiency and transient gene expression in many plant species (Olhoft and Somers 2001; Rogowsky et al. 1987; Roberts et al. 2003; Stachel et al. 1986). It was also envisioned that detergents would also affect both the stable and transient expression systems.
We investigated the effects of several detergents on the transient Agrobacterium-mediated expression in the Arabidopsis leaves. We examined different concentrations of Triton X-100 (Fig. 4a), Tween-20 (Fig. 4b), or Silwet L-77 (Fig. 4c). The detergents were added to the infiltration media at different concentrations. Overall, the effects of the detergents were somewhat variable. However, it is evident that higher concentrations of the detergents, such as 0.5% Triton X-100 and 0.05% Silwet L-77, drastically reduced the reporter gene expression, apparently by causing severe wilting and necrosis symptoms (Fig. 4). The detergents exhibited some degree of positive effects on the reporter gene expression when they were used at lower concentrations. In all the detergents tested, the levels of transient expression were highest at a concentration of 0.01%.
Effect of different detergents on the transient GUS expression. The plant materials used were as described in Fig. 3. The infiltration media were supplemented with varying concentrations of Triton X-100 (a), Tween-20 (b), or with Silwet-L77 (c). Agrobacterial strain LBA4404 cells containing the GUS reporter gene were used. Histochemical GUS staining and fluorometric measurements of the GUS activities were carried out as described in Fig. 1. Bars indicate standard error of the mean. a Effects of Triton X-100. It was included in the infiltration media at the final concentrations of 0, 0.001%, 0.005, 0.01, 0.1, or 0.5%. b Effects of Tween-20. It was included in the infiltration media at the final concentrations of 0, 0.001, 0.005, 0.01, or 0.05%. c Effects of Silwet-L77. It was included in the infiltration media at the final concentrations of 0, 0.005, 0.01, or 0.05
Comparison of the GUS activities between plant samples treated with different detergents revealed that treatments with 0.01% Triton X-100 and 0.01% Tween-20 showed the most promotive effects. However, the effects of Silwet-L-77 were relatively lower than those of Triton X-100 and Tween-20. Together, these observations indicate that detergents, such as Triton X-100 and Tween-20, are also a factor of consideration for the transient Agrobacterium-mediated expression assays in Arabidopsis, particularly when highest expression is required.
Discussion
Arabidopsis thaliana is an annual herb in which floral induction is regulated by multiple flowering genetic pathways that respond to diverse environmental signals, such as changes in daylengths and vernalization (exposure to cold temperature for a long time period). Arabidopsis thaliana belongs to a long day plants: its flowering is promoted under LDs. In other words, it is drastically delayed and vegetative growth is extended under SDs (Greb et al. 2003; Melzer et al. 2008). The molecular mechanisms underlying this discrepancy are currently unclear. Considering that the leaf cells of plants undergoing reproductive growth are developmentally older than those in their vegetative growth, it is envisioned that developmental age of the leaves is critical for higher levels of transient expression.
Humidity in the air surrounding the plants is an important environmental factor that influences diverse aspects of plant growth and development. The most direct effect of humidity is exerted at the rate of transpiration or evaporation of water from the leaves. Both environmental and intrinsic factors significantly affect the rate of transpiration. Environmental factors influencing transpiration rate include relative humidity, air movements, ambient temperature, water supply from the roots, and light intensity. Endogenous regulatory factors include leaf surface area, thickness of epidermis and cuticle layer, stomatal frequency and size, and distribution of stomata (Ares and Fownes 1999; Satisha et al. 2006). Dry conditions are certainly stressful to plants, and physiological activities would be reduced in the leaf cells under these conditions. This view entails that the cell viability is also important for high transient expression.
Under SDs with high relative humidity, vegetative growth is sustained without flowering, but the vegetative-to-reproductive transition is delayed. Transpiration rate would be lower under SDs than under LDs, perhaps due to shorter daylength, supporting high cell viability. It is therefore anticipated that individual leaf cells may be in a physiologically more active state when plants are grown under SDs than under LDs. Under SDs with high relative humidity, the physiological activities of the cells are higher, and thus gene expression would be promoted. Alternatively, the cells undergoing active vegetative growth would be more susceptible to Agrobacterial infections (Gelvin 2003).
Our data indicate that levels of transient expression were higher in plants grown under SDs than in plants grown under LDs. Furthermore, incubation of the plants at high relative humidities (85–90%) for 24 h after infiltration greatly improved the levels of transient expression.
Surfactants are known to function either as enhancers of cuticle penetration of spray mix by making the plant cuticular membrane susceptible to solute transfer or by acting as cosolvents, thereby enhancing the movement of herbicidal active ingredients into the plant cells (Madhou et al. 2006). There are several types of surfactants, depending on their charges: anionic, cationic, amphoteric, and nonionic surfactants. The nonionic surfactants are most commonly used in the horticulture, because they have less toxic to plant growth.
The herbicidal chemicals are used to suppress the growth of unwanted plants. Application of surfactants together with the herbicide solution is often an effective method of improving the performance of herbicides. In Arabidopsis, it has been found that plants treated with 0.02% NUL1026, a nonionic surfactant, undergoes premature leaf senescence, since the chemical activates genes involved in jasmonic acid (JA) biosynthesis, including LYPOXIGENASE 3 (LOX3, At1g17420), ALLENE OXIDE SYNTHASE (AOS, At5g42650), ALLENE OXIDE CYCLASE 2 (AOC2, At3g25780) (Madhou et al. 2006). As a result, JA biosynthesis is induced, and it acts an activator of leaf senescence, signifying that although surfactants improve the efficiency of transient expression by facilitating infection of the plant tissues by Agrobacterial cells, its doses should be tightly controlled.
We also obtained similar results on the effects of a few detergents, such as Triton X-100, Tween 20, and Silwet L-77 on the levels of transient expression. When they were applied at a concentration of 0.01% or lower, the level of transient expression was markedly elevated. However, concentrations of higher than 0.05% caused leaf wilting and yellowing, resulting in drastic reduction of gene expression. It is interesting that while Silwet L-77 is most effective in the Arabidopsis floral transformation system (Chung et al. 2000; Clough and Bent 1998), it is least effective in the transient expression assays in the leaves.
References
Ares A, Fownes JH (1999) Water supply regulates structure, productivity, and water use efficiency of Acacia koa forest in Hawaii. Oecologia 121:458–466
Baulcombe DC (1999) RNA makes no protein. Curr Biol 9:599–601
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cheng M, Fry JE, Pang S, Zhou H, Hironaka CM, Duncan DR, Conner TW, Wan Y (1997) Genetic transformation of wheat mediated by Agrobacterium tumefaciens. Plant Physiol 115:971–980
Christou P (1997) Rice transformation: bombardment. Plant Mol Biol 35:197–203
Chung MH, Chen MK, Pan SM (2000) Floral spray transformation can efficiently generate Arabidopsis transgenic plants. Transgenic Res 9:471–476
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Dessaux Y, Petit A, Tempe J (1992) Opines in Agrobacterium biology. In: Verma DPS (ed) Molecular signals in plant-microbe communications. CRC Press, Boca Raton, pp 109–136
Ditt R, Nester EW, Comai L (2001) Plant gene expression responses to Agrobacterium tumefaciens. Proc Natl Acad Sci USA 98:10954–10959
Fisher R, Emans N (2000) Molecularfarming of pharmaceutical proteins. Transgenic Res 9:279–299
Frederick RD, Thilmony RL, Sessa G, Martin GB (1998) Recognition specificity for the bacterial avirulence protein AvrPto is determined by Thr-204 in the activation loop of the tomato Pto kinase. Mol Cell 2:241–245
Gelvin SB (2003) Agrobacterium-Mediated plant transformation: the biology behind the “Gene-Jockeying” tool. Microbiol Mol Biol Rev 67:16–37
Greb T, Clarenz O, Schäfer E, Muller D, Herrero R, Schmitz G, Theres K (2003) Molecular analysis of the LATERAL SUPPRESSOR gene in Arabidopsis reveals a conserved control mechanism for axillary meristem formation. Genes Dev 17:1175–1187
Hamilton RH, Fall MZ (1971) The loss of tumour-initiating ability in Agrobaeterium tumefaciens by incubation at high temperature. Experientia 27:229–230
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Hoekema A, Hirsch PR, Hooykaas PJJ, Schilperoort RA (1983) A binary plant vector strategy based on separation of vir- and T-region of the Agrobacterium tumefaciens Ti-plasmid. Nature 303:179–180
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusion: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Kapila J, DeRycke R, VanMontagu M, Angenon G (1997) An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Sci 122:101–108
Lindsey J, Jones MGK (1987) Transient gene expression in electroporated protoplasts and intact cells of sugar beet. Plant Mol Biol 10:43–52
Madhou P, Raghavan C, Wells A, Stevenson TW (2006) Genome-wide microarray analysis of the effect of a surfactant application in Arabidopsis. Weed Res 46:275–283
Melzer S, Lens F, Gennen J, Vanneste S, Rohde A, Beeckman T (2008) Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nat Genet 40:1489–1492
Mestre P, Brigneti G, Baulcombe DC (2000) An Ry-mediated resistance response in potato requires the intact active site of the NIa proteinase from potato virus Y. Plant J 23:653–661
Montoya AL, Chilton MD, Gordon MP, Sciaky D, Nester EW (1977) Octopine and nopaline metabolism in Agrobacterium tumefaciens and crown gall tumor cells: role of plasmid genes. J Bacteriol 129:101–107
Nam J, Matthysse AG, Gelvin SB (1997) Differences in susceptibility of Arabidopsis ecotypes to crown gall disease may result from a deficiency in T-DNA integration. Plant Cell 9:317–333
Negrouk V, Eisner G, Lee H, Han K, Taylor D, Wong HC (2005) Highly efficient transient expression of functional recombinant antibodies in lettuce. Plant Sci 169:433–438
Olhoft PM, Somers DA (2001) L-Cysteine increases Agrobacterium-mediated T-DNA delivery into soybean cotyledonarynode cells. Plant Cell Report 20:706–711
Roberts RL, Matz M, Monks DE, Lockwood Mullaney M, Hall T, Nester EW (2003) Purine synthesis and increased Agrobacterium tumefaciens transformation of yeast and plants. Proc Natl Acad Sc USA 100:6634–6639
Rogowsky PM, Close TJ, Chimera JA, Shaw JJ, Kado CI (1987) Regulation of the vir genes of Agrobacterium tumefaciens plasmid pTiC58. J Bacteriol 169:5101–5112
Santos-Rosa M, Poutaraud A, Merdinoglu D, Mestre P (2008) Development of a transient expression system in grapevine via agro-infiltration. Plant Cell Rep 27:1053–1063
Satisha J, Prakash GS, Venugopalan R (2006) Statistical modeling of the effect of physio-biochemical parameters on water use efficiency of grape varieties, rootstocks and their stionic combinations under moisture stress conditions. Turk J Agric For 30:261–271
Sato S, Newell C, Kolacz K, Tredo L, Finer J, Hinchee M (1993) Direct gene transfer in potato: A comparison of particle bombardment of leaf explants and PEG-mediated transformation of protoplasts. Plant Cell Rep 12:408–413
Schob H, Kunz C, Meins F Jr (1997) Silencing of transgenes introduced into leaves by agroinfiltration: a simple, rapid method for investigation of sequence requirements for gene silencing. Mol Gen Genet 256:581–585
Schweizer P, Christoffel A, Dudler R (1999) Transient expression of members of the germin-like gene family in epidermal cells of wheat confers disease resistance. Plant J 20:541–552
Scofield SR, Tobias CM, Rathjen JP, Chang JH, Lavelle DT, Michelmore RW, Staskawicz BJ (1996) Molecular basis of gene for-gene specificity in bacterial speck disease of tomato. Science 274:2063–2065
Sheen J (2001) Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiol 127:1466–1475
Stachel SE, Nester EW, Zambryski PC (1986) A plant cell factor induces Agrobacterium tumefaciens vir gene expression. Proc Natl Acad Sci USA 83:379–383
Van der Hoorn JAL, Laurent F, Roth R, De Wit PJGM (2000) Agroinfiltration is a versatile tool that facilitates comparative analyses of Avr9/cf-9-induced and Avr4/Cf-4-induced necrosis. Mol Plant Microbe Interact 13:439–446
Vaquero C, Sack M, Chandler J, Drossard J, Schuster F, Monecke M, Schillberg S, Fischer R (1999) Transient expression of a tumor-specific single-chain fragment and a chimeric antibody in tobacco leaves. Proc Natl Acad Sci USA 96:11128–11133
Wroblewski T, Tomczak A, Michelmore R (2005) Optimization of Agrobacterium-mediated transient assays of gene expression in lettuce, tomato and Arabidopsis. Plant Biotechnol J 3:259–273
Wu H, Sparks C, Amoah A, Jones HD (2003) Factors influencing successful Agrobacterium-mediated genetic transformation of wheat. Plant Cell Rep 21:659–668
Yang Y, Li R, Qi M (2000) In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J 22:543–551
Yanofsky M, Lowe B, Montoya A, Rubin R, Krul W, Gordon M, Nester E (1985a) Molecular and genetic analysis of factors controlling host range in Agrobacterium tumefaciens. Mol Gen Genet 201:237–246
Yanofsky M, Montoya A, Knauf V, Lowe B, Gordon M, Nester E (1985b) Limited-host-range plasmid of Agrobacterium tumefaciens: molecular and genetic analyses of transferred DNA. J Bacteriol 163:341–348
Acknowledgments
This work was supported by the Brain Korea 21, Biogreen 21 (20080401034001), and National Research Laboratory Programs and by grants from the Plant Signaling Network Research Center, the Korea Science and Engineering Foundation (2007-03415), and from the Agricultural R & D Promotion Center (309017-5), Korea Ministry for Food, Agriculture, Forestry and Fisheries.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. R. Liu.
Rights and permissions
About this article
Cite this article
Kim, M.J., Baek, K. & Park, CM. Optimization of conditions for transient Agrobacterium-mediated gene expression assays in Arabidopsis . Plant Cell Rep 28, 1159–1167 (2009). https://doi.org/10.1007/s00299-009-0717-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-009-0717-z