Abstract
Tissue-specific patterns and levels of protein expression were characterized in transgenic carrot plants transformed with the β-glucuronidase (GUS) gene driven by one of five promoters: Cauliflower mosaic virus 35S (35S) and double 35S (D35S), Arabidopsis ubiquitin (UBQ3), mannopine synthase (mas2) from Agrobacterium tumefaciens or the rooting loci promoter (rolD) from A. rhizogenes. Five independently transformed carrot lines of each promoter construct were assessed for GUS activity. In leaves, activity was highest in plants with the D35S, 35S and UBQ3 promoters, while staining was weak in plants with the mas2 promoter, and only slight visual staining was present in the leaf veins of plants containing rolD promoter . Strong staining was seen in the lateral roots, including root tips, hairs and the vascular tissues of plants expressing the 35S, D35S and UBQ3. Lateral roots of plants containing the rolD construct also showed staining in these tissues while the mas2 promoter exhibited heightened staining in the root tips. Relatively strong GUS staining was seen throughout the tap root with all the promoters tested.. When GUS expression was quantified, the UBQ3 promoter provided the highest activity in roots of mature plants, while plants with the D35S and 35S promoter constructs had higher activity in the leaves. Although plants containing the mas2 promoter had higher levels of activity compared to the rolD plants, these two promoters were significantly weaker than D35S, 35S and UBQ3. The potential for utilization of specific promoters to target expression of desired transgenes in carrot tissues is demonstrated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carrot (Daucus carota L. subsp. sativa), a member of the family Apiaceae, is grown for its edible taproot, which contains high levels of β-carotene (provitamin A), vitamins B1 and C, and provides a good source of dietary fiber (Ammirato 1986). Commercial cultivars of carrot have been developed using traditional breeding methods for improved root growth, root shape and colour, β-carotene levels and smooth skin (Ammirato 1986). Carrot is a model system for tissue culture studies and previous research has demonstrated the utility of somatic embryogenesis, bioreactor scale-up of suspension cultures and protoplast culture and fusion for carrot improvement (Ammirato 1986; Zimmerman 1993; Komamine et al. 2005). In addition, transgenic technology has been used to enhance fungal disease resistance in carrot (Gilbert et al. 1996; Melchers and Stuiver 2000; Takaichi and Oeda 2000; Chen and Punja 2002; Jayaraj and Punja 2007), to create herbicide-resistant plants (Chen and Punja 2002), or for metabolic engineering of designer medical products (Bouche et al. 2003; Marquet-Blouin et al. 2003; Kumar et al. 2004) and novel antioxidant compounds (Jayaraj et al. 2007). In the majority of these studies, the Cauliflower mosaic virus 35S (CaMV 35S) (Odell et al. 1985) was used, while the maize ubiquitin promoter (Christensen et al. 1992) was also utilized to drive the constitutive expression of transgenes in carrot (Chen and Punja 2002). The regulation of transgene expression is crucial for successful commercial genetic engineering to ensure expression levels are high and in the desired tissues. A comparative assessment of promoter tissue specificity and strength in different tissues has not been previously conducted in carrot as they have in other plants (Schledzewski and Mendel 1994; Horloft et al. 1995; Ni et al. 1995; Gandhi et al. 1999; Kamo and Blowers 1999; Samac et al. 2004). In particular, the utility of promoters to provide expression in carrot tap roots, has not been previously assessed.
The objective of this study was to characterize β-glucuronidase (uidA) expression in transgenic carrot tissues under control of three constitutive promoters: the Arabidopsis Ubiquitin promoter 3 (UBQ3) (Norris et al. 1993), the CaMV 35S promoter (Odell et al. 1985) and domain B duplicated CaMV 35S (D35S) (Kay et al. 1987) The promoters mannopine synthase (mas2) (Feltkamp et al. 1995) from Agrobacterium tumefaciens and the rooting loci gene promoter rolD (Leach and Aoyogi 1991) from A. rhizogenes were also evaluated, since previous reports indicated that these promoters had enhanced root activity. Relative strengths of these promoters were measured in the leaves, lateral roots and tap roots of mature greenhouse-grown carrot plants as well as in vitro grown calli, leaves and roots of five independently-derived transgenic lines for each promoter.
Materials and methods
Plasmid DNA and plant transformation
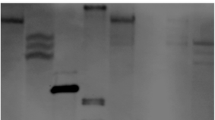
The pCAMBIA 1391 Z plant transformation vector containing the hygromycin resistance gene under control of the CaMV 35S promoter and the two exons of uidA with the catalase intron in front of the puc9 multi-cloning site (MCS) with a nopaline synthase terminator (CAMBIA, Australia) was used. Plasmid DNA was isolated from 2 ml of overnight grown cultures of E. coli using the Qiagen Qiaquick spin column isolation kit (Qiagen, Maryland, USA). The CAMV 35S, D35S (Dr. Shawn Mansfield, University of British Columbia), UBQ3 (Syngeta, Canada), mas2 (Dr. Stephane Garberk, INRA, France) and rolD (Francesca Leach, INRA, France) promoters were cloned into the HindIII and BamHI sites of the MCS (Fig. 1). The ligated plasmids were transformed by electroporation into electromax LBA4404 competent A. tumefaciens cells (Invitrogen, Carlsbad, CA, USA) using established methods (Wally et al. 2006). Sterile ‘Nantes Coreless’ carrot petiole explants were transformed and regenerated as described by Wally et al. (2006). The transgenic callus was maintained on full-strength Murashige and Skoog (MS) medium (Murashige and Skoog 1962) supplemented with 100 mg/l hygromycin and 0.5 mg/l 2,4-D. Regenerated plantlets were maintained on hormone-free half-strength MS with 100 mg/l hygromycin, and transferred to soil and grown in the greenhouse (Wally et al. 2006). Root and leaf tissues from 4–5 month old greenhouse-grown plants were harvested and used for analysis of GUS expression and molecular analysis. In addition, lateral root and leaf tissues from 4–8 week old tissue-culture derived plantlets were also included in the analysis.
Plasmid DNA constructs used for Agrobacterium transformation of carrot. From top to bottom the uidA fusion constructs in pCambia1391Z plasmid, under the control of CaMV 35S promoter (pC35S::GUS), enhanced 35S promoter (pCD35S::GUS), the A. tumefaciens mannopine synthase (mas2) promoter (pCmas2::GUS), the A. rhizogenes rooting loci gene (rolD) promoter (pCrolD::GUS) and the Arabidopsis ubiquitin (UBQ3) promoter (pCUBQ3::GUS). Restriction sites are indicated on figure BamHI (B) and HindIII (H)
Confirmation of gene integration
The presence of the hygromycin phosphotransferase (hph) gene using PCR, was used as the first step for confirmation of transformation. Total genomic DNA was isolated from 200 mg (fresh weight) of leaf tissue that was lyophilized prior to being extracted using established protocols (Wally et al. 2006). The primers used to amplify a 1025 bp fragment of the hph gene were hph-F2 (5′-CTA TTT CTT TGC CCT CGG AC-3′) and hph-R2 (5′-AAG CCT GAA CTC ACC GCG AC-3′). Each reaction (25 μl) contained 50 ng carrot DNA, 50 pM primers, 0.5 units Taq polymerase (Invitrogen) and 1.5 mM MgCl2. The PCR conditions included a 55°C annealing temperature and proceeded for 35 cycles using a PTC-200 thermocylcer (MJ Research, Waltham, MA, USA). Subsequently, Southern analysis was used to demonstrate integration of the uidA gene. Total genomic DNA (12 μg) was digested for 16 h at 37°C with 80 units of either BamHI or HindIII (Promega, Madison, WI, USA), to cut at sites that are both unique in the transgene construct . The digested DNA was run on a 1.2% agarose TBE gel for 20 h and transferred to a nylon membrane under alkaline conditions using established protocols (Sambrook et al. 1989). The blots were probed with a 32P labelled 740 bp fragment of the uidA gene generated by PCR using the primers GUSA-F1 (5′- TGA AGA TGC GGA CTT ACG TG -3′) and GUSA-R1 (5′- CCA GCC ATG CAC ACT GAT AC-3′) under the above PCR conditions with a 58°C annealing temperature. Random primers were used to label the probe using [α-32P] dCTP and Prime-A-gene kit (Promega) following manufacturers protocols. The blots were hybridized with the probes for 16 h at 65°C following a 2 h pre-hybridization at 55°C using Ekono hybridization buffer (Research Products International Corp, Mt. Prospect, IL, USA). Following hybridization, the blots were washed 3 times at room temperature with 2× SSC, 0.1% (w/v) SDS for 5 min each, followed by 2 washes with 1× SSC, 0.1% (w/v) SDS at 60 and 65°C, respectively, for 20 min (Sambrook et al. 1989). The blots were exposed to X-ray film at −80°C for 3–7 days with an intensifying screen.
GUS expression
Histochemical staining of petioles, leaves, lateral and tap roots was performed according to Jefferson et al. (1987). The plant tissues were placed in fixative (10 mM MES, pH 5.6, 0.3 M mannitol and 0.3% formaldehyde) on ice for 30 min, followed by washing in 50 mM NaH2PO4, pH 7.0. The samples were vacuum infiltrated (20 mBar) for 15 min and stained overnight at 37°C in 50 mM NaH2PO4 , 0.5% (v/v) Triton X-100 and 1 mM 5-bromo-3-chloro-3-indoyl-β-D-glucuronide cyclohexamide sodium salt (X-gluc, Inalco Pharmaceuticals, Italy) dissolved in dimethyl-formamide. Petioles, leaves and orange roots were destained by repeated washings with 70% ethanol for 24–48 h, or until all the coloured pigments were removed. Sections were visualized using 40× magnification under a light microscope with white light (Zeiss, Axioskop, Germany).
Specific expression of GUS for each promoter was determined using five independent lines derived from different transformation events and confirmed to be transformed by Southern analysis. Tissue samples were taken a minimum of three times at 1-week intervals from mature greenhouse-grown shoots and roots, callus and tissue-cultured shoots and roots. GUS activity was determined according to Jefferson et al. (1987) by measuring the accumulation of the fluorescent substrate 4-methylumbelliferone (4-MU) cleaved from 4-methylumbelliferone-glucuronide (4-MUG). Samples of 250−750 mg fresh weight of the various carrot tissues were macerated using an ice-cold mortar and pestle with a pinch of sea sand with a 2:1 (v/w) buffer:tissue ratio. The extraction buffer contained 50 mM NaH2PO4, pH 7.0, 10 mM EDTA, 0.1% (v/v) Triton X-100, 0.1% (w/v) sodium lauryl sarcosine, 4% (w/v) polyvinylpolypropadine (PVPP) and 5 mM dithioretitol (DTT) (Jefferson et al. 1987). Macerated samples were centrifuged twice at 4°C for 15 min at 14,000g, and the supernatant was transferred to a new 1.5 ml microcentrifuge tube. Clarified protein extracts were quantified using the Bradford reagent (Sigma, USA) using a Bio-Tek 1200 microplate reader (Fisher, USA). Five μg of protein extract was assayed in a total volume of 50 μl in a 1 mM MUG solution (50 mM NaH2PO4, 10 mM EDTA, 0.1% (v/v) Triton X-100 and 5 mM DTT) on a 96 well micro-titre plate. The reactions were stopped at 1.5, 3 and 4.5 h by adding 250 μl of 0.2 M sodium carbonate. Fluoresence was measured using a 96 well fluorometer (Isoplate-96 Perkin-Elmer Corporation, Norwalk, CT, USA) set at 365 nm for excitation and 455 nm for emission. Non-transformed ‘Nantes Coreless’ carrot roots and leaves served as negative controls/blanks for both histochemical and fluorometric assays.
Results
Molecular analysis
Of the 76 total lines confirmed to be positive for the presence of the hph gene by PCR (not shown), 70 contained one to three copies of the uidA gene, as determined by HindIII and BamHI digestion and Southern blot hybridization (Fig. 2). Up to ten independent lines, with 1–3 uidA copies, from each of the five promoter constructs were then analyzed for GUS activity by histochemical staining (Fig. 3). The majority (65 of 70) had detectable levels of X-gluc staining, and the five visually strongest expressing lines from each promoter were selected for further analysis. The percentage of regenerated plantlets that grew on MS medium with 100 mg/l hygromycin and showed uidA expression was 100% for UBQ3 and mas2, 80% for rolD, 75% for 35S and 50% for D35S. The number of uidA copies had no correlation to the intensity of GUS activity (data not shown).
Southern hybridization analysis of transgenic carrot lines containing the uidA fusion gene under control of either the rolD, mas2, UBQ3, CaMV 35S or D35S promoters. Genomic DNA was digested with BamHI (a) or HindIII (b), the DNA blot was hybridized with the 740 bp uidA gene fragment. Three different lines were shown for each promoter. Size markers from the 1 Kb+ (Invitrogen) ladder are shown on the left. NT non transgenic control plant
Visualization of GUS expression
There were significant differences in the intensity of staining of carrot tissues with X-gluc in plantlets containing the different constitutive promoter constructs. Strong GUS activity was found in the leaves of plants expressing uidA under control of the D35S, UBQ3 and 35S promoters. All leaf tissues stained very darkly, including the trichomes, mesophyll and vascular tissues (Fig. 3). There were only slight visually observable differences between the promoters, with the D35S lines appearing stronger overall. When the lateral roots were stained, these three promoters provided expression throughout the length of the root, including the root tip, root hairs and the vascular tissues (Fig. 3). Cross-sections of the tap roots revealed similar patterns of staining; however, the 35S promoter provided slightly less intense and non-uniform staining throughout the length of the root compared to UBQ3 and D35S (Fig. 3). There was intense GUS staining in the root parenchyma cells, phloem rays, xylem and cambium in plants containing the 35S, D35S and UBQ3 promoters (Fig. 3).
Plantlets expressing uidA under control of the mas2 and rolD promoters showed significant differences in staining intensities in different tissue types of greenhouse-grown plants compared to the smaller tissue culture-grown plants. Only slight staining was visible in the veins of leaves of rolD plants (Fig. 3), while the mas2 promoter showed weak staining throughout the leaves, with more staining near the tip of the leaf. (Fig. 3). In mas2 lines, the cotyledons and hypocotyl tissues exhibited GUS activity, while no activity was seen in these tissues in the rolD lines (not shown). Lateral roots of mas2 and rolD lines stained darkly, with enhanced staining observed at root tips, vascular bundles and root hairs. The rolD roots stained slightly darker than roots of the mas2 lines; however, expression was still significantly lower when compared to the constitutive promoters. The taproots of plants with mas2 and rolD promoters were also stained throughout the different tissue types, with the weakest staining observed in the periderm and more intense staining in the phloem and cambium (Fig. 3).
Quantification of promoter strength
GUS enzyme activity assays were performed on the five lines selected for each of the different promoter constructs. For the CaMV 35S promoter, GUS activity was lowest in callus tissue and highest in leaves of tissue-cultured plants (Fig. 4). High GUS activity was observed in the roots and leaves of mature greenhouse-grown plants, which was similar to the levels measured in the roots of tissue-cultured plants. There was more variation among individual 35S promoter plant lines in leaf expression, compared to root or callus tissue.
Specific activity of GUS in callus, tissue-cultured (TC) roots, tissue-cultured leaves, greenhouse-grown (GH) roots and leaves of transgenic carrot plants expressing the uidA gene under control of five promoters (D35S, UBQ3, 35S, mas2 or rolD). Values represent the mean specific activity in five individual lines for each promoter, with a minimum of three replicates ± standard error for each transgenic line
With the D35S promoter, there was very high GUS activity in all tissue types examined, with highest activities in mature greenhouse-grown leaves and in leaves and roots of tissue culture-grown plants (Fig. 4). The lowest activity was found in callus tissue, which had approximately 50% of the GUS activity compared to the leaves. The D35S promoter had higher GUS activity than the 35S promoter in all tissues examined, ranging from an average of 1.5-fold higher activity in tissue-culture grown leaves to an average of over threefold higher activity in callus tissues (Table 1).
With the UBQ3 promoter, the highest GUS activity was observed in the roots of mature greenhouse-grown plants, with an average of 2.5-fold higher activity than the 35S promoter. The GUS activity was also significantly higher compared to the 35S promoter in the callus tissue, with nearly an average increase of twofold. The UBQ3 promoter GUS activity was significantly lower than 35S promoter in the leaves of tissue-cultured plants, and was similar to that in roots of tissue culture-grown plants. However, in greenhouse-grown plants, the roots had an average of nearly threefold higher GUS activity compared to the leaves.
With the mas2 promoter, the average GUS activity was highest in leaves of tissue culture-grown plants (Fig. 4). There was also fairly high GUS activity in the callus tissue with levels similar to that of the 35S promoter (Table 1). There was an average of fourfold higher activity in tissue-culture grown leaves as compared to roots of the mas2 lines examined. However, in mature greenhouse-grown plants, there was 50% less GUS activity in leaves compared to roots. There was an average of twofold higher activity in mature roots compared to young tissue-culture grown roots. There was approximately fourfold higher activity in young tissue-culture grown leaves compared to mature greenhouse-grown leaves.
With the rolD promoter, GUS activity was highest in callus tissues (Fig. 4). The activity was relatively low in all of the other tissues tested, with GUS levels at only 3–12% of that in the roots and leaves of 35S promoter plants (Table 1). There were no significant differences in GUS activity between tissue-culture grown roots and leaves. However, in mature greenhouse-grown roots, there was nearly a fourfold increase in GUS activity compared to the leaves. GUS activity was similar in greenhouse-grown roots and tissue-cultured roots.
Discussion
The CaMV 35S promoter is the most widely used promoter for transgene expression in plants, and provides very high constitutive levels of expression in dicotyledon species and slightly weaker expression in monocotyledon species (Gandhi et al. 1999). The enhanced D35S promoter has a duplication of the −343 to −90 domain B which has been shown to enhance transgene expression by up to tenfold when compared to the 35S promoter (Kay et al. 1987). As with previous reports from other dicotyledon plants (Comai et al. 1990; Holtorf et al. 1995), the CaMV 35S and enhanced D35S promoters were found to be the strongest promoters overall in carrot tissues. The duplication of the domain B increased the overall expression levels in carrot by an average of 3.1-fold in callus, 2.7 and 2.1-fold in roots, and 1.5 and 2.9-fold in leaves under different growing conditions. These increases are within the ranges reported for other transgenic plants (Potenza 2004). Both 35S and D35S promoters provided strong GUS expression in all carrot tissues examined, including leaves, petioles, cotyledons, lateral roots and tap roots. Both promoters had highest activity in leaves, followed by roots and callus tissue. These findings are consistent with those from Arabidopsis and tobacco, where petiole and leaf expression levels were significantly higher than in roots (Holtorf et al. 1995; Malik 2002) but differs from the expression levels reported in gladiolus and alfalfa (Kamo 2003; Samac et al. 2004), in which the 35S promoter had the highest activity in roots. Overall, the D35S promoter provided the highest level of gene expression in all carrot tissue types tested, except for mature roots.
Ubiquitin is highly conserved across plant species, is highly abundant in the cytoplasm and is involved in many crucial cellular processes. Many of the Ubiquitin genes are constitutively expressed, including the maize (ubi-1) and Arabidopsis (UBQ3) genes (Christensen et al. 1992; Norris et al. 1993). Typically, monocotyledon plants have highest constitutive levels of transient gene expression with monocot-derived actin or ubiquitin promoters, while dicots typically have highest expression with viral promoters or dicot-derived ubiquitin promoters (Horloft et al. 1995; Gandhi et al. 1999; Kamo 2003). In this study, the Arabidopsis UBQ3 promoter provided significantly higher expression levels compared to the CaMV 35S promoter in callus (2.0-fold higher) and mature greenhouse-grown roots (2.5-fold higher). The UBQ3 driven GUS expression levels were very similar to the 35S promoter in tissue-cultured young roots, and slightly lower in both tissue-cultured (0.65 of the 35S) and mature greenhouse leaves (0.7 of the 35S). These findings are similar to those made in transiently-expressing Arabidopsis, where comparable expression was observed in the leaves with genes driven by the 35S and UBQ3 promoters (Norris et al. 1993). The UBQ3 driven GUS expression was very prominent in carrot root tissues, with enhanced activity when compared to the 35S and D35S promoters. When quantified, the GUS activity provided by the UBQ3 promoter in mature roots was significantly higher than that of the D35S promoter. The heightened overall root activity indicates that UBQ3 is ideal for expressing proteins in tap roots, and for post-harvest roles, including suppression of post-harvest diseases by over-expression of pathogenesis-related proteins.
The mannopine synthase gene is a bi-directional gene from Agrobacterium tumefaciens, which requires the activity of a mannopine conjugase (mas2) and reductase (mas1). The mas2 promoter has been analyzed in detail, and contains two enhancer sequences and is reported to provide significantly higher levels of gene expression than the mas1 promoter, which contains only a single enhancer sequence (Guevera-Garcia et al. 1999). In this study, the mas2 promoter drove high levels of GUS expression in callus tissue cultured in vitro, with GUS expression levels approaching that of the 35S promoter. The mas promoters are sensitive to auxin and activity increases when the auxin:cytokinin ratio is increased (Langridge et al. 1989). The carrot calli were maintained on medium containing 2,4-D (0.5 mg/l); therefore, enhanced expression in the callus tissue was expected. Similar to transgenic potato and tobacco transformed with mas2::uidA fusion constructs, there was higher GUS activity in mature greenhouse-grown carrot roots compared to the shoot or leaf tissues (Feltkamp et al. 1995). However, these levels were substantially less than in transgenic tobacco, where root activity levels exceeded those of the 35S promoter (Comai et al. 1990). Our results also differed from the mas2 activity reported in transgenic rapeseed varieties, which exhibited high mas2 driven GUS activities in the leaves, with reduced activity in roots, substantially lower than for the 35S promoter (Pauk et al. 1995). In carrot, the mas2 promoter is potentially useful for use in suspension culture bio-reactors, reflecting the high GUS expression levels observed in callus tissues, and for expression of transgenic proteins in mature roots.
The rooting loci gene (rolD) isolated from the root-inducing plasmid of Agrobacterium rhizogenes has been reported to drive high levels of expression in both the leaves and roots of young transgenic tobacco seedlings (Leach and Aoyagi 1991) and transgenic pea (Fei et al. 2003). The transgenic carrots examined had very low levels of GUS expression in all of the tissues tested. Strongest expression was seen in the callus, roots and leaves of tissue-cultured plants. In mature plants, there was fourfold higher activity in the roots compared to the leaves. Conversely, mature transgenic tobacco containing the rolD::uidA construct had 30-fold higher GUS expression in roots compared to the shoots (Elmayan and Tepfer 1995). In carrot, the overall strength of rolD in mature root tissues was substantially lower than that reported from other plants. Transgenic Gladious plants exhibited strong GUS root expression with rolD, and expression levels were comparable to that of the 35S promoter (Kamo 2003). In transgenic N. plumbaginifolia, a 3–5 fold higher root expression was seen with rolD compared to that of the 35S promoter (Fraisier et al. 2000). Despite lower GUS activity, histochemical staining of GUS was still evident with the rolD promoter in carrot taproots. However, these findings indicate that the rolD promoter will likely not be very useful for expressing transgenic proteins in carrots.
In conclusion, the D35S promoter provided highest levels of GUS activity in carrot leaves followed by the 35S promoter, while the UBQ3 promoter from Arabidopsis provided high levels of GUS activity in all tissues, especially in the tap roots. The previously reported root enhanced promoters mas2 and rolD provided proportionally lower levels of GUS activity in mature carrot roots. Understanding the GUS expression profiles of the different promoters will allow for more precise control of expression levels and organ targeting in both in vitro and field grown transgenic carrots.
References
Ammirato PV (1986) Carrot. In: Evans DA, Sharp WR, Ammirato PV (eds) Handbook of plant cell culture, vol 4. Macmillan, New York, pp 457–499
Bouche FB, Marquet-Blouin E, Yanagi Y, Steinmetz A, Muller CP (2003) Neutralising immunogenity of a polypitote antigen expressed in transgenic food plant: a novel antigen to protect against measles. Vaccine 21:2065–2072
Chen WP, Punja ZK (2002) Transgenic herbicide- and disease-tolerant carrot (Daucus carota L.) plants obtained through Agrobacterium-mediated transformation. Plant Cell Rep 20:929–935
Christensen AH, Sharrock RA, Quail PH (1992) Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol 18:675–689
Comai L, Moran P, Maslyar D (1990) Novel and useful properties of a chimeric plant promoter combining CaMV 35S and MAS elements. Plant Mol Biol 15:373–381
Elmayan T, Tepfer M (1995) Evaluation in tobacco of the organ specificity and strength of the rolD promoter, domain A of the 3 s promoter and the 35S2 promoter. Transgenic Res 4:388–396
Fei H, Chaillou S, Hirel B, Mahon JD, Vessey JK (2003) Overexpresssion of a soybean cytosolic glutamine synthase gene linked to organ-specific promoters in pea plants grown in different concentrations of nitrate. Planta 216:467–474
Feltkamp D, Baumann E, Schmalenbach W, Masterson R, Rosahal S (1995) Expression of the mannopine synthase promoter in roots is dependent on the mas elements and correlates with high transcript levels of mas-binding factor. Plant Sci 109:57–65
Fraisier V, Gojon A, Tillard P, Daniel-Vedele F (2000) Constitutive expression of a putative high-affinity nitrate transporter in Nicotiana plumbaginifolia: evidence for post-transcriptional regulation by a reduced nitrogen source. Plant J 23:489–496
Gandhi R, Maheshwari SC, Khurana P (1999) Transient gene expression and influence on foreign gene expression in Arabidopsis thaliana. In Vitro Cell Dev Biol Plant 35:232–237
Gilbert MO, Zhang YY, Punja ZK (1996) Introduction and expression of chitinase encoding genes in carrot following Agrobacterium-mediated transformation. In Vitro Cell Dev Biol Plant 32:171–178
Guevara-Garcia A, Lopez-Bucio J, Herrera-Estrella L (1999) The mannopine synthase promoter contains vectorial cis-regulatory elements that act as enhancers and silencers. Mol Gen Genet 262:608–617
Holtorf S, Apel K, Bohlmann H (1995) Comparison of different constitutive and inducible promoters for the overexpression of transgenes in Arabidopsis thaliana. Plant Mol Biol 29:637–646
Jayaraj J, Punja ZK (2007) Combined expression of chitinases and lipid transfer protein genes in transgenic carrot plants enhances resistance to foliar fungal pathogens. Plant Cell Rep 26:1539–1546
Jayaraj J, Devlin R, Punja Z (2007) Metabolic engineering of novel ketocarotenoid production in carrot plants. Transgenic Res. doi:10.1007/s11248-007-9120-0
Jefferson RA, Kavanagh RA, Bevan MW (1987) GUS-fusions: β-glucuronidase as a sensitive and versatile fusion marker in higher plants. EMBO J 6:3901–3907
Kamo KK (2003) Long-term expression of the uidA gene in Gladiolus plants under control of either the ubiquitin, rolD, mannopine synthase, or cauliflower mosaic virus promoters following three seasons of dormancy. Plant Cell Rep 21:797–803
Kamo K, Blowers A (1999) Tissue specificity and expression level of GUSA under rolD, mannopine synthase and translation elongation factor 1 subunit alpha promoters in transgenic Gladiolus plants. Plant Cell Rep 18:809–815
Kay R, Chan A, Daly M, McPerson J (1987) Duplication of a CaMV 35S promoter sequences creates a strong enhancer for plant genes. Science 236:1299–1302
Komamine A, Murata N, Nomura K (2005) Mechanisms of somatic embryogenesis in carrot suspension cultures—morphology, physiology, biochemistry, and molecular biology. In Vitro Cell Dev Biol Plant 41:6–10
Kumar S, Dhingra A, Daniell H (2004) Plastid-expressed betaine aldehyde dehydrogenase gene in carrot cultured cells, roots, and leaves confers enhanced salt tolerance. Plant Physiol 136:2843–2854
Langridge WHR, Fitzgerald KJ, Koncz C, Schell J, Szalay AA (1989) Dual promoter of Agrobacterium tumefaciens mannopine synthase genes is regulated by plant growth hormones. Proc Natl Acad Sci USA 86:3219–3223
Leach F, Aoyagi K (1991) Promoter analysis of the highly expressed rolC and rolD root-inducing genes of Agrobacterium-rhizogenes-enhancer and tissue-specific DNA determinants are dissociated. Plant Sci 79:69–76
Malik K, Wu K, Li XQ, Martin-Heller T, Hu M, Foster E, Tian L, Wang C, Ward K, Jordan M, Brown D, Gleddie S, Simmonds D, Zheng S, Simmonds J, Miki B (2002) A constitutive gene expression system derived from the tCUP cryptic promoter elements. Theor Appl Genet 105:505–514
Marquet-Blouin E, Bouche FB, Steinmetz A, Muller CP (2003) Neutralizing immunogenicity of transgenic carrot (Daucus carota L.)-derived measles virus hemagglutin. Plant Mol Biol 51:459–469
Melchers LS, Stuiver MH (2000) Novel genes for disease-resistance breeding. Curr Opin Plant Biol 3:147–152
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–492
Ni M, Cui D, Einstein J, Narasimhulu S, Vergara CE, Gelvin SB (1995) Strength and time specificity of chimeric promoters derived from the octopine and mannopine synthase genes. Plant J 7:661–676
Norris SR, Meyer SE, Callis J (1993) The intron of Arabidopsis thaliana polyubiquitin genes is conserved in location and is a quantitative determinant of chimeric gene expression. Plant Mol Biol 21:895–906
Odell JT, Nagy F, Chua NH (1985) Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. Nature 313:810–812
Pauk J, Stefanov I, Fekete S, Borge L, Karsai I, Feher A, Dudits D (1995) A study of different (CaMV 35S and mas) promoter activities and risk assessment of field use in transgenic rapeseed plants. Euphytica 85:411–416
Potenza C, Aleman L, Sengupta-Copalan C (2004) Targeting transgene expression in research, agricultural, and environmental applications: promoters used in plant transformation. In Vitro Cell Dev Biol Plant 40:1–22
Samac DA, Tesfaye M, Dornbusch M, Saruul P, Temple SJ (2004) A comparison of constitutive promoters for expression of transgenes in alfalfa (Medicago sativa). Transgenic Res 13:349–361
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: A laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Schledzewski K, Mendel RR (1994) Quantitative transient gene expression: comparison of the promoters for maize polyubiquitin1, rice actin1, maize-derived Emu and CaMV 35S in cells of barley, maize and tobacco. Transgenic Res 3:249–255
Takaichi M, Oeda K (2000) Transgenic carrots with enhanced resistance against two major pathogens, Erysiphe heraclei and Alternaria dauci. Plant Sci 153:135–144
Wally O, Jayaraman J, Punja ZK (2006) Carrot (Daucus carota L.) transformation. In: Wang K (eds) Methods in molecular biology. Agrobacterium protocols, vol. 44. Humana Press Inc, NJ, pp 3–15
Zimmerman JL (1993) Somatic embryogenesis: a model for early developments in higher plants. Plant Cell 5:1411–1423
Acknowledgments
Funding for this research was provided by NSERC (Natural Sciences and Engineering Research Council of Canada), Discovery Grants program. We would like to thank Dr. Jin-Gui Chen and Dr. Shucai Wang (University of British Columbia, Canada) for use of their 96-well fluorometer, Dr. Jim Mattsson (Simon Fraser University) for use of a digital imaging microscope, and Lisa Leippi for assistance in preparation of graphs and photos.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Harwood.
Rights and permissions
About this article
Cite this article
Wally, O., Jayaraj, J. & Punja, Z.K. Comparative expression of β-glucuronidase with five different promoters in transgenic carrot (Daucus carota L.) root and leaf tissues. Plant Cell Rep 27, 279–287 (2008). https://doi.org/10.1007/s00299-007-0461-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-007-0461-1