Abstract
We examined the effect of ectopic expression of WUS on the morphology of tobacco seedlings and the segments in vitro. WUS was amplified from Arabidopsis cDNA and introduced into the tobacco genome under the transcriptional control of the β-estradiol-inducible expression system. When 1-week-old transgenic seedlings were cultured in the presence of β-estradiol, only the root tip region developed bulbous tissues followed by shoot formation and plant regeneration, suggesting its applicability for improving the strategy of micropropagation in recalcitrant species. Evident abnormality was not observed in the cotyledons, hypocotyl nor root except for the tip. However, ectopic WUS seemed to be functional in those parts through the observation of gene expression and the behavior of cultured segments. Small root segments with a root tip treated with β-estradiol also showed bulbing but no shoots unless exogenous cytokinin was supplied. These findings suggest the existence of unknown factors regulating ectopic WUS function in the seedling.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Since the early observations of in vitro regeneration from somatic cells (Skoog and Miller 1957; Steward et al. 1958; Takebe et al. 1971), cytokinin and auxin have been always critical factors to induce meristematic cells leading to regeneration. However, recently a number of reports demonstrated that regeneration from somatic cells could be induced by ectopic expression of various genes coding for transcription factors (reviewed by Zhang 2003), including LEAFY COTYLEDON 1 (LEC1, Lotan et al. 1998), LEAFY COTYLEDON 2 (LEC2, Stone et al. 2001), LEC1-LIKE (Kwong et al. 2003), WUSCHEL (WUS, Laux et al. 1996; Zuo et al. 2002; Gallois et al. 2004) and BABY BOOM (BBM, Boutilier et al. 2002). These suggest a possibility of establishing an alternative method for regeneration of general plant species without using phytohormones.
A reliable expression system of a transgene, using a chimeric transcription factor named XVE, was developed by Zuo et al. (2000). The gene coding for the chimeric transcription factor was constructed by fusing the DNAs coding for the DNA-binding domain of the bacterial repressor LexA, the transactivating domain of VP16 and the regulatory region of the human estrogen receptor. The XVE transcription factor was activated by exogenous estrogen and then it increased the expression level of the transgene under the control of a promoter consisting of eight copies of the LexA operator fused upstream of the (−46)35S minimal promoter. The XVE system was successfully applied to screening for a gene whose ectopic expression causes somatic embryogenesis (Zuo et al. 2002). The gene isolated through the screening was identified as WUS (Laux et al. 1996). In Arabidopsis zygotic embryogenesis, WUS is expressed in the 16-cell-stage embryo prior to meristem development within the region that originates the apical meristem (Mayer et al. 1998).
In this study, we examined the effect of ectopic expression of WUS and LEC2 on the morphology of tobacco seedlings and the segments using the XVE system.
Materials and methods
Molecular manipulation and plant materials
The DNA clones coding for WUS and LEC2 were obtained by PCR using first stranded cDNAs synthesized from mRNA prepared from inflorescence of Arabidopsis thaliana var. Columbia. Specific primers for WUS and LEC2 were designed from the available sequence data supplied by DDBJ (http://www.ddbj.nig.ac.jp/) as follows; 5′-ctcgagCTTATTTACCGTTAACTTTGTGAA (small letters indicate Xho I restriction site) and 5′-actagtCACATAACGAGAGATAACTAGTTAAC (small letter, Spe I site) for WUS, and 5′-gtcgacAATGGATAACTTCTTACCCTTTCC (small letter, Sal I site) and 5′-actagtCTATTTTCATAAGGGGGTTATG (small letter, Spe I site) for LEC2. The PCR fragments of WUS (size: 1,208 bp) and LEC2 (size: 1,332 bp) were directly cloned into the vector, pER8 coding XVE (Zuo et al. 2000). The sequences of the resulting constructs were confirmed by DNA sequencing and then transferred into Agrobacterium tumefaciens (strain LBA4404) by electroporation. Agrobacterium-mediated transformations of Nicotiana tabacum cv. Samsun were performed with leaf discs as previously reported (Kyo et al. 2000). The resulting transgenic tobacco plants were selected in the presence of 50 mg l−1 of hygromycin and grown to mature plants to harvest seeds. They were referred to as XVE::WUS and XVE::LEC2 lines, respectively. We obtained six and five lines transgenic for WUS and LEC2, respectively. Among them three XVE::WUS lines and two XVE::LEC2 lines showed abnormality in morphology in the presence of β-estradiol (Sigma–Aldrich, MI, USA). One representative of each of these lines was selected and backcrossed with wild tobacco to harvest their seeds for experiments.
Seedling morphology
Seeds of the transgenic lines were sterilized, sown on LS (Linsmaier and Skoog 1965) medium with 0.8% agar and hygromycin (50 mg l−1) and kept in the dark at 25°C for 1 week. Seedlings showing normal root growth on the medium were selected and transferred to LS agar medium with or without β-estradiol to observe their morphological abnormality.
Segment culture
Young seedlings were cut into segments; cotyledon, hypocotyl with or without shoot apical meristem (SAM), root and root tip with root apical meristem (RAM). These segments were cultured on LS agar medium supplemented with β-estradiol and/or 6-benzyladenine (BA) at 25°C in the dark for 1 month.
Expression analysis
Young seedlings were cultured on LS liquid medium with or without β-estradiol. After the treatment with β-estradiol (for 24 h or 5 days) seedlings were harvested and cut into segments; cotyledon with SAM, hypocotyl and root with RAM. These segments were immediately homogenized for RNA extraction followed by RT-PCR according to the method previously reported (Kyo et al. 2000). The sequences of specific primers for RT-PCR were 5′-CTTATTTACCGTTAACTTTGTGAA and 5′-GGYGGCAACTAYACTAGRAGTGG for WUS; 5′-GCTCTGGATATGGCCGACTTC and 5′-GTAGCCAGCAGCATGTCGAAG for XVE; and 5′-TCCTCCTCCTATGATGATGCCT and 5′-TTCACATCAACCTCCTCTTCAGA for NTH15 (Tamaoki et al. 1997).
Results
Seedling morphology
The 1-week-old seedlings transgenic for WUS gene (XVE::WUS) were transferred onto LS agar medium with or without β-estradiol. Within 3 days, the seedlings formed bulbous tissues at their root tip regions in the presence of β-estradiol but not in its absence (Fig. 1a). Under the 16 h light/8 h dark condition, the bulbous tissues generated green spots within 2 weeks, and they developed to shoots in the following culture (Fig. 1c–e). In the dark condition also, bulbing in root tip regions and regeneration of shoots were observed (Fig. 1f–h), though the frequency of shoot formation was lower than that in a light condition. These results suggest that light is not required for the bulbing but promotes the shoot formation. The developed shoots were detached from original tissue and cultured on LS agar medium under the light condition (Fig. 1i, j). They generated roots and developed to adult plants with fertility (Fig. 1j, k). Their offspring contained individuals that show abnormalities in the presence of β-estradiol as their mother plants (data not shown).
Aspects of the response of the seedling transgenic for XVE::WUS to β-estradiol. a The root tip region after a 3-days culture on the medium without (left) and with 1 μM β-estradiol (right). Bar 150 μm. b Seedlings with severe abnormality, developed from the seeds sown on the medium with β-estradiol and kept for 18 days in the dark. Bar 10 mm. c–h Seedlings cultured on the medium with β-estradiol for 5 days (c and f), 10 days (d and g) and 21 days (e and h). The seedlings on the left in c and f were cultured on the medium without β-estradiol as controls. The seedlings were kept in the light/dark cycle (c–e) and dark (f–h) condition, respectively. c, d and f are shown at the same magnification and the bar in c shows 10 mm. e, g and h are shown at the same magnification and the bar in e shows 10 mm. i–k Shoot formation from bulbous tissue generated on the root tips and their growth to plants. i Different stages of bulbous tissue and shoot generated from root tip regions of seedling cultured on the medium with the inducer. Bar 5 mm. j Regenerated shoots were detached from original plate and cultured for one month on LS agar medium without the inducer in a 9-cm dish. k Plants grown on the soil in glass pots. Bar 50 mm
When seedlings of XVE::LEC2 line were cultured on the medium with β-estradiol, primary roots curved and became thicker within 3 days. The roots continued to grow and coiled themselves generating long root hairs. Gradually those long root hairs covered the coiled root region and cotton-like structures appeared (Fig. 2). Also in this line, morphological abnormality was limitedly observed only in the root tip region of treated seedlings. Stone et al. (2001) reported that over expression of LEC2 in transgenic Arabidopsis induced somatic embryogenesis around the SAM. However, in our study, no regeneration was observed from those seedlings.
In both transgenic lines, morphological abnormalities were observed in their root tip regions but not in the aerial parts even when treated with β-estradiol during the first period of culture (Figs. 1, 2). When the seedlings were imbibed under liquid medium with various concentrations of β-estradiol (0.1–10 μM), the morphological abnormality was limited to the root tip region (data not shown).
Expression of the transgenes
We examined the expression of transgenes in different parts of XVE::WUS seedlings cultured on the medium with or without β-estradiol. As shown in Fig. 3, the constitutive expression of XVE gene was detected (amplified fragment size: approx. 600 bp) in all parts of treated and untreated seedlings. In contrast, expression of WUS gene was detected (amplified fragment size: approx. 900 bp) only in the seedlings treated with β-estradiol. In the presence of β-estradiol, WUS was expressed in all parts of the seedling, but as described above, morphological abnormality was observed only in the root tip region. These results suggest that the WUS function in the root tip region was not suppressed for an unknown reason, although it was suppressed in other regions.
Expression analysis of transgenes in XVE::WUS seedlings. Young seedlings were cultured in the presence (+) or absence (−) of β-estradiol for 24 h. cDNAs were separately prepared from the three types of segments; cotyledon with SAM (C), hypocotyl (H) and root with RAM (R). The approximate length of amplified transcript is indicated at the left. M: 0.1 kbp DNA ladder
Segment culture
Segments from XVE::WUS seedlings were cultured as described in Materials and methods. In the absence of β-estradiol, cotyledons, hypocotyl and root segments generated adventitious or lateral roots, and root segments with RAM continued to grow (Fig. 4a–e). In the presence of β-estradiol, both root generation and the root growth were suppressed (Fig. 4f–j) probably due to the expression of WUS though the translation for WUS was not examined. Figure 5 shows the frequency of root generation and root growth in each tissue summarizing the results in this experiment (20 segments each). The suppression was observed in each part in the presence of the inducer. Using the segments from non-transgenic seedlings, the suppression was not observed in the presence of the inducer (supplementary Fig. 1).
Segment culture of XVE::WUS seedlings. The upper (a–e) and lower (f–j) lanes represent segment cultures under the absence or presence of β-estradiol, respectively. Young seedlings were cut into segments; cotyledon (a, f), upper part of hypocotyl with SAM (b, g), lower part of hypocotyl (c, h), upper part of root (d, i) root tip with RAM (e, j) and cultured for 1 month. Bars 5 mm
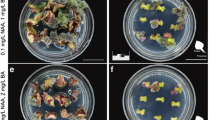
Bulbing of the root tip region was observed in the presence of β-estradiol (Fig. 4j) as in the seedlings (Fig. 1c, f), but no shoot regeneration was observed even after 2 months of culture. This suggested that shoot formation requires some endogenous hormones or specific nutrients supplied from the other regions including the aerial parts. We preliminarily examined the effects of auxin, cytokinin, abscisic acid and gibberellin on the growth of the segments from XVE::WUS seedlings (data not shown), and found the specific effect of 6-benzyladenine (BA). In the presence of 10 μM BA and 1 μM β-estradiol, we observed bulbing of the root tip followed by quick shoot formation (Fig. 6d), but no shoot formation in other conditions (Fig. 6a–c).
After a longer culture period (2 months and more), in the presence of BA (without β-estradiol), we occasionally observed development of calli with leaf primordia. Such calli were generated from the cut-end of root segment but never from the root tip side (data not shown).
Expression of NTH15
Nicotiana tabacum homeobox 15 (NTH15) gene was identified as a marker gene for the SAM region and its ectopic expression caused abnormality in tobacco leaf morphology accompanied with decrease of gibberellin level and increase of cytokinin level (Tamaoki et al. 1997). We examined the expression of NTH15 in different parts of transgenic seedlings by RT-PCR. In the absence of β-estradiol, the expression was detected in cotyledons with SAM and hypocotyl but not in root (Fig. 7). These expression patterns were similar to the previous result (Tamaoki et al. 1997). However, in the presence of β-estradiol, the expression was detected also in the root generating bulbous tissues, indicating that cells specific to SAM were generated and the marker gene was expressed in the bulbous tissue within 5 days after the β-estradiol treatment (Fig. 1c, f).
The expression analysis of NTH15 and XVE in XVE::WUS seedlings. Young seedlings were cultured in the presence (+) or absence (−) of β-estradiol for 5 days. cDNAs were separately prepared from the three types of segments; cotyledon with SAM (C), hypocotyl (H) and root with RAM (R). The approximate lengths of amplified sequences are indicated on the left side. M: 0.1 kbp DNA ladder
Discussion
Appearance of shoots from root, so called “radical bud”, is observed in a number of genera: for example, Ipomoea batatas, Armoracia rusticana, Cirsium arvense, Convolvulus arvensis and Coronilla varia, though the radical buds originated from the pericycle and not from the root tip (Rauh 1950). This strategy is very efficient for vegetative reproduction in natural conditions. If such a specific capacity can be given to recalcitrant plant species by transferring the single gene, it is advantageous for efficient micropropagation. By inducing ectopic expression of WUS originated from Arabidopsis, we could regenerate fertile plants from the root tip region in tobacco (Fig. 1). Our observation suggests a potential utilization of the gene in the strategy for producing clonal plants from roots in various crops.
Shoot formation from the root tip (not from pericycle) seems to be a rare phenomenon in natural conditions (Wareing 1978) and also in vitro. We observed adventitious shoot formation from the transgenic tobacco root tips though we did not confirm whether the root meristem directly changed to the adventitious shoot meristem. From the viewpoint of plant developmental biology, we are interested in this phenomenon, especially in terms of commitment and transdifferentiation. As shown in Fig. 1a, in the early period of the response to β-estradiol, the root tip first enlarged in size and developed to a clump prior to form one to several shoot buds. When small root segments with RAM were treated with β-estradiol they also developed a clump but no shoot buds unless exogenous cytokinin was supplied (Fig. 6). We speculate that the WUS function deprived the RAM cells of their identity in the process of bulbing. This process is distinguishable from the following process of generating SAM, which requires endogenous (in case of whole seedlings) or exogenous (in case of small root tip segments) cytokinins. The canceling of developmental commitment seems to be essential for the transdifferentiation from RAM to SAM.
In A. thaliana, ectopic expression of WUS successfully induced adventitious organs by the XVE system (Zuo et al. 2002) and a Cre-loxP-recombination system, which confers random and mosaic expression pattern in tissues (Gallois et al. 2004). In the former system, adventitious embryos were generated mainly in the root region and the embryos developed to plants. In the latter system, WUS-expressing sectors were induced mainly near the root tips and the sectors generated adventitious leaves, embryos (in the presence of NAA) or inflorescences (with cooperative expression of LEAFY). However, the development of those adventitious organs did not continue probably because of constitutive expression of WUS driven by 35S promoter after Cre-loxP recombination. A similar developmental arrest was also reported in the 35S::WUS transgenic plants by Zuo et al. (2002).
For comparison with the gene expression pattern conferred by Cre-loxP-recombination system (Gallois et al. 2004), we preliminarily examined the gene expression pattern by the XVE system using a fusion reporter gene (H3sGFP) consisting of synthetic green fluorescent protein gene and tobacco H3 histone gene (Yamaji and Kyo 2006). In the root tip region of tobacco seedlings treated with the inducer, green fluorescence was observed in the nuclei of the whole organ (supplementary Fig. 2), being very different from the mosaic expression pattern as shown previously (Gallois et al. 2004). The expression pattern of WUS in the tobacco root tip seems to be identical to that of H3sGFP driven by the XVE system. The XVE system was probably suitable for obtaining regenerants from the adventitious organs. In tobacco XVE::WUS line we observed shoot formation but no somatic embryogenesis as in A. thaliana. Whether somatic embryogenesis can be induced by using tobacco orthologs for WUS remains to be examined.
In A. thaliana (Zuo et. al. 2002; Gallois et al. 2004) and tobacco (this paper), the ectopic WUS expression induces specific cells possessing the identity of embryonic and/or shoot stem cells. However, depending on the expression manner (level and duration), the expressing area (site and size) and additional factors (phytohormones and other gene expression), the specific cells did not show the same behavior. The factors effective for the behavior of the adventitious stem cells remain to be identified in the future.
As is frequently observed in A. thaliana (Zuo et. al. 2002; Gallois et al. 2004), somatic embryogenesis or adventitious organogenesis was observed most frequently on root explants or root tip region. In tobacco, as described above, the expression of WUS was detected in all parts of tobacco seedlings treated with the inducer (Fig. 3) and, in the segment culture experiment, root generation and root growth were inhibited depending on the possession of the transgene (supplementary Fig. 1) and the presence of the inducer (Figs. 4, 5). Therefore, WUS were expressed and the translational products seemed to be functional not only in the root tip but in all parts of the tobacco seedling. Additionally, when seeds of tobacco XVE::WUS lines were sown on the medium with the inducer, germination was delayed and severe abnormality was observed in the whole seedling both in the dark (Fig. 1b) and in the light condition (supplementary Fig. 3). Also in the XVE::LEC2 line severe abnormality was observed in the whole seedling when the seeds were sown in the presence of the inducer (data not shown) but when 7-day-old seedlings were treated the abnormality was limited to the root tip region (Fig. 2).
As for the organ-specific and developmental stage-specific responses to the ectopic WUS expression, existence of putative co-factors and antagonistic factors were speculated (Zuo et al. 2002). We suggest two possibilities, one is that putative co-factors for WUS decreased gradually after the seed germination and finally remained only in the root tip region. As another possibility, a putative antagonistic factor was accumulated in whole seedlings except for root tips. In previous study in A. thaliana (Schoof et al. 2000; Lenhard et al. 2002; Lohmann et al. 2001), even if WUS was overexpressed around the shoot apical meristem by using meristem-specific promoters somatic embryogenesis was not induced probably because antagonistic factors regulated the activity of WUS, like a negative feedback loop including CLAVATA1.
In the A. thaliana shoot region, microarray analysis revealed that 104 genes were up-regulated and 44 genes were down-regulated in response to the overexpression of WUS (Leibfried et al. 2005). The latter down-regulated genes include four type-A ARR (Arabidopsis response regulator) genes, which are known to show a quick response to exogenous cytokinin (D’Agostino et al. 2000), suggesting that the WUS function and cytokinin effect are antagonistic to each other in the shoot region. However, as described in the segment culture using tobacco XVE::WUS line, the small root segments with RAM required both the WUS and exogenous cytokinin for generating shoot buds (Fig. 6). At present, it is difficult to trace the context from the primary events to the appearance of markers for the shoot meristem in the root tip region. Our future target is to find some substantial changes on chromatin that are associated with the transdifferentiation of root tip cells induced by ectopic WUS function.
References
Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T et al (2002) Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14:1737–1749
D’Agostino IB, Deruere J, Kieber JJ (2000) Characterization of the response of the Arabidopsis response regulator gene family to cytokinin. Plant Physiol 124:1706–1717
Gallois J-L, Nora FR, Mizukami Y, Sablowski R (2004) WUSCHEL induces shoot stem cell activity and developmental plasticity in the root meristem. Genes Dev 18:375–380
Kwong RW, Bui AQ, Kwong LW, Fischer RL, Goldberg RW, Harada JJ (2003) LEAFY COTYLEDON1-LIKE defines a class of regulators essential for embryo development. Plant Cell 15:5–18
Kyo M, Miyatake H, Mamezuka K, Amagata K (2000) Cloning of cDNA encoding NtEPc, a marker protein for the embryogenic dedifferentiation of immature tobacco pollen grains cultured in vitro. Plant Cell Physiol 41:129–137
Laux T, Mayer KF, Berger J, Jurgens G (1996) The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122:87–96
Leibfried A, To JPC, Busch W, Stehling S, Kehle A, Demar M, Kieber JJ, Lohmann JU (2005) WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nat Lett 438:1172–1175
Lenhard M, Jurgens G, Laux T (2002) The WUSCHEL and SHOOTMERISTEMLESS genes fulfill complementary roles in Arabidopsis shoot meristem regulation. Development 129:3195–3206
Linsmaier EM, Skoog F (1965) Organic growth factor requirements in tobacco tissue cultures. Physiol Plant 18:100–128
Lohmann J, Hong RL, Hobe M, Busch MA, Parcy F, Simon R, Weigel D (2001) A molecular link between stem cell regulation and floral patterning in Arabidopsis. Cell 105:793–803
Lotan T, Ohto M, Yee KM et al (1998) Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell 93:1195–1205
Mayer KFX, Schoof H, Haecker A, Lenhard M, Jurgens G, Laux T (1998) Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95:805–815
Rauh W (1950) Morphologie der nutzpflanzen. Quelle and Meyer Verlag, Wiesbaden (Japanese translation, 1994)
Schoof H, Lenhard M, Haecker A, Mayer KFX, Jurgens G, Laux T (2000) The stem cell population of Arabidopsis shoot meristem is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100:635–644
Skoog F, Miller CO (1957) Chemical regulation of growth and organ formation in plant tissue cultivated in vitro. Sym Soc Exp Biol 11:118–131
Steward F, Mapes M, Mears K (1958) Growth and organized development of cultured cells. II. Organization in cultures grown from freely suspended cells. Am J Bot 45:705–708
Stone SL, Kwong LW, Yee KM, Pelletier J, Lepiniec L, Fischer RL, Goldberg RB, Harada JJ (2001) LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc Natl Acad Sci USA 98:11806–11811
Takebe I, Labib G, Melchers G (1971) Regeneration of whole plants from isolated mesophyll protoplasts of tobacco. Naturwissenschaften 58:318–320
Tamaoki M, Kusaba S, Kano-Murakami Y, Matsuoka M (1997) Ectopic expression of a tobacco homeobox gene, NTH15, dramatically alters leaf morphology and hormone levels in transgenic tobacco. Plant Cell Physiol 38(8):917–927
Wareing PF (1978) Determination in plant development. Bot Mag Tokyo Special Issue 1:3–17
Yamaji N, Kyo M (2006) Two promoters conferring active gene expression in vegetative nuclei of tobacco immature pollen undergoing embryogenic dedifferentiation. Plant Cell Rep 25:749–757
Zhang JZ (2003) Overexpression analysis of plant transcription factors. Curr Opin Plant Biol 6:1–11
Zuo J, Niu Q-W, Chua N-H (2000) An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J 24(2):265–273
Zuo J, Niu Q-W, Frugis G, Chua N-H (2002) The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J 30(3):349–359
Acknowledgments
We thank Prof. N-H. Chua (Rockefeller University) for his kind gift of the plasmid pER8, harboring the XVE expression system. We thank S. Nagata and S. Hayashi for their cooperation in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Sato.
Electronic supplementary material
Below is the link to the electronic supplementary material.
299_2007_342_MOESM1_ESM.tif
[Segment culture of non-transgenic tobacco seedlings. Young seedlings were cut into segments; cotyledon, hypocotyl, root and root tip with RAM, and were cultured on LS agar medium with or without b-estradiol in the dark for three weeks. Those segments were arranged in the order from cotyledon to root tip. Neither root generation nor root growth was inhibited by b-estradiol in wild-type tobacco]
299_2007_342_MOESM2_ESM.tif
[Fluorescent microscopic observation of a root tip region of XVE::H3sGFP line. A young seedling was treated with b-estradiol (1 mM) for 24 h. Note the presence of green fluorescence in the nuclei of the whole organ (right)]
299_2007_342_MOESM3_ESM.tif
[XVE::WUS seedlings showing severe abnormality in the light condition. Transgenic seeds were sown on the medium with b-estradiol and kept for 20 d in the light]
Rights and permissions
About this article
Cite this article
Rashid, S.Z., Yamaji, N. & Kyo, M. Shoot formation from root tip region: a developmental alteration by WUS in transgenic tobacco. Plant Cell Rep 26, 1449–1455 (2007). https://doi.org/10.1007/s00299-007-0342-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-007-0342-7