Abstract
Helianthus maximiliani is one of the wild Helianthus species with the genes for resistance to many pathogens including Sclerotinia sclerotiorum. Unfortunately, a transfer of disease resistance genes from this species into the cultivated sunflower is limited by its poor crossability with the cultivated sunflower and sterility of interspecific hybrids. To overcome this problem, mesophyll protoplasts of Sclerotinia sclerotiorum-resistant clone of H. maximiliani were electrically fused with etiolated hypocotyl protoplasts of the cultivated sunflower inbred line PH-BC1-91A. Fusion products were embedded in agarose droplets and subjected to different regeneration protocols. Developed microcalluses were released from the agarose and transferred into solid media. Shoot regeneration was achieved by culture of calluses on regeneration medium containing 2.2 mg l−1 BAP and 0.01 mg l−1 NAA after the treatment with a high concentration of 2,4 D for a limited period of time. A morphological and RAPD analysis confirmed a hybrid nature of the regenerated plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wild sunflowers (Helianthus spp.) constitute an important source of resistance against several major sunflower (Helianthus annuus L.) diseases including white rot, caused by a fungus Sclerotinia sclerotiorum (Lib.) de Bary. White rot is the major disease of sunflower in countries with the humid climate, whereas in countries with the moderate climate it causes the yield loss in rainy years (Masirevic and Gulya 1992). There are no suitable cultural control methods against this disease and resistant genotypes of cultivated sunflower have not yet been found or developed. The breeding for Sclerotinia resistance is complicated task since the fungus attacks most parts of the plant: the root, stem, capitulum, leaf and terminal bud. Sclerotinia reaction is under polygenic control and different resistance mechanisms that generally appear independent can be involved in different plant organs (Robert et al. 1987; Castano et al. 1992; Roenicke et al. 2004). Beside genetic factors, accumulation of the soluble phenolic compounds, melanisation and lignification of tissues also play an important part in the plant resistance (Hemery-Tardin et al. 1998; Prats et al. 2003). The complexity of the resistance mechanisms prevented development of fully tolerant or resistant genotypes (Bazzalo et al. 1991; Vasic et al. 2002). One of the wild sunflower species with the highest resistance to Sclerotinia infections is Helianthus maximiliani (Schrader) (Skoric and Rajcan 1992; Henn et al. 1997). Several attempts have been made to cross this species with cultivated sunflower using conventional methods, but with limited success due to the poor crossability and sterility of interspecific hybrids (Atlagic et al. 1995).
Somatic hybridization by protoplast fusion is known as a mean for overcoming sexual incompatibility and production of hybrids of species that could not be crossed using conventional methods, as well as for the introduction of desirable traits from the wild into the cultivated species of the same genus (Waara and Glimelius 1995). It has been successfully used for the introduction of resistance to viruses (Valkonen and Rokka 1998), bacterial (Collonier et al. 2003), and fungal pathogens (Liu et al. 1995; Furuta et al. 2004) from one plant species to another. Somatic hybridization via polyethylene glycol (PEG)-mediated fusion has been used to introduce cytoplasmic male sterility in the cultivated sunflower (Trabace et al. 1996) and for the production of interspecific hybrids between the cultivated sunflower and Helianthus giganteus L. (Henn et al. 1998a), as well as H. maximiliani (Henn et al. 1998a; Binsfeld and Schnabl 2002). Since the sensitivity of Helianthus protoplasts, especially mesophyll protoplasts of wild species, to PEG treatment is very high (Krasnyanski and Menczel 1995; Henn et al. 1998a), we tried to improve the existing fusion protocols and to produce somatic hybrid plants by using the electrofusion of protoplasts. Electrofusion is the most frequently used technique for generation of somatic hybrid plants between different species, as it helps in the maintenance of protoplast viability, reduces membrane damage, and protoplast distortion and disruption (Davey et al. 2005). Electrofusion has also been used for the fusion of sunflower protoplasts that resulted in formation of the calluses whose hybrid nature was subsequently confirmed by isozyme analysis (Barth et al. 1993). Also, it has been used for the fusion of sunflower and H. maximiliani protoplasts where formation of the hybrid calluses was confirmed by the random amplified polymorphic DNA (RAPD) analysis (Vasic 2003). However, plant regeneration from these calluses was not reported.
In this paper, we report the first successful regeneration of the somatic hybrid plants between the sunflower and H. maximiliani obtained by the electrofusion of protoplasts, and also provide a confirmation of their hybrid origin by morphological and RAPD analysis.
Materials and methods
Plant material
Helianthus annuus inbred line code number PH-BC1-91A (obtained from the Institute of Field and Vegetable Crops in Novi Sad, Serbia and Montenegro) has been used for the protoplast isolation due to its high regeneration capacity, which was tested using the protocol of Paterson and Everett (1985) (unpublished results). The seeds were surface sterilized with 14% commercial bleach for 20 min, rinsed three times in the sterile distilled water, and dehulled. Dehulled seeds were sterilized again by soaking in 5% commercial bleach (for 60 min), rinsed three times in the sterile distilled water, and dry sterilized in thermostat at 45°C for 1 h (Taski and Vasic, 2005). The seeds were germinated on MS medium (Murashige and Skoog 1962) supplemented with 2% sucrose and solidified with 0.8% agar. The seedlings were grown at 25°C in the dark.
H. maximiliani, accession 1631, was obtained from the wild Helianthus species collection of the Institute of Field and Vegetable Crops in Novi Sad, Serbia and Montenegro. This accession was found to be highly tolerant to the inoculation with Sclerotinia mycelium in field conditions (Skoric and Rajcan 1992). Apical shoots and nodal segments from a single plant were sterilized and propagated in vitro using a culture of apical shoots (Vasic et al. 2001b). Prior to transfer on propagation medium, the clones were dipped into 0.1% 4-(3-Indolyl)butanoic acid (IBA) solution for 4 min. The explants were grown on MS medium at 24°C and photoperiod 16 h (light)/8 h (dark). The same procedure was repeated every 2 weeks.
Protoplast isolation
Etiolated hypocotyls from the 7-day-old seedlings were cut longitudinally and plazmolysed in washing solution (M medium) (Vasic et al. 2001b). After 90 min, the M medium was replaced with a fresh one, supplemented with cell wall degrading enzymes according to Aslane-Chanabe (1991), and left for 17 h at 25°C. Protoplast mixture was filtered through 100 μm sieve and pelleted by centrifugation (70 g, 5 min). Pelleted protoplasts were purified by centrifugation in 10% Ficoll gradient according to Chanabe et al. (1989) at 1000 g for 20 min. Mesophyll protoplasts from H. maximiliani leaves were isolated according to the protocol of Vasic et al. (2001b). Protoplasts were purified by floating in 15% Ficoll gradient solution, and irradiated by UV rays (2 μmol m−2 s−1) for 15 min.
Electrofusion
Protoplasts of sunflower and H. maximiliani were mixed at a ratio of 1:1. The protoplast density was adjusted to 6.7×105 ml−1 with TF solution (Aslane-Chanabe 1991). Electrofusion was carried out in Eppendorf Multiporator, in which protoplasts were fused with three pulses of 1250 V cm−1 for 30 μs. Electrofused protoplasts were incubated overnight in the dark at 25°C.
Protoplast culture and plant regeneration
Fusion products were embedded in 200 μl agarose solidified droplets (Shillito et al. 1983) at the final density of 105 ml−1 and incubated at 4°C for 1 h. The droplets were cultured according to the regeneration protocols of Krasnyanski and Menczel (1993), Wingender et al. (1996), and Trabace et al. (1995). The cultures were maintained in the dark at 25°C.
Microcalluses were released from the agarose droplets and transferred onto solid differentiation medium containing 2.2 mg l−1 6-Benzylaminopurine (BAP) and 0.01 mg l−1 1-Naphthylacetic acid (NAA) (Krasnyanski et al. 1992), but with the addition of silver nitrate (KR-R-Ag medium) (Vasic et al. 2001a), and incubated at 24°C with the photoperiod 16 h (light)/8 h (dark).
Well-developed calluses were transferred to the KR-R-Ag medium without BAP and NAA, but with 10 mg ml−1 2,4-Dichlorophenoxyacetic acid (2,4 D) for 3 days and then transferred onto the KR-R-Ag medium. When the shoot elongation started, shoots were excised from the surrounding callus and placed onto the MS medium supplemented with 2% sucrose and solidified with 0.8% agar. The rooting of shoots occurred on the MS medium after the shoots were dipped into 0.1% IBA solution for 4 min.
Isozyme and RAPD analysis
The leaves of in vitro grown plants were homogenized in 0.5 M Tris-HCl buffer pH 6.8 with 1% 2-Mercaptoethanol and the crude extract was absorbed onto paper wicks (2×11 mm; Whatman 3MM). The samples were subjected to the starch electrophoresis. After electrophoresis, the gel was sliced horizontally into 1 mm slabs that were separately incubated in the specific staining solutions for visualization of the following enzymes: malate dehydrogenase (MDH; E.C. 1.1.1.37), acid phosphatase (ACP; E.C. 3.1.3.2), phosphohexose isomerase (PHI; E.C. 5.3.1.9), 6-phosphogluconate dehydrogenase (PGD; E.C. 1.1.1.44), phosphogluco mutase (PGM; E.C. 5.4.2.2), and aconitase (ACO; E.C. 4.2.1.3). Both electrophoresis and enzyme staining were performed according to Stuber et al. (1988).
DNA for RAPD analysis was isolated from the leaves of both parents and somatic hybrid, according to the protocol of Gentzbittel et al. (1994). The analysis was performed using ten 10-base primers from Operon Technologies, which were found previously to be unique markers for the wild Helianthus species (Sossey-Alaoui et al. 1998): A11 (5′-CAATCGCCGT-3′), A14 (5′-TCTGTGCTGG-3′), C02 (5′-GTGAGGCGTC-3′), C04 (5′-CCGCATCTAC-3′), C14 (5′-TGCGTGCTTG-3′), C15 (5′-GACGGATCAG-3′), C19 (5′-CCTCTAGACC-3′), E03 (5′-CCAGATGCAC-3′), E05 (5′-TCAGGGAGGT-3′), E09 (5′-CTTCACCCGA-3′), and E15 (5′-ACGCACAACC-3′).
All polymerase chain reactions (PCR) were carried out in a 25-μl reaction volume containing 2.5 μl buffer (Amersham Pharmacia Biotech); 0.2 mM dNTP; 0.5 μM primer; two units of Taq polymerase (Amersham Pharmacia Biotech) and 30 ng DNA. In the second PCR reaction, 2 μl of the PCR products were used instead of DNA. DNA was amplified in a termocycler (Biometra Tpersonal) at 94°C for 1 min followed by 35 cycles at 93°C for 1 min, 38°C for 30 s, 72°C for 30 s and final elongation at 72°C for 6 min. The PCR products were separated on 2% agarose gels containing 0.005% ethidium bromide. The gels were analysed under UV light.
Results and discussion
Protoplast isolation
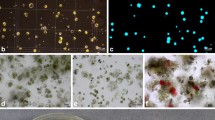
The crude pellet containing isolated protoplasts obtained after the elimination of the enzyme solution was contaminated by debris from the broken cells. Moreover, H. maximiliani protoplasts were very heterogeneous in size. Flotation in Ficoll gradient enabled the recovery of pure homogenous protoplast fractions in both species. The leaves of H. maximiliani grown in vitro gave high protoplast yield of about 5×106 g−1 of fresh weight, which is in accordance with the results obtained with the other wild Helianthus species: 3×106 g−1 of fresh weight from H. giganteus, 2×106 g−1 of fresh weight from H. nuttallii (Henn et al. 1998b), and 1.5×106 g−1 of fresh weight from H. maximiliani (Vasic et al. 2001b). H. maximiliani protoplasts were small with lots of chloroplasts (Fig. 1b). The yield of purified protoplasts isolated from the cultivated sunflower hypocotyls was 4–5×105 g−1 of fresh weight, which agrees with the results of Schmitz and Schnabl (1989), while Wingender et al. (1996) and Krasnyanski and Menczel (1993) reported yield of 2–3×106 g−1 of fresh weight. The sunflower protoplasts were bigger then those of H. maximiliani, etiolated and with a large number of vacuoles (Fig. 1a).
Electrofusion and agarose droplets culture
Successful electrofusions resulted in the formation of heterokaryons (Fig. 1c). The fusion products were embedded in agarose droplets and subjected to different regeneration protocols. When regeneration protocols of Krasnyanski and Menczel (1993) and Wingender et al. (1996) were used, no callus formation was observed. Using the protocol of Trabace et al. (1995) during the first week of culture, symmetrical divisions of the protoplasts were observed and the protoplasts developed into small, macroscopic colonies (Fig. 1d). After 3–4 weeks in the culture, the number of white microcalluses became visible.
Callus cultivation and plant regeneration
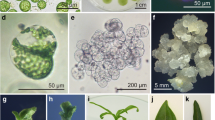
Developed microcalluses that were transferred onto solid KR-R-Ag medium and exposed to the light continued to grow. Since it is known that the exposure of callus colonies to the high concentration of 2,4 D for a limited period of time induces subsequent development of somatic embryos (Krasnyanski and Menczel 1993), some of the vigorously growing calluses were treated with this hormone. The 2,4 D treatment improved the organogenic response of protoplast-derived calluses, since the shoot formation has been observed on two out of 350 calluses (regeneration frequency of 0.57%) when the calluses were returned onto the KR-R-Ag medium (Fig. 2a). Beside 2,4 D treatment, genotype of the recipient parent could also be one of the factors that affected the shoot regeneration (Davey et al. 2005). Like Krasnyanski and Menczel (1993), we also used the cultivated sunflower genotype that was pre-screened for its regeneration capacity using the protocol of Paterson and Everett (1985). Trabace et al. (1995) also used a genotype of known regeneration capacity as a recipient in the fusion experiments.
In total, five shoots were obtained, three from one and two from the other regenerating callus. The shoots were further multiplied with varying success and fifteen clones were produced (Table 1). The clones showed signs of hyperhydration, although they were grown on KR-R-Ag medium that contained silver nitrate, which is known to be an inhibitor of ethylene action and agent that significantly reduces hyperhydration (Krasnyanski and Menczel 1993; Wingender et al. 1996). Krasnyanski and Menczel (1993) have reported that the shoots started to exhibit hyperhydration even in the presence of silver nitrate, if they were left on the regeneration medium for prolonged periods. In our study, the hyperhydration continued even when the shoots were excised from the surrounding callus and placed onto hormone-free MS medium containing silver nitrate (Fig. 2b). Since it is known that different factors can promote hyperhydration and silver nitrate is not always effective against it (Fischer et al. 1992), it was assumed that in our case hyperhydration was not the consequence of the gas exchange, but caused by an excess of cytokinins. Therefore, the shoots were transferred onto hormone-free MS medium without silver nitrate. It seems that time triggered the decrease of cytokinin level in the shoots, as hyperhydration was surpassed and the normal shoot elongation continued after the prolonged culture of shoots on the hormone-free medium (Fig. 2c). Well-developed shoots were rooted by dipping in a high-auxin solution, but rooting efficiency depended on the clone (Table 1). In contrast to Burrus et al. (1991) and Wingender et al. (1996), we used IBA instead of 3-Indolylacetic acid (IAA) or NAA. The root formation on auxin-treated shoots was observed after 3–4 weeks of culture on the hormone-free medium (Fig. 2d). The plantlets with well-developed roots were transferred to the growth chambers for acclimatization. In all the shoots in which a problem of hyperhydration was not completely solved, the treatment with IBA always resulted in callusing. According to the work of other authors, when regenerated shoots could not have been rooted they were grafted in order to be transferred in ex vitro conditions (Fischer et al. 1992; Krasnyanski and Menczel 1993).
Characterization of hybrid plants
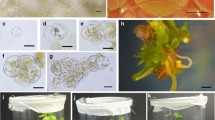
Morphological comparisons between parents and R1 plants were done. The R1 plants were intermediate in the leaf length and width between the parents (Fig. 3). All plants were branched resembling those of the H. maximiliani parent. Zymograms of ACO, ACP and PGD did not reveal any polymorphism between the parents and regenerated plants. A polymorphism between the parents was observed on MDH, PGM and PHI (Fig. 4) zymograms, but the regenerated plants had the same allelic variant as the sunflower.
In order to prove the hybrid nature of regenerated plants, a molecular investigation of the regenerated plants was performed using RAPD markers. This type of markers is very useful in work with genus Helianthus, since the knowledge of the genome of the majority of wild species is quite sparse. The band patterns of the somatic hybrids obtained by primers A11, A14, C02, C04, C14 C15, C19, E03 (Fig. 5), E09, and E15 showed all characteristics of PH-BC1-91A. The band characteristic for H. maximiliani was detected only on the RAPD profile generated with E05 primer, indicating the presence of a part of its genome in the hybrid plants (Fig. 6). The absence of bands characteristic for H. maximiliani in band patterns of the somatic hybrids generated by all, but one primer indicates asymmetric nature of these hybrids. It is probably the reason why there could not be detected any difference on isozyme level between the regenerated plants and sunflower. This is the result of the treatment with UV rays, which fragmentises the nuclear genome and has been successfully used for the production of asymmetric somatic hybrids in some other species (Vlahova et al. 1997; Forsberg et al. 1998).
In our work, we obtained the first somatic hybrid plants produced by electrofusion between the cultivated sunflower and its wild relative—H. maximiliani. The absence of bands characteristic for the wild parent in RAPD profiles generated by the majority of used primers indicates that produced hybrids are asymmetric, thus confirming the efficiency of UV light treatment. The studies are in progress in order to determine exact nature of the hybrids as well as their tolerance to Sclerotinia sclerotiorum. Since the introduction of tolerance to this pathogen into the cultivated sunflower is the ultimate goal of our work, in further experiments the fusion products will be cultured in the presence of oxalic acid, a known toxin of S. sclerotiorum, in order to increase the chances of regeneration of tolerant plants.
Abbreviations
- 2,4 D:
-
2,4-Dichlorophenoxyacetic acid
- ACO:
-
Aconitase
- ACP:
-
Acid Phosphatase
- BAP:
-
6-Benzylaminopurine
- IAA:
-
3-Indolylacetic acid
- IBA:
-
4-(3-Indolyl)butanoic acid
- MDH:
-
Malate Dehydrogenase
- NAA:
-
1-Naphthylacetic acid
- PCR:
-
Polymerase Chain Reaction
- PEG:
-
Polyethylene Glycol
- PGD:
-
6-Phosphogluconate Dehydrogenase
- PGM:
-
Phosphogluco Mutase
- PHI:
-
Phosphohexose Isomerase
- RAPD:
-
Random Amplified Polymorphic DNA
References
Aslane-Chanabe C (1991) Regeneration de plantes a partir de protoplastes chez le genre Helianthus et hybridation somatique entre le tournesol cultive et les tournesols sauvages. Doctoral Thesis, INP, Toulouse, France
Atlagic J, Dozet B, Skoric D (1995) Meiosis and pollen grain viability in Helianthus mollis, H. salicifolius, H. maximiliani and their F1 hybrids with cultivated sunflower. Euphytica 81:259–263
Barth S, Voeste D, Wingender R, Schnabl H (1993) Somatic hybrids of sunflower (Helianthus annuus L.) identified at the callus by isozyme analysis. Bot Acta 106:100–102
Bazzalo ME, Dimarco P, Martinez F, Daleo GR (1991) Indictors of resistance of sunflower plant to basal rot (Sclerotinia sclerotiorum): symptological, biochemical, anatomical and morphological characters of the host. Euphytica 57:195–205
Binsfeld P, Schnabl H (2002) Molecular and cytogenetic constitution of plants obtained via two different somatic hybridization methods. Plant Cell Rep 21:58–62
Burrus M, Chanabe C, Alibert G, Bidney D (1991) Regeneration of fertile plants from protoplasts of sunflower (Helianthus annuus L.). Plant Cell Rep 10:161–166
Castano F, Hemery-Tardin MC, Tourvieille de Labrouhe D, Vear F (1992) The inheritance and biochemistry of resistance to Sclerotinia sclerotiorum leaf infections in sunflower (Helianthus anuus L.). Euphytica 58:209–219
Chanabe C, Burrus M, Alibert G (1989) Factors affecting the improvement of colony formation from sunflower protoplast. Plant Sci 64:125–132
Collonier C, Fock I, Daunay MC, Servaes A, Vedel F, Siljak-Yakovljev S, Souvannavong V, Sihachackr D (2003) Somatic hybrids between Solanum melongena and S. sismbrifoilium, as a useful source of resistance against bacterial and fungal wilts. Plant Sci 164(5):849–861
Davey MR, Anthony P, Power JB, Lowe KC (2005) Plant protoplasts: status and biotechnological perspectives. Biotechnol Adv 23:131–171
Fischer C, Klethi P, Hahne G (1992) Protoplasts from cotyledon and hypocotyl of sunflower (Helianthus annuus L.): shoot regeneration and seed production. Plant Cell Rep 11:623–636
Forsberg J, Lagercrantz U, Glimelius K (1998) Comparison of UV light, X-ray and restriction enzyme treatment as tools in production of asymmetric somatic hybrids between Brassica napus and Arabidopsis thaliana. Theor Appl Genet 96:1178–1185
Furuta H, Shinoyama H, Nomura Y, Maeda M, Makara K (2004) Production of intergeneric somatic hybrids of chrysanthemum (Dendranthema x grandiflorum (Ramat.)) Kitamura) and wormwood (Artemisia sieversiana J.F.Ehrh. ex. Willd) wild rust (Puccinia horiana Hening) resistance by electrofusion of protoplasts. Plant Sci 166:695–702
Gentzbittel L, Zhang G, Vear F, Griveau Y, Nicolas P (1994) RFLP studies of genetic relationships among inbred lines of cultivated sunflower (Helianthus annuus L.): evidence for distinct restorer and maintainer germplasm pools. Theor Appl Genet 89:19–425
Hemery-Tardin M, Tourvieille D, Vear F (1998) Effect of infection by Sclerotinia spp. on the phenolic metabolism of sunflower capitula and leaves. Helia 29:19–32
Henn HJ, Steiner R, Wingender R, Schnabl H (1997) Wild type sunflower clones: source of resistance against Sclerotinia sclerotiorum (Lib.) de Bary stem infection. Angew Bot 71:5–9
Henn HJ, Wingender R, Schnabl H (1998a) Regeneration of fertile interspecific hybrids from protoplast fusions between Helianthus annuus L. and wild Helianthus species. Plant Cell Rep 18:220–224
Henn HJ, Wingender R, Schnabl H (1998b) Regeneration of fertile plants from Helianthus nuttallii T&G and Helianthus giganteus L. mesophyll protoplast. Plant Cell Rep 18:288–291
Krasnyanski S, Polgar Z, Nemeth G, Menczel L (1992) Plant regeneration from callus and protoplast cultures of Helianthus giganteus L. Plant Cell Rep 11:7–10
Krasnyanski S, Menczel L (1993) Somatic embryogenesis and plant regeneration from hypocotyls protoplast of sunflower (Helianthus annuus L.). Plant Cell Rep 12:260–263
Krasnyanski S, Menczel L (1995) Production of fertile somatic hybrid plants of sunflower and Helianthus giganteus L. by protoplast fusion. Plant Cell Rep 14:232–235
Liu JH, Dixelius C, Eriksson I., Glimelius K (1995) Brassica napus (+) B. tourneforti, a somatic hybrid containing traits of agronomic importance for rapeseed breeding. Plant Sci 109(1):75–86
Masirevic S, Gulya TJ (1992) Sclerotinia and Phomopsis—two devastating sunflower pathogens. Field Crops Res 30:271–300
Murashige T, Skoog F (1962) A revised medium for growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Paterson KE, Everett NP (1985) Regeneration of Helianthus annuus inbred plants from callus. Plant Sci 42:125–132
Prats E, Bazzalo ME, Leon A, Jorrin JV (2003) Accumulation of soluble phenolic compounds in sunflower capitula correlates with resistance to Sclerotinia sclerotiorum. Euphytica 132:321–329
Robert N, Vear F, Tourvieille de Labrouche D (1987) L'heredite de la resistance au Sclerotinia sclerotiorum (Lib.) de Bary chez le tournesol. Etude des reaction a deux test myceliens. Agronomie 4:423–429
Roenicke S, Hahn V, Horn R, Groene I, Brahm L, Schnabl H, Friedt W (2004) Interspecific hybrids of sunflower as a source of Sclerotinia resistance. Plant Breed 123:152–157
Schmitz P, Schnabl H (1989) Regeneration and evaluation of protoplasts from mesophyll, hypocotyl and petioles from Helianthus annuus L. J Plant Physiol 135:223–227
Shillito RD, Paszkowski J, Potrykus I (1983) Agarose plating and a bead type culture technique enable and stimulate development of protoplast derivated colonies in a number of plant species. Plant Cell Rep 2:244–247
Skoric D, Rajcan I (1992) Breeding for Sclerotinia resistance in sunflower. In: Proceedings of the 13th International Sunflower Conference, Pisa, Italy, pp. 1257–1262
Sossey-Alaoui K, Serieys H, Tersac M, Lambert P, Schilling E, Griveau Y, Kaan F, Berville A (1998) Evidence for several genomes in Helianthus. Theor Appl Genet 97:422–430
Stuber CW, Wendel JF, Goodman MM, Smith JSC (1988) Techniques and scoring procedures for starch gel electrophoresis of enzymes from maize (Zea mays L.). Technical bulletin 286, North Carolina agricultural research service, pp 95
Taski K, Vasic D (2005) Different sterilization methods for overcoming internal bacterial infection in sunflower seeds. Proc Nat Sci, Matica Srpska 109:59–64
Trabace T, Fiore MC, D'Ambrosio C, Vanadia S, Sunseri F (1996) Sunflower cytoplasmic hybrids revealed by PCR assay using male sterility as selectable marker. J Genet Breed 50:29–34
Trabace T, Vischi M, Fiore MC, Sunseri F, Vanadia S, Marchetti S, Olivieri AM (1995) Plant regeneration from hypocotyl protoplast in sunflower (Helianthus annuus L). J Genet Breed 49:51–54
Valkonen JPT, Rokka V-M (1998) Combination and expression of two virus resistance mechanisms in interspecific somatic hybrids of potato. Plant Sci 131(1):85–94
Vasic D, Alibert G, Skoric D (2001a) Protocol for efficient repetitive and secondary somatic embryogenesis in the Helianthus maximiliani (Schrader). Plant Cell Rep 20:121–125
Vasic D, Skoric D, Gilbert A, Miklic V (2001b) Micropropagation of Helianthus maximiliani (Schrader) by shoot apex culture. Helia 24:63–68
Vasic D, Skoric D, Taski K, Stosic Lj (2002) Use of oxalic acid for screening intact sunflower plants for resistance to Sclerotinia in vitro. Helia 36:145–152
Vasic D (2003) Sunflower somatic hybridization. Andrejevic Endowment, Belgrade
Vlahova M, Hinnisdaels S, Frulleux F, Claeys M, Atanassov A, Jacobs M (1997). UV irradiation as a tool for obtaining asymmetric somatic hybrids between Nicotiana plumbaginifolia and Lycopersicon esculentum. Theor Appl Genet 94:184–191
Waara S, Glimelius K (1995) The potential of somatic hybridization in crop breeding. Euphytica 85:217–255
Wingender R, Henn HJ, Barth S, Voeste D, Machlab H, Schnabl H (1996) A regeneration protocol for sunflower (Helianthus annuus L.) protoplasts. Plant Cell Rep 15:742–745
Acknowledgements
This work was supported by the Serbian Ministry of Science, Technology and Development as a part of the project BTR.5.02.0401.B.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Atanassov
Rights and permissions
About this article
Cite this article
Taski-Ajdukovic, K., Vasic, D. & Nagl, N. Regeneration of interspecific somatic hybrids between Helianthus annuus L. and Helianthus maximiliani (Schrader) via protoplast electrofusion. Plant Cell Rep 25, 698–704 (2006). https://doi.org/10.1007/s00299-006-0134-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-006-0134-5